Deposition of PM2.5 Sulfate in the Spring on Urban Forests in Beijing, China
Abstract
:1. Introduction
2. Experimental Description and Methodology
2.1. Experimental Description
2.2. Sample Collection
2.3. Calculation of Deposition Velocity
2.4. Empirical Models
3. Results and Discussion
3.1. Meteorological Conditions
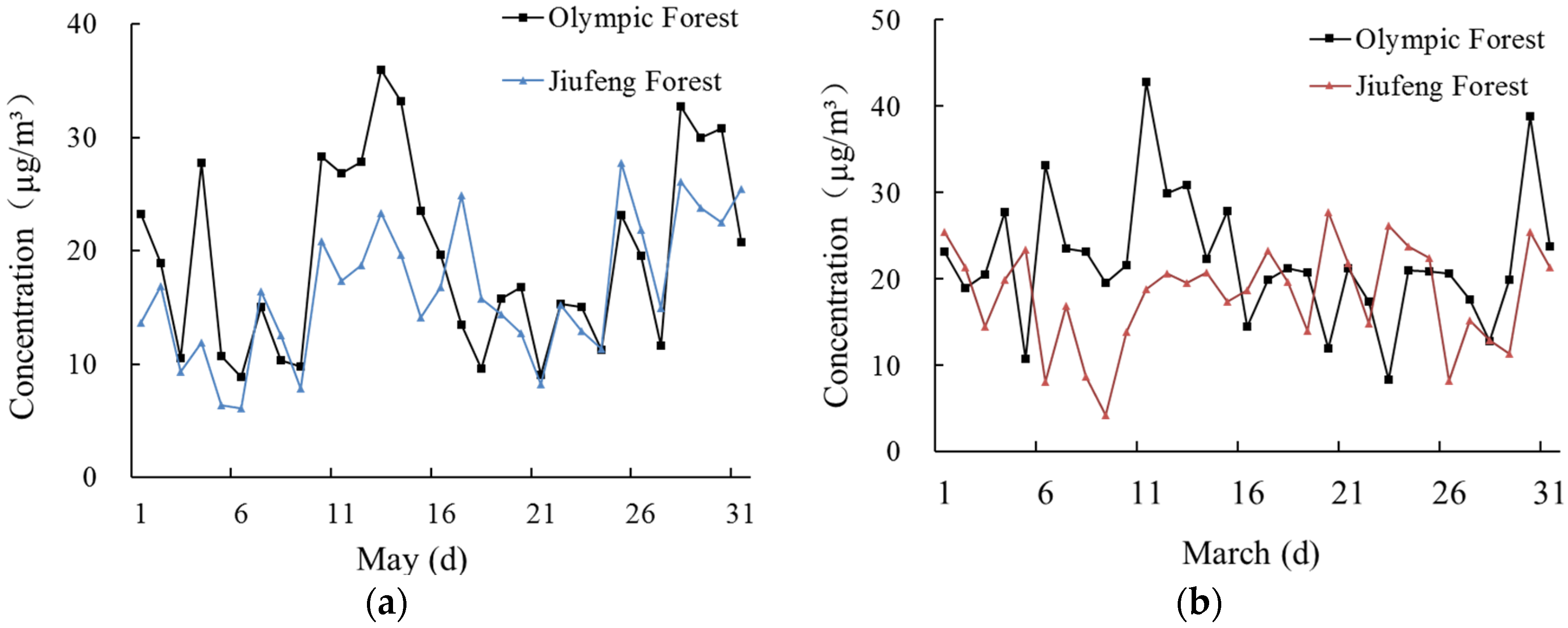
3.2. Sulfate Concentrations and Vertical Gradients
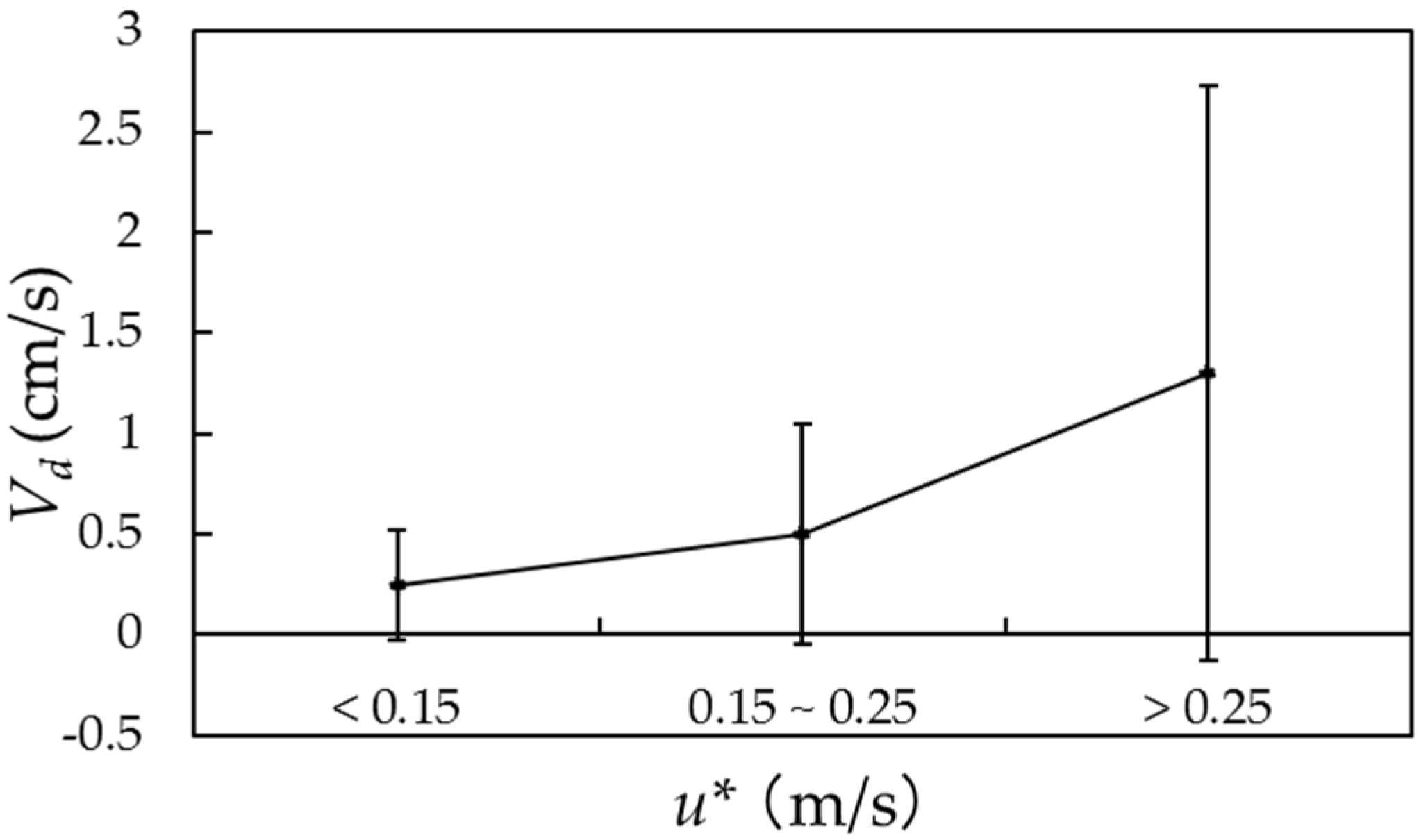
3.3. Deposition Velocity of PM2.5 Sulfate
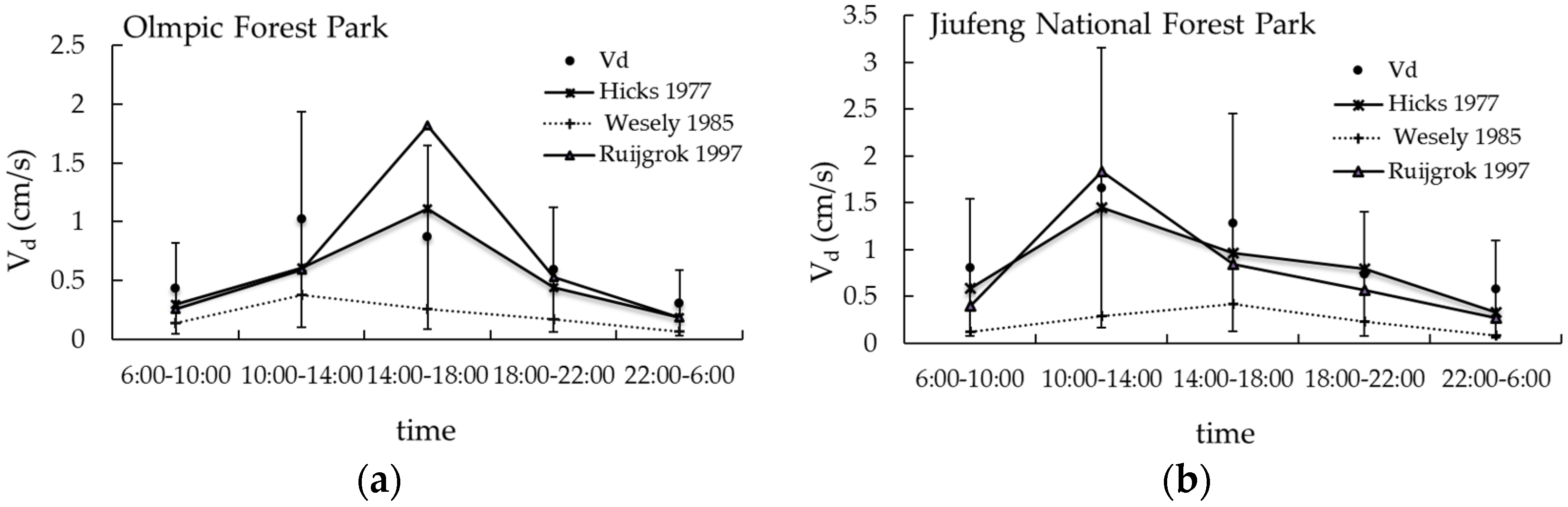
3.4. Comparison between Measured and Other Calculated Vd by Model
3.5. Comparison between Measured and Parameterized Vd
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yan, S.; Li, H. Harm and prevention of particulate matter 2.5. Sci. Technol. Inf. 2013, 15, 38–39. [Google Scholar]
- Yuet, L.L.; Sequeira, R. Water-soluble aerosol and visibility degradation in Hong Kong during autumn and early winter, 1998. Environ. Pollut. 2002, 116, 225–233. [Google Scholar]
- EANET. Second Periodic Report on the State of Acid Deposition in East Asia (Part III)—Executive Summary. Available online: http://www.eanet.asia/product/ (accessed on 29 December 2016).
- Yang, S.; Cao, J.; Shen, Z.; Liu, S.; Zhang, S.; Wang, Q.; Qiang, J.; Li, W. Variations of water-soluble ions in PM2.5 at Xi’an between summer and winter. Environ. Chem. 2012, 31, 1179–1188. [Google Scholar]
- Gu, J.; Wu, L.; Huo, G.; Bai, Z.; Du, S.; Liu, A.; Xie, Y. Pollution character and source of water-soluble inorganic ions in PM2.5 over tianjin. Environ. Monit. China 2013, 29, 30–34. [Google Scholar]
- Zhang, B.; Zhang, C.; Zhou, B.; Jiao, J.; Jiang, J.; Tao, L. Time series characteristics of atmospheric particulate pollution PM2.5 and its water-soluble inorganic ion during the heating period of Xi’an. Chin. J. Environ. Eng. 2012, 6, 1643–1646. [Google Scholar]
- Katul, G.G.; Gronholm, T.; Launiainen, S.; Vesala, T. The effects of the canopy medium on dry deposition velocities of aerosol particles in the canopy sub-layer above forested ecosystems. Atmos. Environ. 2011, 45, 1203–1212. [Google Scholar] [CrossRef]
- Wyers, G.P.; Duyzer, J.H. Micrometeorological measurement of the dry deposition flux of sulfate and nitrate aerosols to coniferous forest. Atmos. Environ. 1997, 31, 333–343. [Google Scholar] [CrossRef]
- Elshout, A.J.; Viljeen, J.W.; van Duuren, H. Sulphates and sulphuric acid in the atmosphere in the years 1971–1976 in The Netherlands. Atmos. Environ. 1978, 12, 785–790. [Google Scholar] [CrossRef]
- Gallagher, M.W.; Beswick, K.M.; Duyzer, J.; Westrate, H.; Choularton, T.W.; Hummelshoj, P. Measurements of aerosol fluxes to speulder forest using a micrometeorological technique. Atmos. Environ. 1997, 31, 359–373. [Google Scholar] [CrossRef]
- Vong, R.J.; Vong, I.J.; Vickers, D.; Covert, D.S. Size-dependent aerosol deposition velocities during bearpex’07. Atmos. Chem. Phys. 2010, 10, 5749–5758. [Google Scholar] [CrossRef]
- Wesely, M.L.; Cook, D.R.; Hart, R.L.; Speer, R.E. Measurement and parameterization of particulate sulfur dry deposition over grass. J. Geophys. Res. 1985, 90, 2131–2143. [Google Scholar] [CrossRef]
- Hicks, B.B.; Wesely, M.L.; Durham, J.L.; Brown, M.A. Some direct measurements of atmospheric sulfur fluxes over a pine plantation. Atmos. Environ. 1982, 16, 2899–2903. [Google Scholar] [CrossRef]
- Hicks, B.B.; Matt, D.R.; McMillan, R.T.; Womack, J.D.; Wesely, M.L.; Hart, R.L.; Cook, D.R.; Lindberg, S.E.; De Pena, R.G.; Thomsom, D.W. A field investigation of sulfate fluxes to a deciduous forest. J. Geophys. Res. 1989, 94, 13003–13011. [Google Scholar] [CrossRef]
- Tsai, J.H.; Chang, L.P.; Chiang, H.L. Size mass distribution of water-soluble ionic species and gas conversion to sulfate and nitrate in particulate matter in southern Taiwan. Environ. Sci. Pollut. Res. Int. 2013, 20, 4587–4602. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Fujimura, Y.; Hayashi, K.; Takahashi, A.; Nakaya, K. Deposition velocity of PM2.5 sulfate in the summer above a deciduous forest in central Japan. Atmos. Environ. 2010, 44, 4582–4587. [Google Scholar] [CrossRef]
- Cheng, M.T.; Horng, C.L.; Lin, Y.C. Characteristics of atmospheric aerosol and acidic gases from urban and forest sites in central Taiwan. Bull. Environ. Contam. Toxicol. 2007, 79, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Yin, Z.; Lun, X.; Zhao, Y.; Li, R.; Shi, F.; Yu, X. Deposition velocity of PM2.5 in the winter and spring above deciduous and coniferous forests in Beijing, China. PLoS ONE 2014, 9, e97723. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, L.; Wang, H.; Yang, Y.; Liu, J.; Qiu, D.; Ma, W.; Zhang, Z.; Liu, J. Dry deposition of particulate matter at an urban forest, wetland and lake surface in Beijing. Atmos. Environ. 2016, 125, 178–187. [Google Scholar] [CrossRef]
- Zhang, L.; Gong, S.; Padro, J.; Barrie, L. A size-segregated particle dry deposition scheme for an atmospheric aerosol module. Atmos. Environ. 2001, 35, 549–560. [Google Scholar] [CrossRef]
- Erisman, J.W.; Draaijers, G.P.J. Atmospheric Deposition in Relation to Acidification and Eutrophication; Elsevier Science: Amsterdam, Netherlands, 1995; Volume 63, p. 405. [Google Scholar]
- Wesely, M.L.; Hicks, B.B. Some factors that affect the deposition rates of sulfur dioxide and similar gases on vegetation. J. Air Pollut. Control Assoc. 1977, 27, 1110–1116. [Google Scholar] [CrossRef]
- Walter, R.; Hans, T.; Peter, E. The dry deposition of particles to a forest canopy: A comparison of model and experimental results. Atmos. Environ. 1997, 31, 399–415. [Google Scholar]
- Garland, J.A. Dry and wet removal of sulphur from the atmosphere. Atmos. Environ. 1978, 12, 349–362. [Google Scholar] [CrossRef]
- Hicks, B.B.; Baldocchi, D.D.; Meyers, T.P.; Hosker, R.P., Jr.; Matt, D.R. A preliminary multiple resistance routine for deriving dry deposition velocities from measured quantities. Water Air Soil Pollut. 1987, 36, 311–330. [Google Scholar] [CrossRef]
- Wang, L.-L.; Wang, Y.-S.; Wang, Y.-H.; Sun, Y.; Ji, D.-S.; Ren, Y.-F. Relationship between different synoptic weather patterns and concentration of NOX, O3 and PM2.5/10 in beijing during summer and autumn. China Environ. Sci. 2010, 30, 924–930. [Google Scholar]
- Yarnal, B. Synoptic climatology in environmental analysis, a primer. J. Prev. Med. Inf. 1993, 347, 170–180. [Google Scholar]
- Petroff, A.; Mailliat, A.; Amielh, M.; Anselmet, F. Aerosol dry deposition on vegetative canopies. Part I: Review of present knowledge. Atmos. Environ. 2008, 42, 3625–3653. [Google Scholar] [CrossRef]
- Slinn, W.G.N. Predictions for particle deposition to vegetative canopies. Atmos. Environ. 1982, 16, 1785–1794. [Google Scholar] [CrossRef]
- Xu, Y.; Gregory, R.C. Modeling the dry deposition velocity of sulfur dioxide and sulfate in Asia. Appl. Meteorol. 1998, 37, 1084–1099. [Google Scholar] [CrossRef]


| Olympic Forest Park | Jiufeng National Forest Park | |||||||
|---|---|---|---|---|---|---|---|---|
| March | May | March | May | |||||
| Daytime | Nighttime | Daytime | Nighttime | Daytime | Nighttime | Daytime | Nighttime | |
| Concentration of SO42− (μg/m3) | 29.8 | 15.0 | 26.8 | 15.0 | 22.5 | 14.7 | 21.3 | 13.7 |
| Temperature (°C) | 4.16 ± 4.23 | 1.38 ± 2.78 | 23.81 ± 5.23 | 20.04 ± 4.57 | 4.58 ± 4.98 | 1.73 ± 2.99 | 20.96 ± 4.97 | 18.12 ± 4.23 |
| Wind speed (m/s) | 1.12 ± 0.5 | 0.86 ± 0.43 | 1.06 ± 0.64 | 0.79 ± 0.49 | 0.97 ± 0.49 | 0.7 ± 0.38 | 1.28 ± 0.73 | 0.96 ± 0.51 |
| Relative Humidity (%) | 44 ± 17 | 50 ± 19 | 53 ± 20 | 59 ± 22 | 42 ± 18 | 47 ± 18 | 56 ± 21 | 62 ± 23 |
| Solar radiation (w) | 221 ± 187 | −1.24 ± 1.3 | 312 ± 251 | 0.94 ± 1.6 | 208 ± 199 | −1.63 ± 1.4 | 343 ± 231 | 1.21 ± 1.6 |
| Olympic Forest Park | Jiufeng National Forest Park | |||
|---|---|---|---|---|
| Daytime | Nighttime | Daytime | Nighttime | |
| u* (m/s) | 0.35 ± 0.22 | 0.19 ± 0.12 | 0.42 ± 0.25 | 0.31 ± 0.18 |
| L (m) | −0.18 ± 9.7 | 27.97 ± 14.3 | −4.37± 7.8 | 22.80 ± 6.4 |
| Vd (cm/s) | 0.9 ± 1.0 | 0.4 ± 0.3 | 1.3 ± 1.2 | 0.8 ± 0.8 |
| Surface | Species | LAI | Sampling Time | Method | Vd (cm/s) | Location | Reference |
|---|---|---|---|---|---|---|---|
| Deciduous forest | PM2.5 Sulfate | 1.8 | May 2013 | Gradient method | Daytime 0.9 ± 1.0, Nighttime 0.4 ± 0.3 | Beijing Olympic, China | This work |
| Coniferous forest | 3.8 | May 2013 | Daytime 1.3 ± 1.2, Nighttime 0.8 ± 0.8 | Beijing Jiufeng, China | |||
| Deciduous forest | PM2.5 Sulfate | 5–7 | Early summer (2 to 8 July) in 2009 | Gradient method | Daytime 0.9± 1.0, Nighttime 0.3 ± 0.3 | Mt. Asama, Japan | [16] |
| Deciduous forest | Particulate sulfate | / | May 1983 | Eddy correlation | Daytime 1.0, Nighttime 0, Mean 0.6 | Oak Ridge, Tennessee | [14] |
| Coniferous forest | Sulfate | 11 | April, June/July, September and November/December 1993 | Gradient method | Stable ~0.0 Unstable < 4.0 Mean 0.7 ± 0.3 | The Netherlands | [8] |
| Pine forest | Particulate sulfur | / | June 1981 | Eddy correlation | Moderately unstable 0.90 ± 0.09 Neutral 0.48 ± 0.04 | America | [12] |
| Coniferous/Deciduous Forest | Sulfate | / | May 1990 | Eddy correlation | All day mean 0.11 | Asia | [30] |
| Coniferous forest | Particulate sulfur | / | July 1977 | Eddy correlation | Daytime 0.5 ± 1.0, Mean 0.7 | North Carolina | [13] |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Lun, X.; Li, R.; Cao, Y.; Sun, F.; Yu, X. Deposition of PM2.5 Sulfate in the Spring on Urban Forests in Beijing, China. Atmosphere 2017, 8, 3. https://doi.org/10.3390/atmos8010003
Zhao L, Lun X, Li R, Cao Y, Sun F, Yu X. Deposition of PM2.5 Sulfate in the Spring on Urban Forests in Beijing, China. Atmosphere. 2017; 8(1):3. https://doi.org/10.3390/atmos8010003
Chicago/Turabian StyleZhao, Lu, Xiaoxiu Lun, Renna Li, Yingying Cao, Fengbin Sun, and Xinxiao Yu. 2017. "Deposition of PM2.5 Sulfate in the Spring on Urban Forests in Beijing, China" Atmosphere 8, no. 1: 3. https://doi.org/10.3390/atmos8010003





