Concentration and Size Distribution of Fungi Aerosol over Oceans along a Cruise Path during the Fourth Chinese Arctic Research Expedition
Abstract
:1. Introduction
2. Experimental Methods
2.1. Sample Collection and Fungi Cultivation
| Site | Date | Time | Latitude/Longitude | C | RH | T | RV | Weather |
|---|---|---|---|---|---|---|---|---|
| 1 | 28 June | 11:20 | 24°02.17′N 118°04.15′E | 320.4 | 80 | 26.3 | / | Sunny |
| 2 | 1 July | 11:25 | 25°03.511′N 120°06.776′E | 5.3 | 91 | 27.2 | 6.7 | Sunny |
| 3 | 4 July | 01:40 | 33°13.686′N 126°35.142′E | 190.8 | 100 | 24.2 | 5.1 | Heavy fog |
| 4 | 8 July | 01:40 | 48°22.811′N 149°54.791′E | 224.4 | 90 | 11.5 | 25.5 | Light fog |
| 5 | 11 July | 07:30 | 54°34.097′N 171°22.092′E | 7.1 | 81 | 7.6 | 8.8 | Sunny |
| 6 | 14 July | 11:05 | 59°39.629′N 179°25.230′E | 0.0 | 100 | 7.2 | 10.5 | Heavy fog |
| 7 | 18 July | 04:40 | 62°42.473′N 167°38.746′W | 63.6 | 100 | 7.5 | 31.0 | Sprinkle |
| 8 | 21 July | 08:45 | 69°11.664′N 167°54.724′W | 24.7 | 84 | 6.9 | 4.6 | Sunny |
| 9 | 24 July | 06:20 | 71°59.675′N 168°7.530′W | 1.8 | 86 | 3.3 | 13.3 | Sunny |
| 10 | 28 July | 09:00 | 73°41.119′N 156°22.381′W | 24.7 | 100 | 2.7 | 15.3 | Light fog |
| 11 | 1 August | 09:15 | 79°56.070′N 158°54.212′W | 15.9 | 88 | 0.8 | 12.3 | Light fog |
| 12 | 7 August | 04:35 | 86°41.363′N 179°16.989′E | 1.8 | 86 | −0.9 | 16.7 | Little snow |
| 13 | 20 August | 05:55 | 88°22.974′N 177°11.646′W | 1.8 | 87 | 0 | 19.0 | Sunny |
| 14 | 22 August | 03:20 | 85°37.956′N 171°29.714′W | 0.0 | 100 | 0 | 16.7 | Light fog |
| 15 | 25 August | 05:20 | 79°43.302′N 169°3.438′W | 8.8 | 100 | 0 | 2.7 | Light fog |
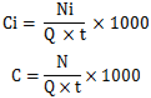
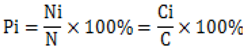
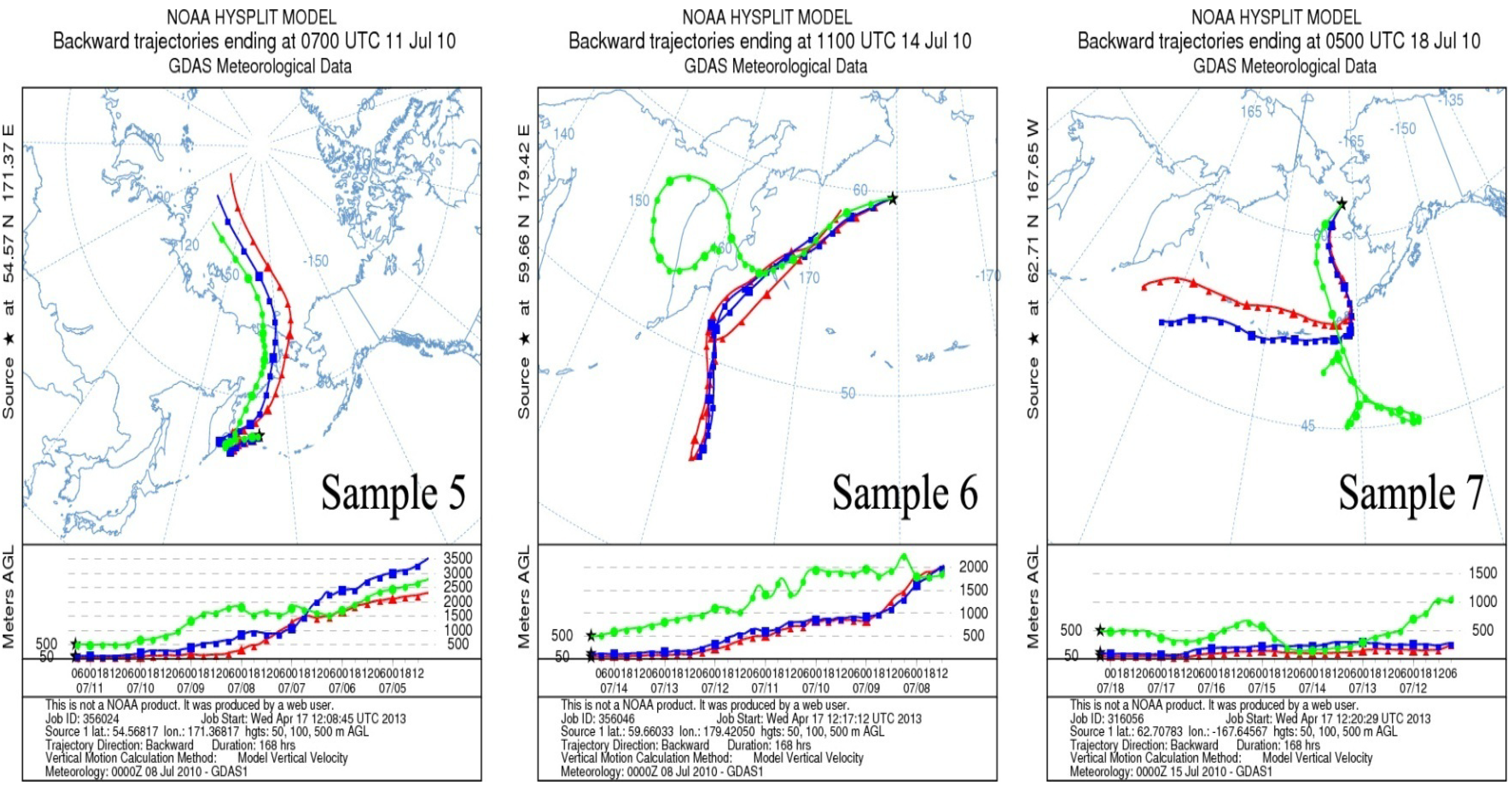
2.2. Air Mass Back Trajectories
3. Results and Discussion
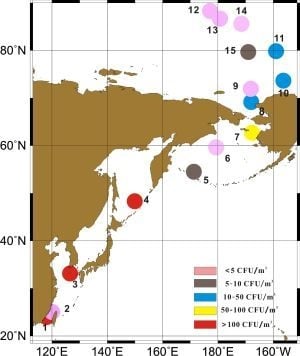
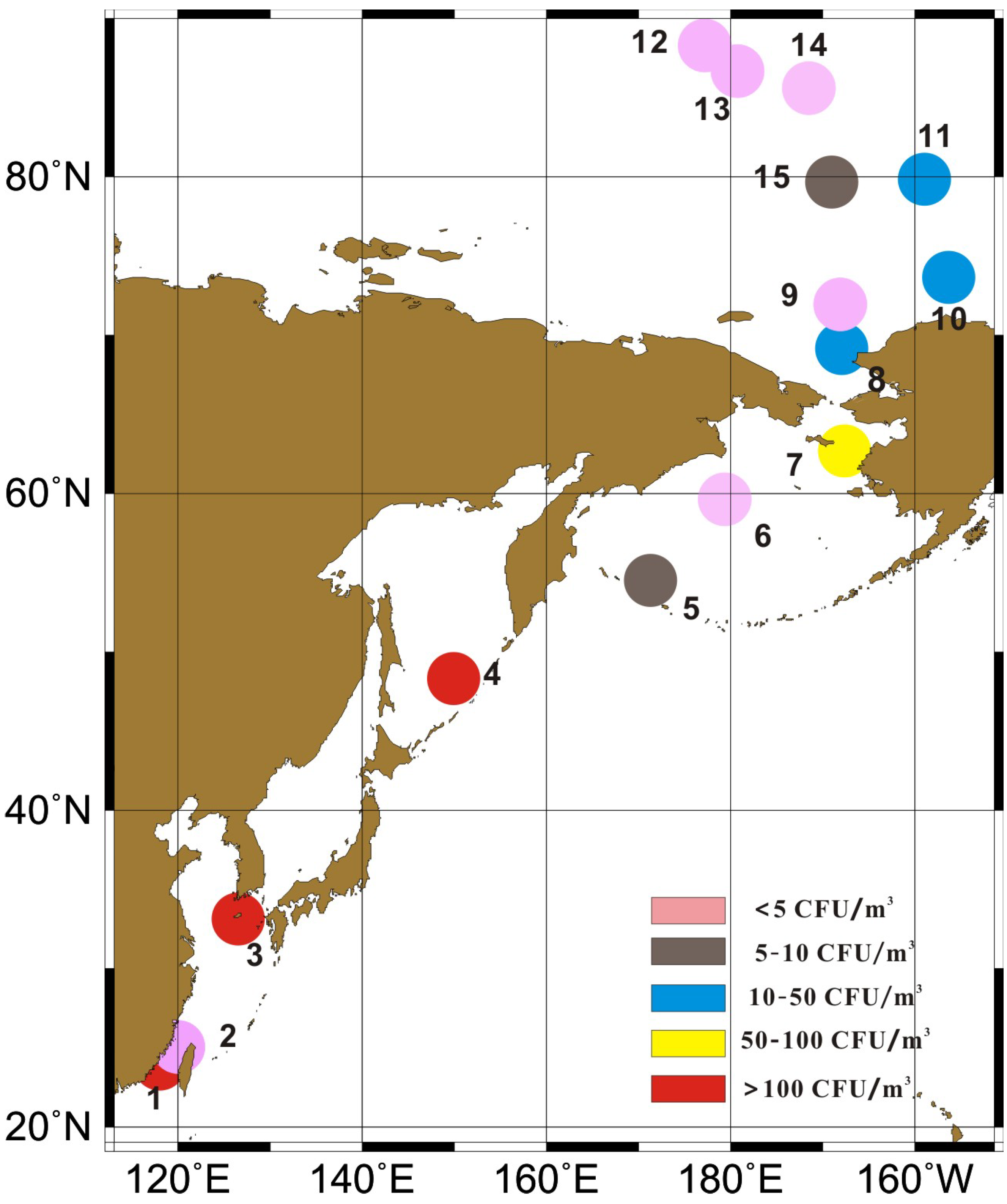
3.1. Concentrations of Cultivable Fungi
| Region | Sample Site | CR CFU/m3 | Mean ± SD CFU/m3 | Sea ice Area |
|---|---|---|---|---|
| Offshore China | 1,2,3 | 5.3–320.4 | 172.2 ± 158.4 | / |
| Northwestern Pacific Ocean | 4,5,6,7 | 0–224.4 | 73.8 ± 104.4 | / |
| Chuckchi Sea | 8,9 | 1.8–24.7 | 13.3 ± 16.2 | Open sea |
| Canada Basin | 10,11,15 | 8.8–24.7 | 16.5 ± 8.0 | Floating sea ice |
| Central Arctic ocean | 12,13,14 | 0–1.8 | 1.2 ± 1.0 | Pack ice region |
3.2. Size Distributions
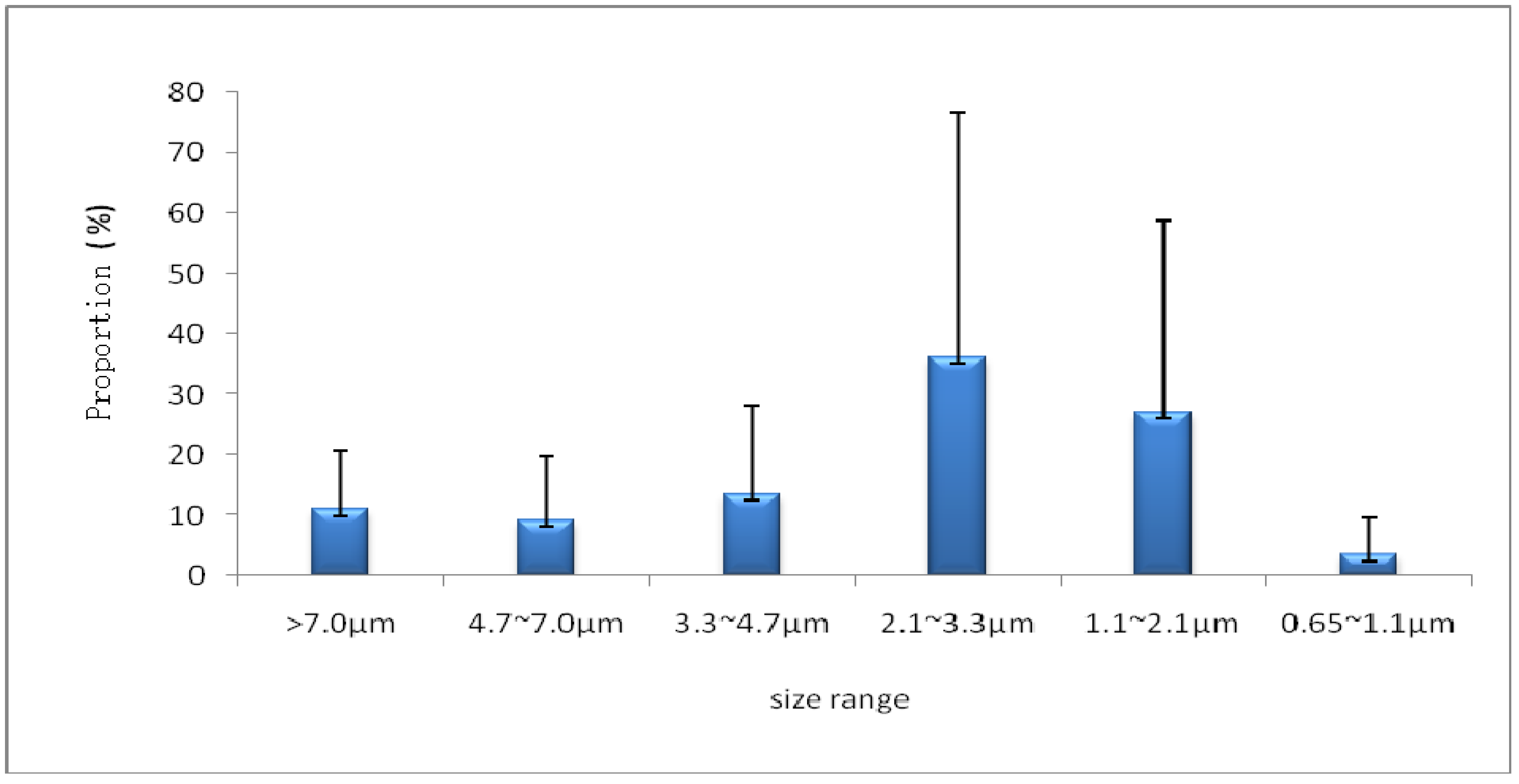
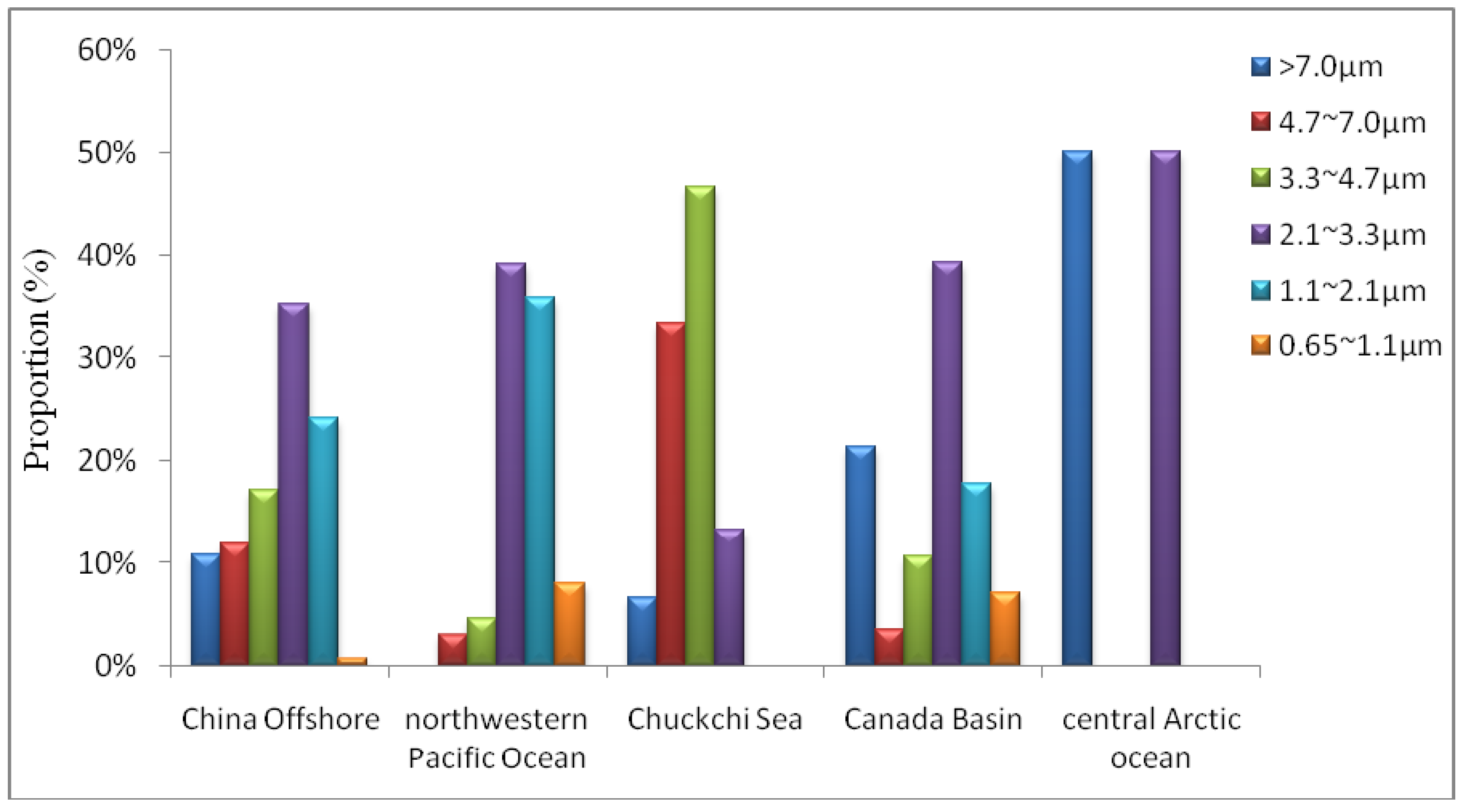
4. Impact of Potential Factors on Airborne Fungi
4.1. Air Mass Origin
4.2. Influence of Meteorological Conditions
4.2.1. Temperature
| Pearson | Concentration | RH | T | RV |
|---|---|---|---|---|
| Concentration | 1.000 | 0.185 | 0.496 * | 0.246 |
4.2.2. Weather
4.3. Sea Ice
5. Summary
Acknowledgments
Conflicts of Interest
References
- Li, M.; Qi, J.; Zhang, H.; Huang, S.; Li, L.; Gao, D. Concentration and size distribution of bioaerosols in an outdoor environment in the Qingdao coastal region. Sci. Total Environ. 2011, 409, 3812–3819. [Google Scholar] [CrossRef]
- Ariya, P.A.; Amyot, M. New directions: The role of bioaerosols in atmospheric chemistry and physics. Atmos. Environ. 2004, 38, 1231–1232. [Google Scholar] [CrossRef]
- Urbano, R.; Palenik, B.; Gaston, C.; Prather, K. Detection and phylogenetic analysis of coastal bioaerosols using culture dependent and independent techniques. Biogeosciences 2011, 8, 301–309. [Google Scholar] [CrossRef] [Green Version]
- Shelton, B.G.; Kirkland, K.H.; Flanders, W.D.; Morris, G.K. Profiles of airborne fungi in buildings and outdoor environments in the United States. Appl. Environ. Microbiol. 2002, 68, 1743–1753. [Google Scholar] [CrossRef]
- Zuraimi, M.; Fang, L.; Tan, T.; Chew, F.; Tham, K. Airborne fungi in low and high allergic prevalence child care centers. Atmos. Environ. 2009, 43, 2391–2400. [Google Scholar] [CrossRef]
- Morris, C.; Sands, D.; Bardin, M.; Jaenicke, R.; Vogel, B.; Leyronas, C.; Ariya, P.; Psenner, R. Microbiology and atmospheric processes: Research challenges concerning the impact of airborne micro-organisms on the atmosphere and climate. Biogeosciences 2011, 8, 17–25. [Google Scholar] [CrossRef]
- Georgakopoulos, D.; Després, V.; Fröhlich-Nowoisky, J.; Psenner, R.; Ariya, P.; Pósfai, M.; Ahern, H.; Moffett, B.; Hill, T. Microbiology and atmospheric processes: Biological, physical and chemical characterization of aerosol particles. Biogeosciences 2009, 6, 721–737. [Google Scholar] [CrossRef]
- Dusek, U.; Frank, G.; Hildebrandt, L.; Curtius, J.; Schneider, J.; Walter, S.; Chand, D.; Drewnick, F.; Hings, S.; Jung, D. Size matters more than chemistry for cloud-nucleating ability of aerosol particles. Science 2006, 312, 1375–1378. [Google Scholar] [CrossRef]
- Pöhlker, C.; Wiedemann, K.T.; Sinha, B.; Shiraiwa, M.; Gunthe, S.S.; Smith, M.; Su, H.; Artaxo, P.; Chen, Q.; Cheng, Y. Biogenic potassium salt particles as seeds for secondary organic aerosol in the Amazon. Science 2012, 337, 1075–1078. [Google Scholar] [CrossRef]
- Fang, Z.; Ouyang, Z.; Hu, L.; Wang, X.; Zheng, H.; Lin, X. Culturable airborne fungi in outdoor environments in Beijing, China. Sci. Total Environ. 2005, 350, 47–58. (in Chinese). [Google Scholar] [CrossRef]
- Menetrez, M.; Foarde, K.; Esch, R.; Schwartz, T.; Dean, T.; Hays, M.; Cho, S.; Betancourt, D.; Moore, S. An evaluation of indoor and outdoor biological particulate matter. Atmos. Environ. 2009, 43, 5476–5483. [Google Scholar] [CrossRef]
- Kalogerakis, N.; Paschali, D.; Lekaditis, V.; Pantidou, A.; Eleftheriadis, K.; Lazaridis, M. Indoor air quality—Bioaerosol measurements in domestic and office premises. J. Aerosol Sci. 2005, 36, 751–761. [Google Scholar] [CrossRef]
- Prospero, J.M.; Blades, E.; Mathison, G.; Naidu, R. Interhemispheric transport of viable fungi and bacteria from Africa to the Caribbean with soil dust. Aerobiologia 2005, 21, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Wang, B. Research on airborne microbes over some areas of the East China Sea and the Yellow Sea. Donghai Marine Sci. 1998, 16, 33–39. [Google Scholar]
- Pady, S.; Kapica, L. Air-borne fungi in the arctic and other parts of Canada. Can. J. Bot. 1953, 31, 309–323. [Google Scholar] [CrossRef]
- Marks, R.; Kruczalak, K.; Jankowska, K.; Michalska, M. Bacteria and fungi in air over the Gulf of Gdańsk and Baltic sea. J. Aerosol Sci. 2001, 32, 237–250. [Google Scholar] [CrossRef]
- Smith, D.J.; Jaffe, D.A.; Birmele, M.N.; Griffin, D.W.; Schuerger, A.C.; Hee, J.; Roberts, M.S. Free tropospheric transport of microorganisms from Asia to North America. Microb. Ecol. 2012, 64, 973–985. [Google Scholar] [CrossRef]
- Kramer, C.L.; Holzapfel, E.P. Air biota of the upper atmosphere over the Pacific Ocean and continental United States. Agric. Meteorol. 1973, 12, 83–93. [Google Scholar] [CrossRef]
- Raisi, L.; Aleksandropoulou, V.; Lazaridis, M.; Katsivela, E. Size distribution of viable, cultivable, airborne microbes and their relationship to particulate matter concentrations and meteorological conditions in a Mediterranean site. Aerobiologia 2013, 29, 223–248. [Google Scholar]
- Fröhlich-Nowoisky, J.; Burrows, S.; Xie, Z.; Engling, G.; Solomon, P.; Fraser, M.; Mayol-Bracero, O.; Artaxo, P.; Begerow, D.; Conrad, R. Biogeography in the air: Fungal diversity over land and oceans. Biogeosciences 2012, 9, 1125–1136. [Google Scholar] [CrossRef] [Green Version]
- Rolph, G.D.; Draxler, R.R. HYSPLIT (HYbrid Single-Particle Lagrangian Integrated Trajectory); NOAA Air Resources Laboratory: College Park, MD, USA, 2013. [Google Scholar]
- Sesartic, A.; Dallafior, T. Global fungal spore emissions, review and synthesis of literature data. Biogeosciences 2011, 8, 1181–1192. [Google Scholar] [CrossRef] [Green Version]
- Després, V.R.; Huffman, J.A.; Burrows, S.M.; Hoose, C.; Safatov, A.S.; Buryak, G.; Fröhlich-Nowoisky, J.; Elbert, W.; Andreae, M.O.; Pöschl, U. Primary biological aerosol particles in the atmosphere: A review. Tellus B 2012, 64, 15598. [Google Scholar]
- Pady, S.; Kelly, C. Aerobiological studies of fungi and bacteria over the Atlantic Ocean. Can. J. Bot. 1954, 32, 202–212. [Google Scholar] [CrossRef]
- Jaenicke, R. Abundance of cellular material and proteins in the atmosphere. Science 2005, 308, 73. [Google Scholar] [CrossRef]
- Hyvärinen, A.; Vahteristo, M.; Meklin, T.; Jantunen, M.; Nevalainen, A.; Moschandreas, D. Temporal and spatial variation of fungal concentrations in indoor air. Aerosol Sci. Technol. 2001, 35, 688–695. [Google Scholar]
- Fang, Z.; Ouyang, Z.; Hu, L.; Lin, X.; Wang, X. Granularity distribution of airborne microbes in summer in Beijing. Huan Jing Ke Xue 2004, 25, 1–5. (in Chinese). [Google Scholar]
- Xu, W.; Qi, J.; Jin, C.; Gao, D.; Li, M.; Li, L.; Huang, S.; Zhang, H. Concentration distribution of bioaerosol in summer and autumn in the Qingdao coastal region. Huan Jing Ke Xue 2011, 32, 9–17. (in Chinese). [Google Scholar]
- Jiang, H.; Dong, H.; Zhang, G.; Yu, B.; Chapman, L.R.; Fields, M.W. Microbial diversity in water and sediment of Lake Chaka, an athalassohaline lake in northwestern China. Appl. Environ. Microbiol. 2006, 72, 3832–3845. [Google Scholar] [CrossRef]
- Baleux, B.; Caro, A.; Lesne, J.; Got, P.; Binard, S.; Delpeuch, B. Survival and virulence changes in the VNC state of Salmonella Typhimurium in relation to simultaneous UV radiation, salinity and nutrient deprivation exposure. Oceanol. Acta 1998, 21, 939–950. [Google Scholar] [CrossRef]
- Robinson, C.H. Cold adaptation in Arctic and Antarctic fungi. New Phytol. 2001, 151, 341–353. [Google Scholar] [CrossRef]
- Kahl, J.D.W.; Martinez, D.A. Long-term variability in the low-level inversion layer over the Arctic Ocean. Int. J. Climatol. 1996, 16, 1297–1313. [Google Scholar] [CrossRef]
- Dueker, M.E.; O’Mullan, G.D.; Weathers, K.C.; Juhl, A.R.; Uriarte, M. Coupling of fog and marine microbial content in the near-shore coastal environment. Biogeosciences 2012, 9, 803–813. [Google Scholar] [CrossRef]
- Fuzzi, S.; Mandrioli, P.; Perfetto, A. Fog droplets—an atmospheric source of secondary biological aerosol particles. Atmos. Environ. 1997, 31, 287–290. [Google Scholar] [CrossRef]
- Lu, P.; Li, Z.; Cheng, B.; Lei, R.; Zhang, R. Sea ice surface features in Arctic summer 2008: Aerial observations. Remote Sens. Environ. 2010, 114, 693–699. [Google Scholar] [CrossRef]
- Gunde-Cimerman, N.; Sonjak, S.; Zalar, P.; Frisvad, J.C.; Diderichsen, B.; Plemenitaš, A. Extremophilic fungi in arctic ice: A relationship between adaptation to low temperature and water activity. Phys. Chem. Earth Parts A/B/C 2003, 28, 1273–1278. [Google Scholar] [CrossRef]
- Wösten, H.; van Wetter, M.; Lugones, L.; van der Mei, H.; Busscher, H.; Wessels, J. How a fungus escapes the water to grow into the air. Curr. Biol.: CB 1999, 9, 85–88. [Google Scholar] [CrossRef]
- Blanchard, D.C.; Syzdek, L. Mechanism for the water-to-air transfer and concentration of bacteria. Science 1970, 170, 626–628. [Google Scholar]
- Schlichting, H.E., Jr. Ejection of microalgae into the air via bursting bubbles. J. Allergy Clin. Immunol. 1974, 53, 185–188. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yu, J.; Hu, Q.; Xie, Z.; Kang, H.; Li, M.; Li, Z.; Ye, P. Concentration and Size Distribution of Fungi Aerosol over Oceans along a Cruise Path during the Fourth Chinese Arctic Research Expedition. Atmosphere 2013, 4, 337-348. https://doi.org/10.3390/atmos4040337
Yu J, Hu Q, Xie Z, Kang H, Li M, Li Z, Ye P. Concentration and Size Distribution of Fungi Aerosol over Oceans along a Cruise Path during the Fourth Chinese Arctic Research Expedition. Atmosphere. 2013; 4(4):337-348. https://doi.org/10.3390/atmos4040337
Chicago/Turabian StyleYu, Juan, Qihou Hu, Zhouqing Xie, Hui Kang, Ming Li, Zheng Li, and Peipei Ye. 2013. "Concentration and Size Distribution of Fungi Aerosol over Oceans along a Cruise Path during the Fourth Chinese Arctic Research Expedition" Atmosphere 4, no. 4: 337-348. https://doi.org/10.3390/atmos4040337