Size-Dependent Characterization of Atmospheric Particles during Winter in Beijing
Abstract
:1. Introduction
2. Experimental Methods
2.1. Sampling Site
2.2. Instrumentation
3. Results
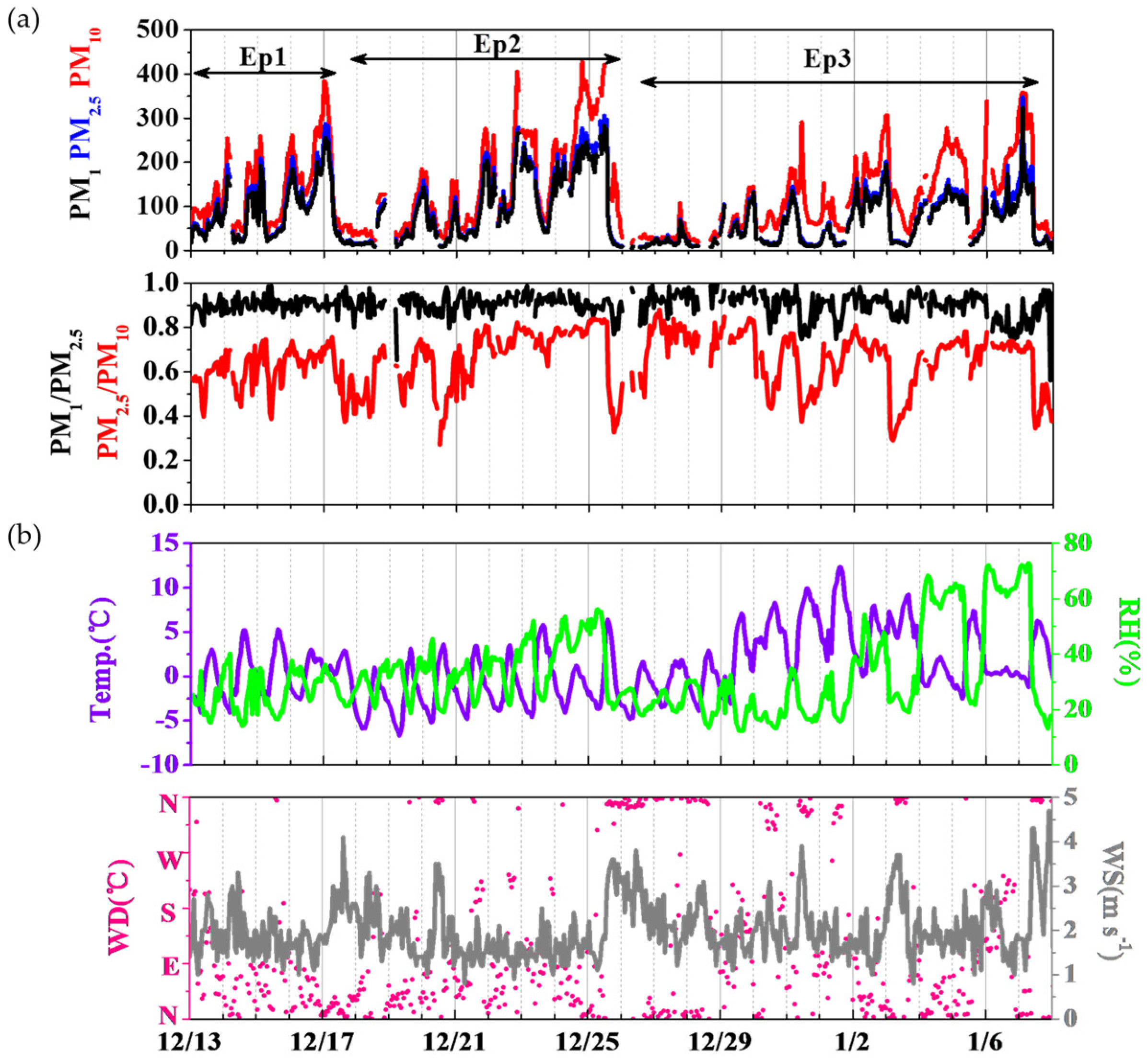
3.1. General Description of the PM1 Evolution
3.2. Chemical Composition of PM1 and the Influence of Meteorology
3.3. Relationship of PM1, PM2.5 and PM10
3.4. Mass-Size Distribution of Sulfate and Nitrate
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schwartz, J.; Dockery, D.W.; Neas, L.M. Is daily mortality associated specifically with fine particles? J. Air Waste Manag. Assoc. 1996, 46, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.E.; Suh, H.H. Fine particles and coarse particles: Concentration relationships relevant to epidemiologic studies. J. Air Waste Manag. Assoc. 1997, 47, 1238–1249. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A., III. Review: Epidemiological basis for particulate air pollution health standards. Aerosol Sci. Technol. 2000, 32, 4–14. [Google Scholar] [CrossRef]
- Michaels, R.A.; Kleinman, M.T. Incidence and apparent health significance of brief airborne particle excursions. Aerosol Sci. Technol. 2000, 32, 93–105. [Google Scholar] [CrossRef]
- Dockery, D.W. Epidemiologic evidence of cardiovascular effects of particulate air pollution. Environ. Health Perspect. 2001, 109, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.; Laden, F.; Zanobetti, A. The concentration-response relation between PM2.5 and daily deaths. Environ. Health Perspect. 2002, 110, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Peplow, M. Beijing smog contains witches’ brew of microbes. Nature 2014. [Google Scholar] [CrossRef]
- Lestari, P.; Oskouie, A.K.; Noll, K.E. Size distribution and dry deposition of particulate mass, sulfate and nitrate in an urban area. Atmos. Environ. 2003, 37, 2507–2516. [Google Scholar] [CrossRef]
- Gong, Z.H.; Lan, Z.J.; Xue, L.; Zeng, L.W.; He, L.Y.; Huang, X.F. Characterization of submicron aerosols in the urban outflow of the central Pearl River Delta region of China. Front. Environ. Sci. Eng. 2012, 6, 725–733. [Google Scholar] [CrossRef]
- Wang, X.F.; Wang, W.X.; Xue, L.K.; Gao, X.M.; Nie, W.; Yu, Y.C.; Zhou, Y.; Yang, L.X.; Zhang, Q.Z.; Wang, T. Size-resolved aerosol ionic composition and secondary formation at Mount Heng in South Central China. Front. Environ. Sci. Eng. 2013, 7, 815–826. [Google Scholar] [CrossRef]
- Ariola, V.; D’Alessandro, A.; Lucarelli, F.; Marcazzan, G.; Mazzei, F.; Nava, S.; Garcia-Orellana, I.; Prati, P.; Valli, G.; Vecchi, R.; et al. Elemental characterization of PM10, PM2.5 and PM1 in the town of Genoa (Italy). Chemosphere 2006, 62, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Vallius, M.J.; Ruuskanen, J.; Mirme, A.; Pekkanen, J. Concentrations and estimated soot content of PM1, PM2.5, and PM10 in a subarctic urban atmosphere. Environ. Sci. Technol. 2000, 34, 1919–1925. [Google Scholar] [CrossRef]
- Spindler, G.; Bruggemann, E.; Gnauk, T.; Gruner, A.; Muller, K.; Herrmann, H. A four-year size-segregated characterization study of particles PM10, PM2.5 and PM1 depending on air mass origin at Melpitz. Atmos. Environ. 2010, 44, 164–173. [Google Scholar] [CrossRef]
- Guo, S.; Hu, M.; Wang, Z.B.; Slanina, J.; Zhao, Y.L. Size-resolved aerosol water-soluble ionic compositions in the summer of Beijing: Implication of regional secondary formation. Atmos. Chem. Phys. 2010, 10, 947–959. [Google Scholar] [CrossRef]
- Song, S.; Wu, Y.; Jiang, J.; Yang, L.; Cheng, Y.; Hao, J. Chemical characteristics of size-resolved PM2.5 at a roadside environment in Beijing, China. Environ. Pollut. 2012, 161, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.J.; Duan, F.K.; Su, H.; Ma, Y.L.; Cheng, Y.; Zheng, B.; Zhang, Q.; Huang, T.; Kimoto, T.; Chang, D.; et al. Exploring the severe winter haze in Beijing: The impact of synoptic weather, regional transport and heterogeneous reactions. Atmos. Chem. Phys. 2015, 15, 2969–2983. [Google Scholar] [CrossRef]
- Kimoto, H.; Ueda, A.; Tsujimoto, K.; Mitani, Y.; Toyazaki, Y.; Kimoto, T. Development of a Continuous Dichotomous Aerosol Chemical Speciation Analyzer. Clean Technol. 2013, 23, 49–52. [Google Scholar]
- He, K.; Zhao, Q.; Ma, Y.; Duan, F.; Yang, F.; Shi, Z.; Chen, G. Spatial and seasonal variability of PM2.5 acidity at two Chinese megacities: Insights into the formation of secondary inorganic aerosols. Atmos. Chem. Phys. 2012, 12, 1377–1395. [Google Scholar] [CrossRef]
- Ziemba, L.D.; Fischer, E.; Griffin, R.J.; Talbot, R.W. Aerosol acidity in rural New England: Temporal trends and source region analysis. J. Geophys. Res. Atmos. 2007, 112, D10S22. [Google Scholar] [CrossRef]
- Atmospheric Environment Monitoring Network. Available online: http://113.108.142.147:20035/emcpublish/ (accessed on 13 December 2013).
- Tang, G.; Wang, Y.; Li, X.; Ji, D.; Hsu, S.; Gao, X. Spatial-temporal variations in surface ozone in Northern China as observed during 2009–2010 and possible implications for future air quality control strategies. Atmos. Chem. Phys. 2012, 12, 2757–2776. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, Q.; Wang, Z.; Fu, P.; Li, J.; Yang, T.; Yin, Y. Investigation of the sources and evolution processes of severe haze pollution in Beijing in January 2013. J. Geophys. Res. Atmos. 2014, 119, 4380–4398. [Google Scholar] [CrossRef]
- Jia, Y.; Rahn, K.A.; He, K.; Wen, T.; Wang, Y. A novel technique for quantifying the regional component of urban aerosol solely from its sawtooth cycles. J. Geophys. Res. Atmos. 2008, 113, 21309. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Z.; Dong, H.; Yang, T.; Li, J.; Pan, X.; Chen, P.; Jayne, J.T. Characterization of summer organic and inorganic aerosols in Beijing, China with an Aerosol Chemical Speciation Monitor. Atmos. Environ. 2012, 51, 250–259. [Google Scholar] [CrossRef]
- Shen, Z.; Arimoto, R.; Cao, J.; Zhang, R.; Li, X.; Du, N.; Okuda, T.; Nakao, S.; Tanaka, S. Seasonal variations and evidence for the effectiveness of pollution controls on water-soluble inorganic species in total suspended particulates and fine particulate matter from Xi’an, China. J. Air Waste Manag. Assoc. 2008, 58, 1560–1570. [Google Scholar] [PubMed]
- Air Quality Index. Available online: http://kjs.mep.gov.cn/hjbhbz/bzwb/dqhjbh/jcgfffbz/201203/t20120302_224166.htm?COLLCC=2906016564& (accessed on 6 September 2014).
- Sun, Y.; Zhuang, G.; Tang, A.; Wang, Y.; An, Z. Chemical characteristics of PM2.5 and PM10 in haze-fog episodes in Beijing. Environ. Sci. Technol. 2006, 40, 3148–3155. [Google Scholar] [CrossRef] [PubMed]
- Arimoto, R.; Duce, R.A.; Savoie, D.L.; Prospero, J.M.; Talbot, R.; Cullen, J.D.; Tomza, U.; Lewis, N.F.; Ray, B.J. Relationships among aerosol constituents from Asia and the North Pacific during PEM-West A. J. Geophys. Res. Atmos. 1996, 101, 2011–2023. [Google Scholar] [CrossRef]
- Yao, X.; Chan, C.K.; Fang, M.; Candle, S.; Chan, T.; Mulawa, P.; He, K.; Ye, B. The water-soluble ionic composition of PM2.5 in Shanghai and Beijing, China. Atmos. Environ. 2002, 36, 4223–4234. [Google Scholar] [CrossRef]
- He, K.; Yang, F.; Ma, Y.; Zhang, Q.; Yao, X.; Chan, C.K.; Cadle, S.; Chan, T.; Mulawa, P. The characteristics of PM2.5 in Beijing, China. Atmos. Environ. 2001, 35, 4959–4970. [Google Scholar] [CrossRef]
- Yu, X.; He, K.; Ma, Y.; Duan, F.; Yang, F.; Cadle, S.H.; Chan, T.; Mulawa, P.A. Water-soluble anionic species of PM2.5 in Beijing, China. In Proceedings of the 225th American Chemical Society (ACS) National Meeting, New Orleans, LA, USA, 23–27 March 2003; Volume 43, pp. 880–885.
- Sun, Y.L.; Wang, Z.F.; Fu, P.Q.; Yang, T.; Jiang, Q.; Dong, H.B.; Li, J.; Jia, J.J. Aerosol composition, sources and processes during wintertime in Beijing, China. Atmos. Chem. Phys. 2013, 13, 4577–4592. [Google Scholar] [CrossRef]
- McMurry, P.H.; Zhang, X.Q. Size distributions of ambient organic and elemental carbon. Aerosol Sci. Technol. 1989, 10, 430–437. [Google Scholar] [CrossRef]
- Contini, D.; Cesari, D.; Genga, A.; Siciliano, M.; Ielpo, P.; Guascito, M.; Conte, M. Source apportionment of size-segregated atmospheric particles based on the major water-soluble components in Lecce (Italy). Sci. Total Environ. 2014, 472, 248–261. [Google Scholar] [CrossRef] [PubMed]






| Entire Study | Ep1 | Ep2 | Ep3 | |
|---|---|---|---|---|
| Meteorological Parameters | ||||
| WS(m·s−1) | 2.0 | 1.9 | 1.9 | 2.1 |
| Temp(°C) | 0.94 | 0.26 | −1.1 | 2.5 |
| RH(%) | 32.2 | 26.0 | 34.3 | 32.9 |
| PM Data (μg·m−3) | ||||
| PM1 | 76.2 | 91.2 | 94.9 | 58.1 |
| PM2.5 | 84.4 | 101.3 | 104.2 | 64.9 |
| PM10 | 130.1 | 141.9 | 146.4 | 114.8 |
| PM1/PM2.5 | 0.90 | 0.90 | 0.91 | 0.90 |
| PM2.5/PM10 | 0.65 | 0.62 | 0.65 | 0.66 |
| Aerosol Composition (μg·m−3) | ||||
| PM1(NO3) | 8.7 | 9.8 | 11.8 | 6.3 |
| PM1(SO4) | 6.8 | 5.5 | 8.6 | 6.3 |
| Gas Data (μg·m−3) | ||||
| CO (mg·m−3) | 1.9 | 1.4 | 2.7 | 1.5 |
| NO2 | 69.1 | 54.2 | 86.9 | 59.5 |
| O3 | 27.7 | 15.0 | 20.5 | 31.9 |
| SO2 | 49.1 | 31.9 | 63.2 | 41.4 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Duan, F.; He, K.; Ma, Y.; Kimoto, T.; Huang, T. Size-Dependent Characterization of Atmospheric Particles during Winter in Beijing. Atmosphere 2016, 7, 36. https://doi.org/10.3390/atmos7030036
Li H, Duan F, He K, Ma Y, Kimoto T, Huang T. Size-Dependent Characterization of Atmospheric Particles during Winter in Beijing. Atmosphere. 2016; 7(3):36. https://doi.org/10.3390/atmos7030036
Chicago/Turabian StyleLi, Haiyan, Fengkui Duan, Kebin He, Yongliang Ma, Takashi Kimoto, and Tao Huang. 2016. "Size-Dependent Characterization of Atmospheric Particles during Winter in Beijing" Atmosphere 7, no. 3: 36. https://doi.org/10.3390/atmos7030036
APA StyleLi, H., Duan, F., He, K., Ma, Y., Kimoto, T., & Huang, T. (2016). Size-Dependent Characterization of Atmospheric Particles during Winter in Beijing. Atmosphere, 7(3), 36. https://doi.org/10.3390/atmos7030036





