A Real-Time Approach to Detect PM2.5 in a Seriously Polluted Environment Based on Pressure Drop
Abstract
:1. Introduction
2. Experimental
2.1. Instrumentation
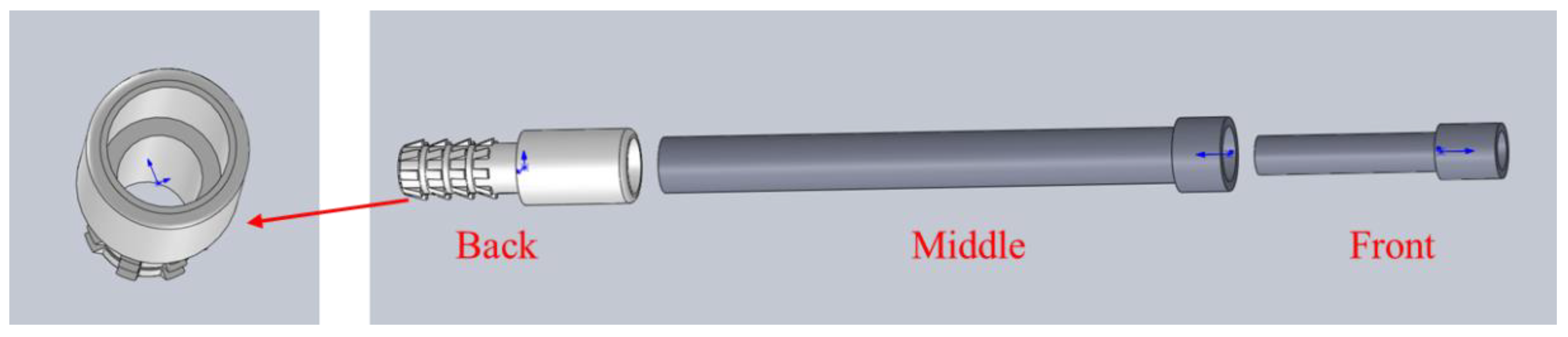
2.1.1. Sampling Device
2.1.2. Pump
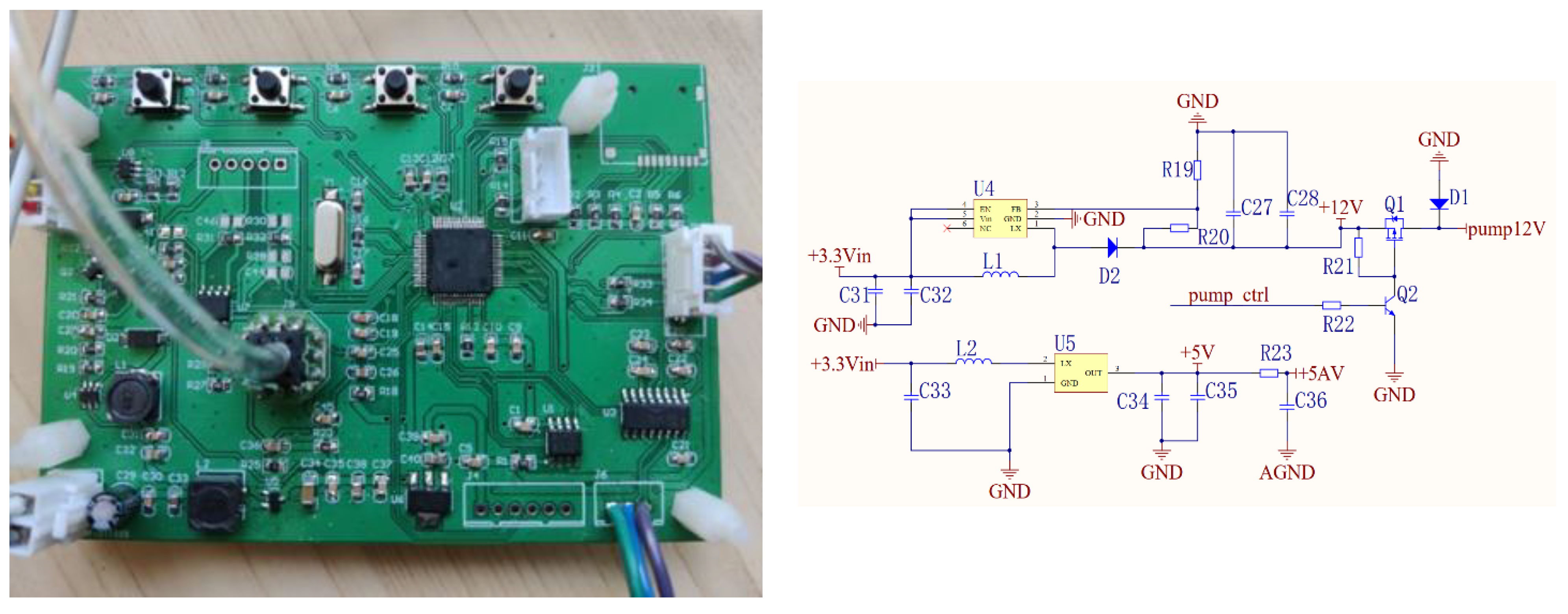
2.1.3. Control Circuit and Pressure Sensor
2.2. Calibration
3. Results and Discussions
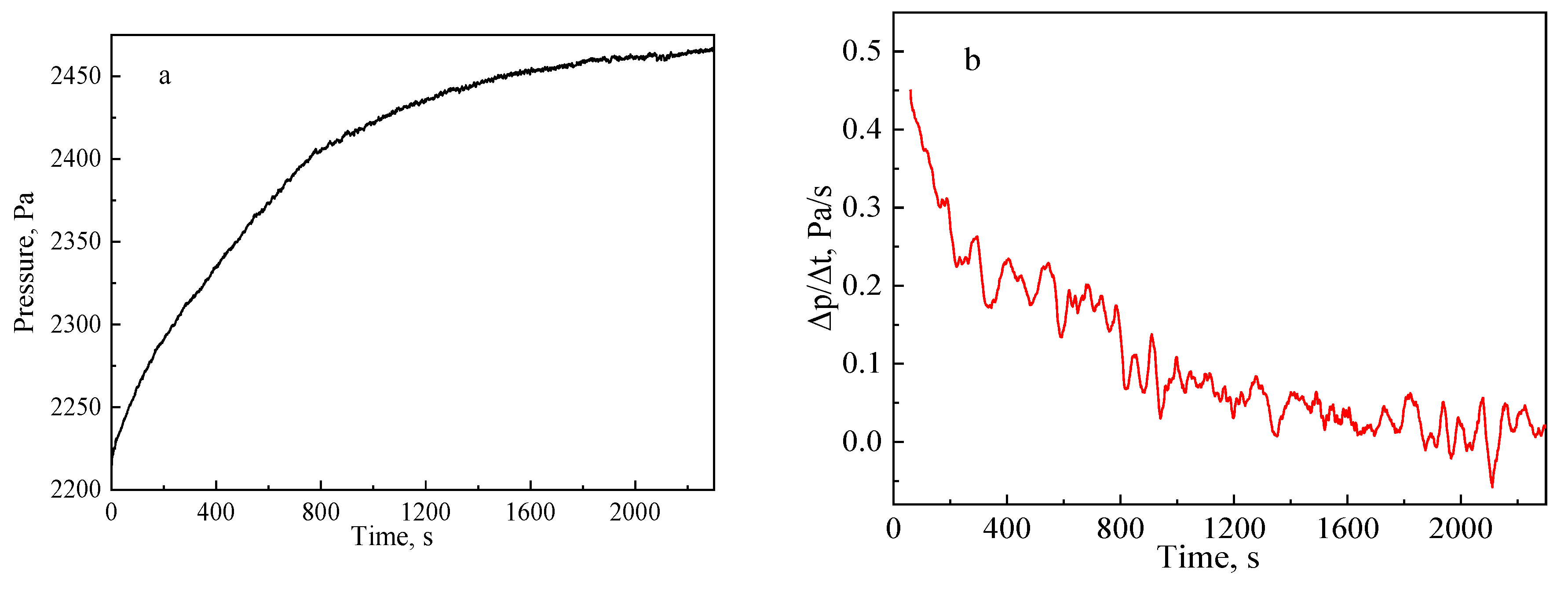
3.1. Calibration in an Atmospheric Environment
3.1.1. Calibration Equation
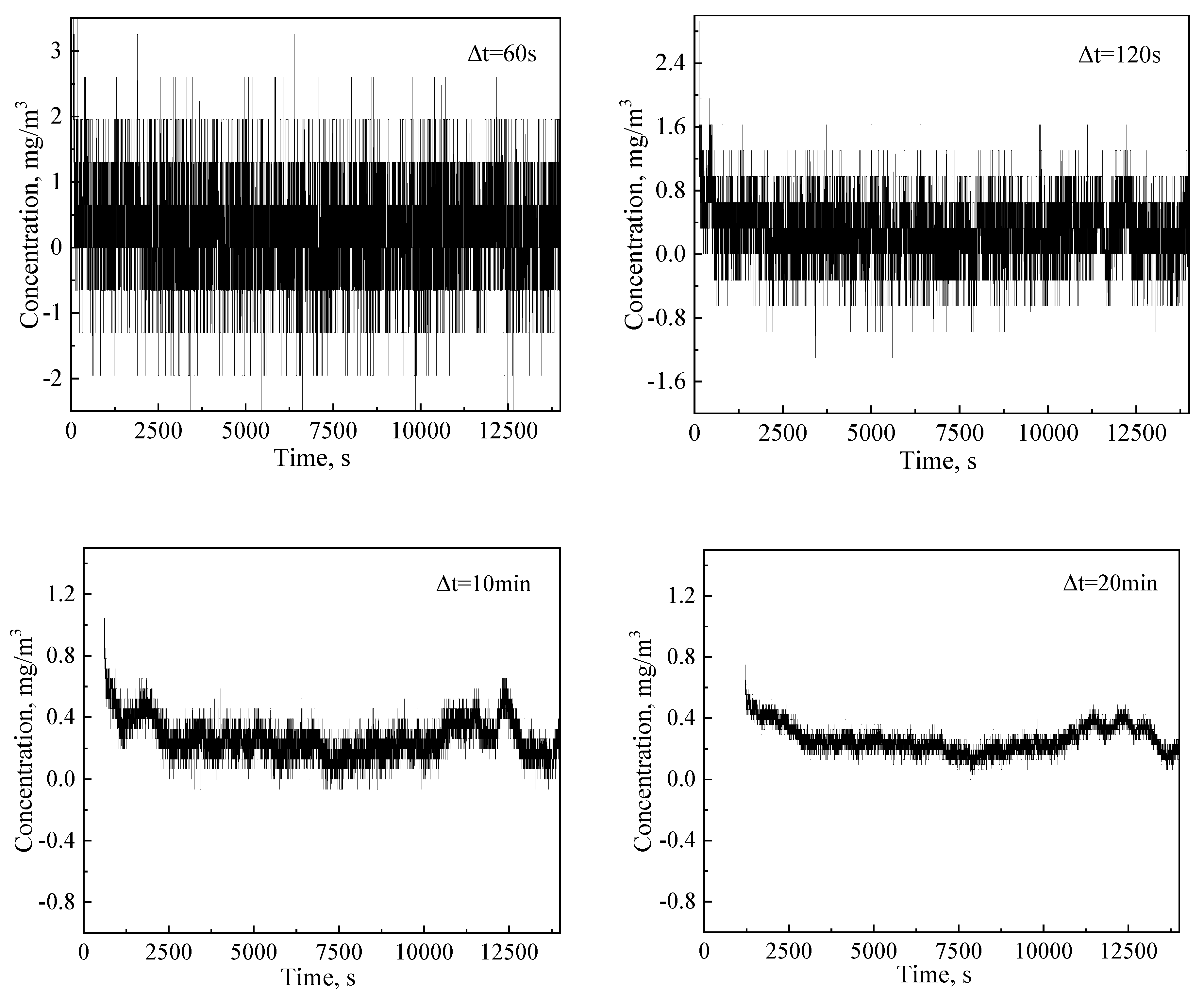
3.1.2. Effects of Time Interval
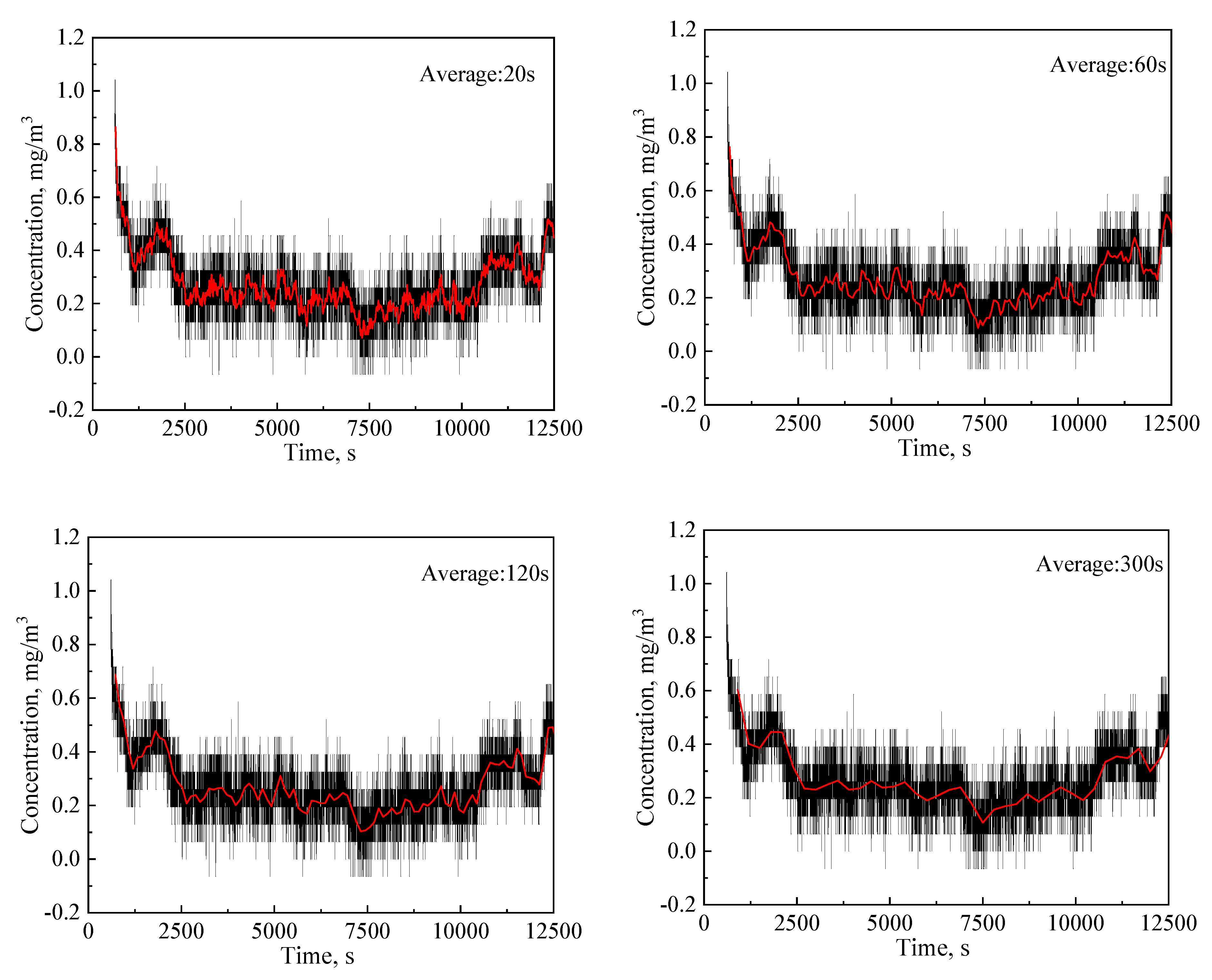
3.1.3. Effects of Average Times
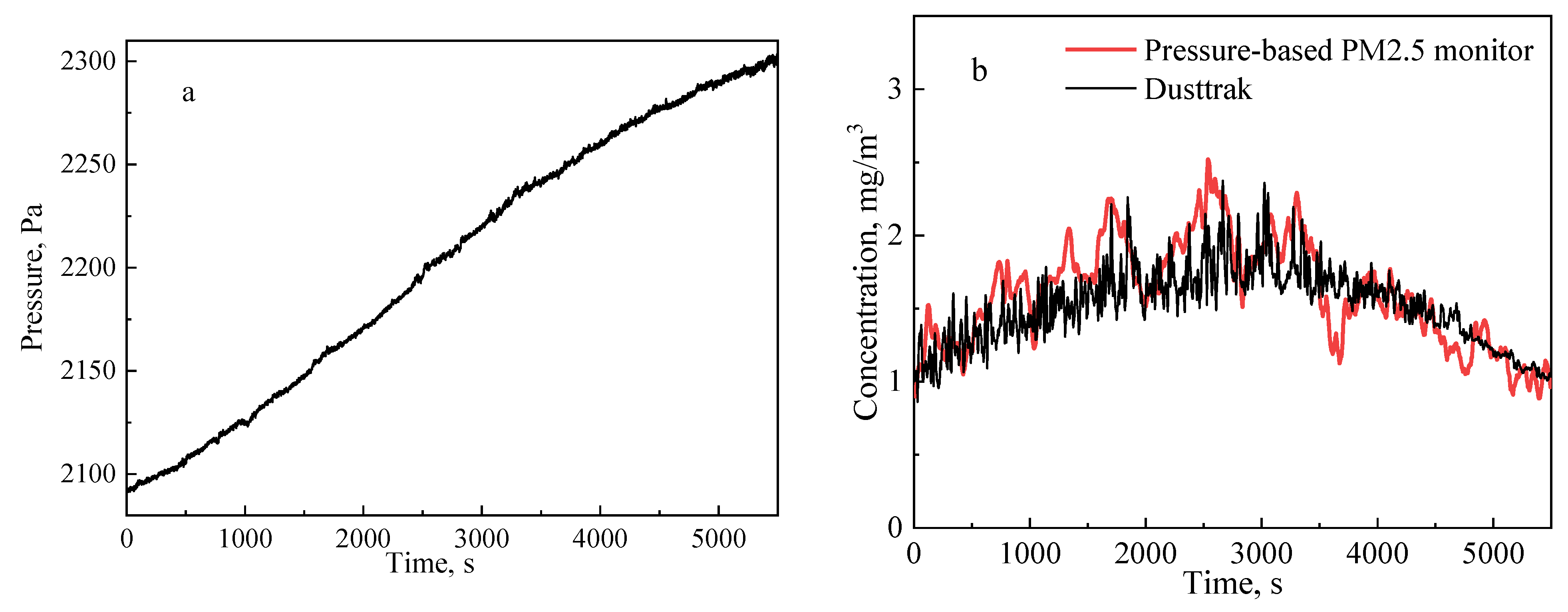
3.2. Calibration in a Laboratory Environment with High PM2.5 Concentration
3.3. Application of the Pressure-Based PM2.5 Measurement Method
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Henschel, S.; Atkinson, R.; Zeka, A.; Le Tertre, A.; Analitis, A.; Katsouyanni, K.; Chanel, O.; Pascal, M.; Forsberg, B.; Medina, S. Air pollution interventions and their impact on public health. Int. J. Public Health 2012, 57, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Bartonova, A.; Schindler, M.; Sharma, M.; Behera, S.N.; Katiyar, K.; Dikshit, O.; Health, O. Respiratory disease in relation to outdoor air pollution in Kanpur, India. Arch. Environ. Occup. Health 2013, 68, 204–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, M.; Brokamp, C.; Balachandran, S. Clustering and regression-based analysis of PM2.5 sensitivity to meteorology in Cincinnati, Ohio. Atmosphere 2022, 13, 545. [Google Scholar] [CrossRef]
- Wang, X.H.; Wang, B.Z. Research on prediction of environmental aerosol and PM2.5 based on artificial neural network. Neural Comput. Appl. 2019, 31, 8217–8227. [Google Scholar] [CrossRef]
- Kulmala, M.; Cai, R.; Stolzenburg, D.; Zhou, Y.; Dada, L.; Guo, Y.; Yan, C.; Petaja, T.; Jiang, J.; Kerminen, V.-M. The contribution of new particle formation and subsequent growth to haze formation. Environ. Sci. Atmos. 2022, 2, 352–361. [Google Scholar] [CrossRef]
- Liu, H.; Dunea, D.; Iordache, S.; Pohoata, A. A review of airborne particulate matter effects on young children’s respiratory symptoms and diseases. Atmosphere 2018, 9, 150. [Google Scholar] [CrossRef] [Green Version]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef]
- Potter, N.A.; Meltzer, G.Y.; Avenbuan, O.N.; Raja, A.; Zelikoff, J.T. Particulate matter and associated metals: A link with neurotoxicity and mental health. Atmosphere 2021, 12, 425. [Google Scholar] [CrossRef]
- Patel, P.; Aggarwal, S.G. On the techniques and standards of particulate matter sampling. J. Air Waste Manag. Assoc. 2022, 72, 791–814. [Google Scholar] [CrossRef]
- Watson, J.G.; Tropp, R.J.; Kohl, S.D.; Wang, X.; Chow, J.C. Filter processing and gravimetric analysis for suspended particulate matter samples. Aerosol Sci. Eng. 2017, 1, 93–105. [Google Scholar] [CrossRef]
- Kelly, K.E.; Whitaker, J.; Petty, A.; Widmer, C.; Dybwad, A.; Sleeth, D.; Martin, R.; Butterfield, A. Ambient and laboratory evaluation of a low-cost particulate matter sensor. Environ. Pollut. 2017, 221, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Gramsch, E.; Oyola, P.; Reyes, F.; Vasquez, Y.; Rubio, M.A.; Soto, C.; Perez, P.; Moreno, F.; Gutierrez, N. Influence of particle composition and size on the accuracy of low cost PM sensors: Findings from field campaigns. Front. Environ. Sci. 2021, 9, 751267. [Google Scholar] [CrossRef]
- Gębicki, J.; Szymańska, K. Comparative field test for measurement of PM10 dust in atmospheric air using gravimetric (reference) method and β-absorption method (Eberline FH 62-1). Atmos. Environ. 2012, 54, 18–24. [Google Scholar] [CrossRef]
- Wanjura, J.D.; Shaw, B.W.; Parnell, C.B.; Lacey, R.E.; Capareda, S.C. Comparison of continuous monitor (TEOM) and gravimetric sampler particulate matter concentrations. Trans. ASABE 2008, 51, 251–257. [Google Scholar] [CrossRef]
- Sullivan, B.; Allawatt, G.; Emery, A.; Means, P.; Kramlich, J.; Posner, J. Time-resolved particulate emissions monitoring of cookstove biomass combustion using a tapered element oscillating microbalance. Combust. Sci. Technol. 2017, 189, 923–936. [Google Scholar] [CrossRef]
- Ngo, N.D.; Jang, J. Long-term measurement of PM2.5 mass concentration using an electrostatic particle concentrator-based quartz crystal microbalance integrated with carbon dioxide aerosol jets for PM sensing in remote areas. IEEE Access 2021, 9, 90715–90726. [Google Scholar] [CrossRef]
- Ngo, N.D.; Lee, J.; Kim, M.W.; Jang, J. Measurement of PM2.5 mass concentration using an electrostatic particle concentrator-based quartz crystal microbalance. IEEE Access 2019, 7, 170640–170647. [Google Scholar] [CrossRef]
- Ayers, G.P. Potential for simultaneous measurement of PM10, PM2.5 and PM1 for air quality monitoring purposes using a single TEOM. Atmos. Environ. 2004, 38, 3453–3458. [Google Scholar] [CrossRef]
- Patra, S.S.; Ramsisaria, R.; Du, R.; Wu, T.; Boor, B.E. A machine learning field calibration method for improving the performance of low-cost particle sensors. Build. Environ. 2021, 190, 107457. [Google Scholar] [CrossRef]
- Vogt, M.; Schneider, P.; Castell, N.; Hamer, P. Assessment of low-cost particulate matter sensor systems against optical and gravimetric methods in a field co-location in Norway. Atmosphere 2021, 12, 961. [Google Scholar] [CrossRef]
- Palmisani, J.; Di Gilio, A.; Viana, M.; de Gennaro, G.; Ferro, A. Indoor air quality evaluation in oncology units at two European hospitals: Low-cost sensors for TVOCs, PM2.5 and CO2 real-time monitoring. Build. Environ. 2021, 205, 108237. [Google Scholar] [CrossRef]
- He, R.; Han, T.; Bachman, D.; Carluccio, D.J.; Jaeger, R.; Zhang, J.; Thirumurugesan, S.; Andrews, C.; Mainelis, G. Evaluation of two low-cost PM monitors under different laboratory and indoor conditions. Aerosol Sci. Technol. 2020, 55, 316–331. [Google Scholar] [CrossRef]
- Manikonda, A.; Zíková, N.; Hopke, P.K.; Ferro, A.R. Laboratory assessment of low-cost PM monitors. J. Aerosol Sci. 2016, 102, 29–40. [Google Scholar] [CrossRef]
- Hapidin, D.A.; Munir, M.M.; Suprijadi; Khairurrijal, K. Development of a new personal air filter test system using a low-cost particulate matter (PM) sensor. Aerosol Sci. Technol. 2020, 54, 203–216. [Google Scholar] [CrossRef]
- Choe, Y.; Shin, J.-s.; Park, J.; Kim, E.; Oh, N.; Min, K.; Kim, D.; Sung, K.; Cho, M.; Yang, W. Inadequacy of air purifier for indoor air quality improvement in classrooms without external ventilation. Build. Environ. 2022, 207, 108450. [Google Scholar] [CrossRef]
- Demanega, I.; Mujan, I.; Singer, B.C.; Anđelković, A.S.; Babich, F.; Licina, D. Performance assessment of low-cost environmental monitors and single sensors under variable indoor air quality and thermal conditions. Build. Environ. 2021, 187, 107415. [Google Scholar] [CrossRef]
- Shao, Y.; Kavi, L.; Boyle, M.; Louis, L.M.; Pool, W.; Thomas, S.B.; Wilson, S.; Rule, A.M.; Quiros-Alcala, L. Real-time air monitoring of occupational exposures to particulate matter among hairdressers in Maryland: A pilot study. Indoor Air 2021, 31, 1144–1153. [Google Scholar] [CrossRef]
- Liu, H.; Schneider, P.; Haugen, R.; Vogt, M. Performance assessment of a low-cost PM2.5 sensor for a near four-month period in Oslo, Norway. Atmosphere 2019, 10, 41. [Google Scholar] [CrossRef] [Green Version]
- Dobroski, H.; Tuchman, D.P.; Vinson, R.P.; Timko, R.J. Differential pressure response of 25-mm-diameter glass fiber filters challenged with coal and limestone dust mixtures. Appl. Occup. Environ. Hyg. 2002, 17, 96–103. [Google Scholar] [CrossRef]
- Volkwein, J.C.; Schoeneman, A.L.; Page, S.J. Laboratory evaluation of pressure differential-based respirable dust detector tube. Appl. Occup. Environ. Hyg. 2000, 15, 158–164. [Google Scholar] [CrossRef]
- Volkwein, J.C.; Thimons, E.D. New tools to monitor personal exposure to respirable coal mine dust. In Proceedings of the Seventh International Mine Ventilation Congress, Crakow, Poland, 17–22 June 2001. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zheng, L.; Xuan, P.; Huo, R. A Real-Time Approach to Detect PM2.5 in a Seriously Polluted Environment Based on Pressure Drop. Atmosphere 2022, 13, 1237. https://doi.org/10.3390/atmos13081237
Li J, Zheng L, Xuan P, Huo R. A Real-Time Approach to Detect PM2.5 in a Seriously Polluted Environment Based on Pressure Drop. Atmosphere. 2022; 13(8):1237. https://doi.org/10.3390/atmos13081237
Chicago/Turabian StyleLi, Jialin, Lina Zheng, Peng Xuan, and Ruiyan Huo. 2022. "A Real-Time Approach to Detect PM2.5 in a Seriously Polluted Environment Based on Pressure Drop" Atmosphere 13, no. 8: 1237. https://doi.org/10.3390/atmos13081237




