Studies of the Dispersed Composition of Atmospheric Aerosol and Its Relationship with Small Gas Impurities in the Near-Water Layer of Lake Baikal Based on the Results of Ship Measurements in the Summer of 2020
Abstract
:1. Introduction
2. Materials and Methods
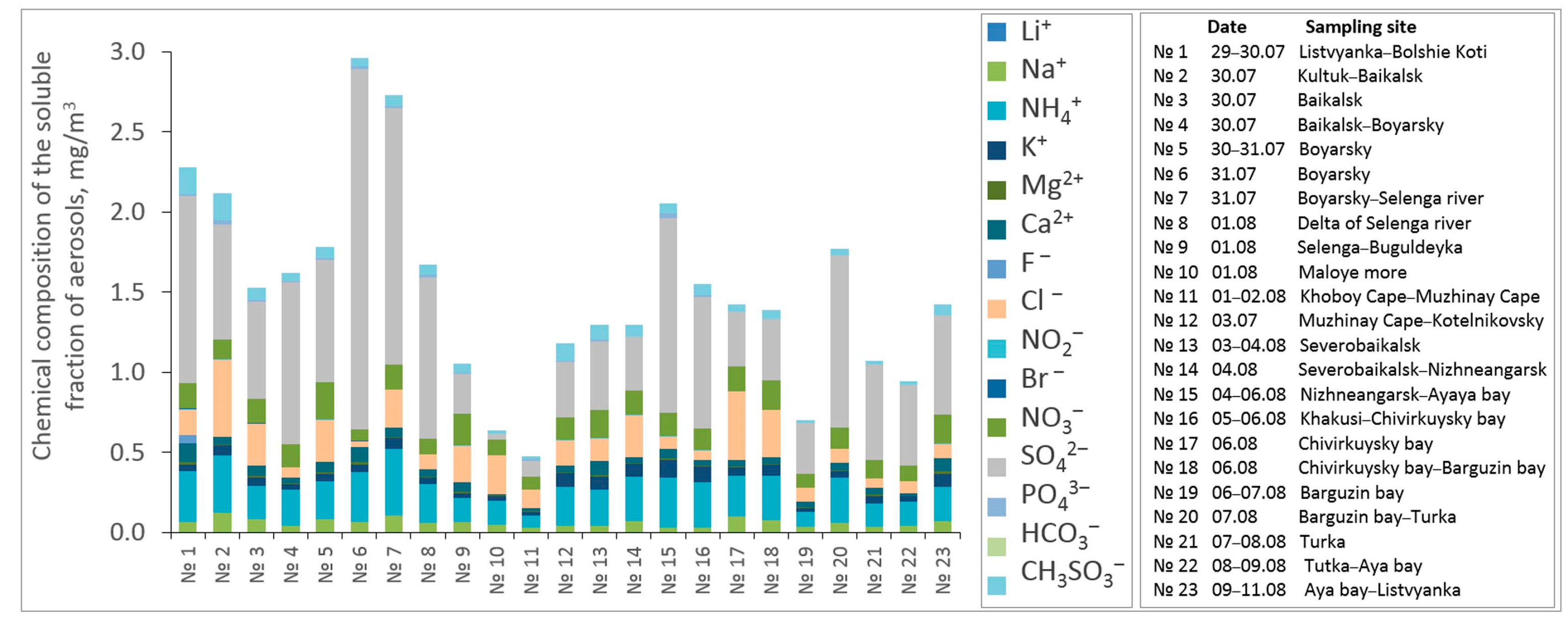
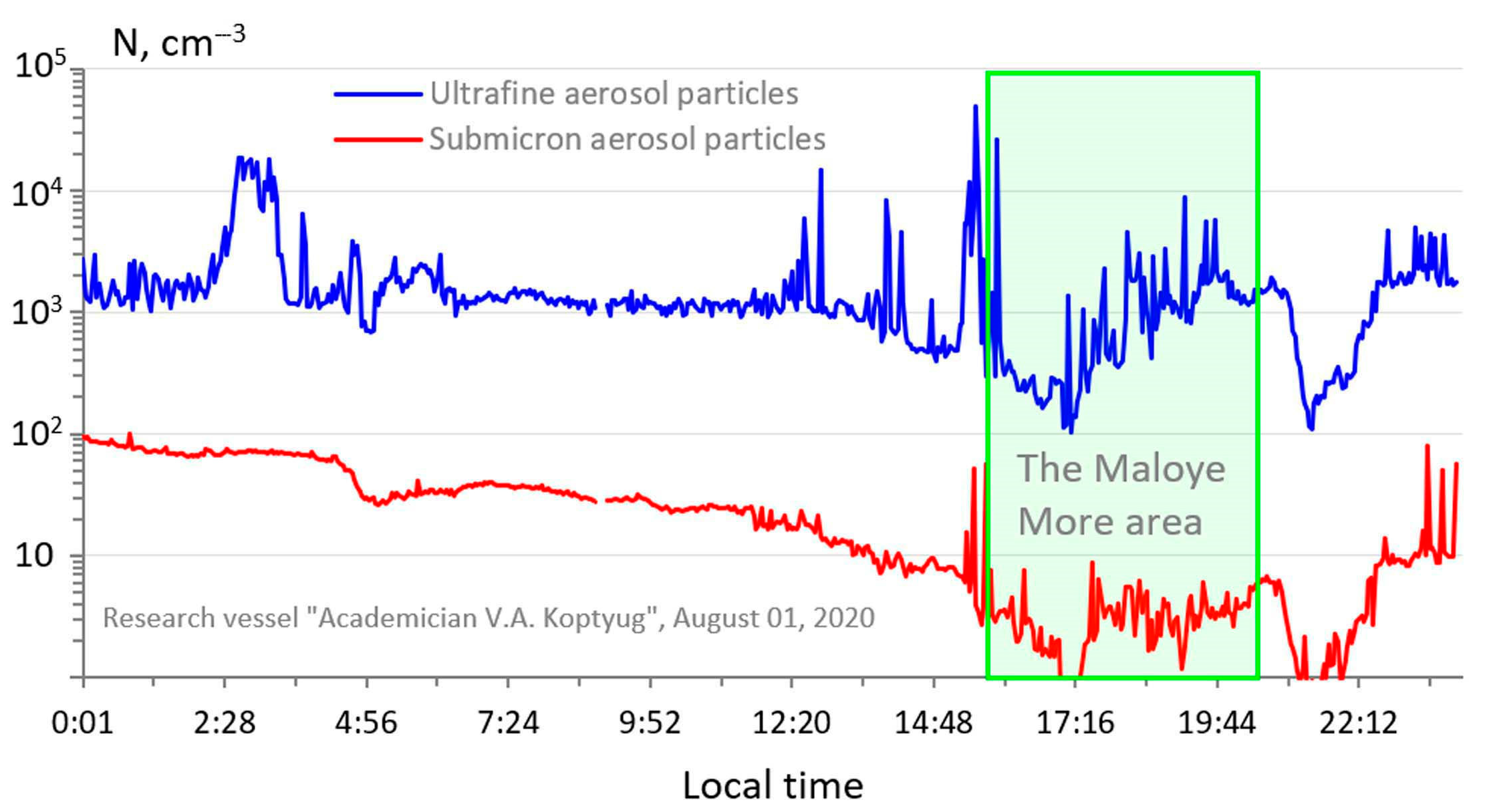
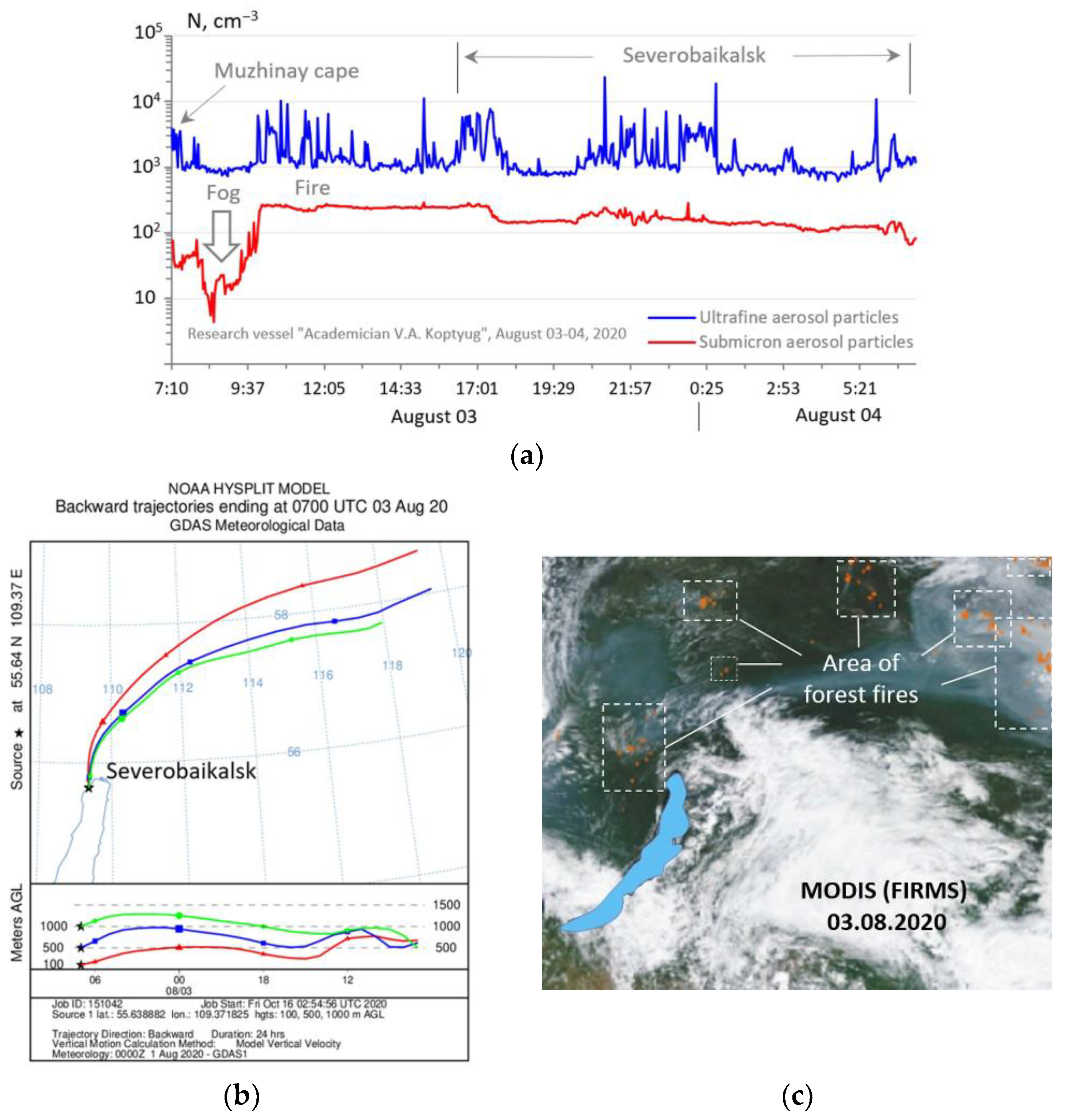
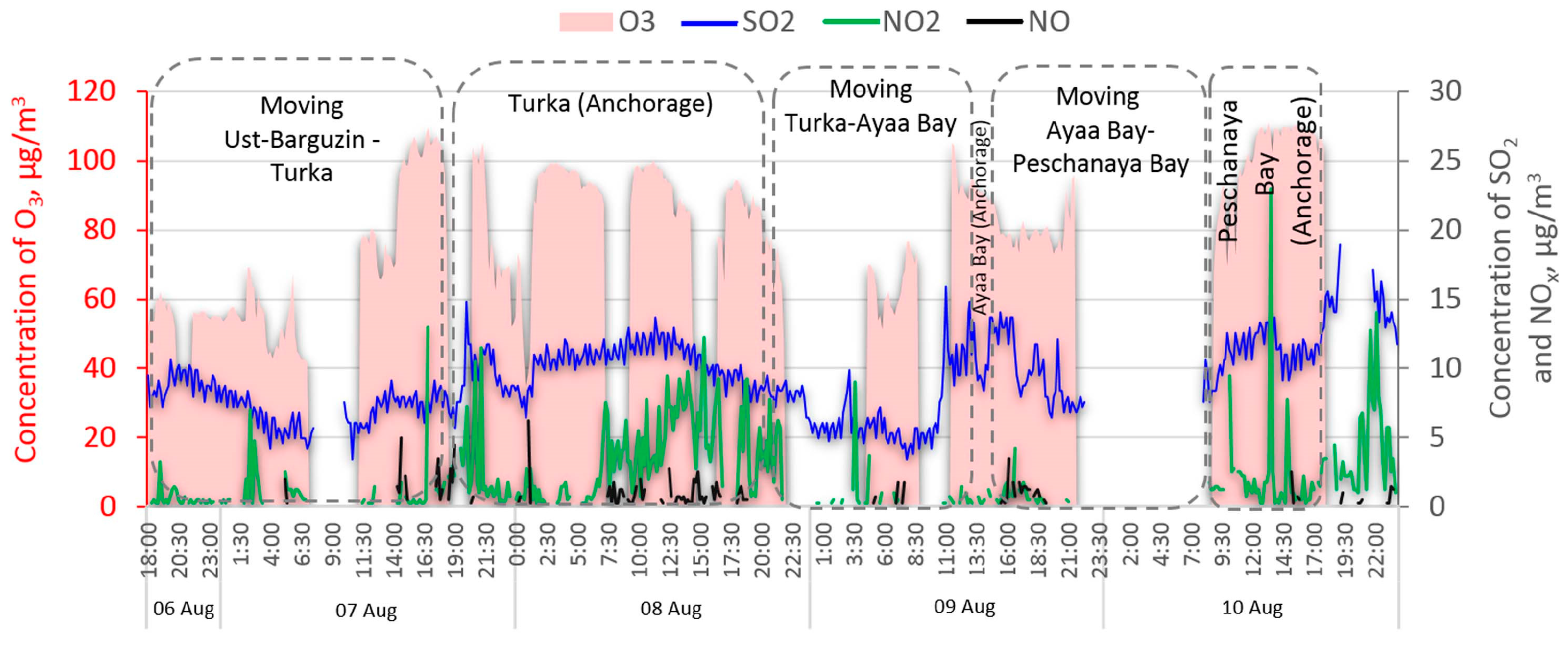
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abbatt, J.P.D.; Leaitch, W.R.; Aliabadi, A.A.; Bertram, A.K.; Blanchet, J.P.; Boivin-Rioux, A.; Bozem, H.; Burkart, J.; Chang, R.Y.W.; Charette, J.; et al. Overview paper: New insights into aerosol and climate in the Arctic. Atmos. Chem. Phys. 2019, 19, 2527–2560. [Google Scholar] [CrossRef] [Green Version]
- Leonardi, A.; Ricker, H.M.; Gale, A.G.; Ball, B.T.; Odbadrakh, T.T.; Shields, G.C.; Navea, J.G. Particle formation and surface processes on atmospheric aerosols: A review of applied quantum chemical calculations. Int. J. Quantum Chem. 2020, 120, 18. [Google Scholar] [CrossRef]
- Warneck, P. Chemistry of the Natural Atmosphere; Academic Press: Cambridge, MA, USA, 1988. [Google Scholar] [CrossRef]
- Schröder, F.; Kärcher, B.; Fiebig, M.; Petzold, A. Aerosol states in the free troposphere at northern midlatitudes. J. Geophys. Res. Atmos. 2002, 107, LAC-8. [Google Scholar] [CrossRef] [Green Version]
- Penner, J.E.; Andreae, M.; Annegarn, H.; Barrie, L.; Feichter, J.; Hegg, D.; Jayaraman, A.; Leaitch, R.; Murphy, D.; Nganga, J.; et al. Aerosols, their Direct and Indirect Effects. In Climate Change 2001: The Scientific Basis: Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change; Houghton, J.T., Ding, Y., Griggs, D.J., Noguer, M., van der Linden, P.J., Dai, X., Maskell, K., Johnson, C.A., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 289–348. [Google Scholar]
- Hallquist, M.; Wenger, J.C.; Baltensperger, U.; Rudich, Y.; Simpson, D.; Claeys, M.; Dommen, J.; Donahue, N.M.; George, C.; Goldstein, A.H.; et al. The formation, properties and impact of secondary organic aerosol: Current and emerging issues. Atmos. Chem. Phys. 2009, 9, 5155–5236. [Google Scholar] [CrossRef] [Green Version]
- Ivlev, L.S. Chemical Composition and Structure of Atmospheric Aerosols; Publishing House of Leningrad State University: Leningrad, Russia, 1982; pp. 233–278. [Google Scholar]
- Smirnov, V.V. Nature and evolution of ultrafine aerosol particles in the atmosphere. Izv. Atmos. Ocean. Phys. 2006, 42, 663–687. [Google Scholar] [CrossRef]
- Miikka, D.M.; Markku, K.; Ilona, R.; Robert, W.; Tareq, H.; Pasi, P.A.; Kari, E.J. Formation and growth of fresh atmospheric aerosols: Eight years of aerosol size distribution data from SMEAR II, Hyytiälä, Finland. Boreal Environ. Res. 2005, 10, 323–336. [Google Scholar]
- Kulmala, M.; Vehkamäki, H.; Petäjä, T.; Dal Maso, M.; Lauri, A.; Kerminen, V.M.; Birmili, W.; McMurry, P. Formation and growth rates of ultrafine atmospheric particles: A review of observations. J. Aerosol Sci. 2004, 35, 143–176. [Google Scholar] [CrossRef]
- Petaja, T.; Vakkari, V.; Pohja, T.; Nieminen, T.; Laakso, H.; Aalto, P.P.; Keronen, P.; Siivola, E.; Kerminen, V.M.; Kulmala, M.; et al. Transportable Aerosol Characterization Trailer with Trace Gas Chemistry: Design, Instruments and Verification. Aerosol Air Qual. Res. 2013, 13, 421–435. [Google Scholar] [CrossRef] [Green Version]
- Hidy, G.M. Aerosols and Atmospheric Chemistry; Academic Press: Cambridge, MA, USA, 1972; pp. 120–127. [Google Scholar]
- World Health Organization. Air Quality Guidelines: Global Update 2005: Particulate Matter, Ozone, Nitrogen Dioxide, and Sulfur Dioxide; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Guerreiro, C.B.B.; Foltescu, V.; de Leeuw, F. Air quality status and trends in Europe. Atmos. Environ. 2014, 98, 376–384. [Google Scholar] [CrossRef] [Green Version]
- Zagaynov, V.A.; Kulmala, M.; Lyubovtseva, Y.S.; Lushnikov, A.A.; Sogacheva, L.; Khodzher, T.V. Nucleation bursts in the atmosphere of Central Siberia. In Proceedings of the NOSA 2006 Aerosol Symposium, Helsinki, Finland, 8–10 November 2006; Finnish-Czech Aerosol Symposium: Helsinki, Finland, 2006; pp. 419–423. [Google Scholar]
- Zagaynov, V.A.; Lushnikov, A.A.; Nikitin, O.N.; Kravchenko, P.E.; Khodzher, T.V.; Petryanov-Sokolov, I.V. Background aerosol over Lake of Baikal. Dokl. Akad. Nauk. 1989, 308, 1087–1090. [Google Scholar]
- Documents of Ministry of Natural Resources and Environment of the Russian Federation. Available online: http://www.mnr.gov.ru/docs/gosudarstvennye_doklady (accessed on 2 September 2021).
- Izmest’eva, L.R.; Moore, M.V.; Hampton, S.E.; Ferwerda, C.J.; Gray, D.K.; Woo, K.H.; Pislegina, H.V.; Krashchuk, L.S.; Shimaraeva, S.V.; Silow, E.A. Lake-wide physical and biological trends associated with warming in Lake Baikal. J. Great Lakes Res. 2016, 42, 0380–1330. [Google Scholar] [CrossRef] [Green Version]
- Shvidenko, A.Z.; Schepaschenko, D.G. Climate change and wildfires in Russia. Contemp. Probl. Ecol. 2013, 6, 683–692. [Google Scholar] [CrossRef]
- Semenov, M.Y.; Marinaite, I.I.; Golobokova, L.P.; Khuriganova, O.I.; Khodzher, T.V.; Semenov, Y.M. Source apportionment of polycyclic aromatic hydrocarbons in Lake Baikal water and adjacent air layer. Chem. Ecol. 2017, 33, 977–990. [Google Scholar] [CrossRef]
- Zayakhanov, A.S.; Zhamsueva, G.S.; Tsydypov, V.V.; Balzhanov, T.S.; Balin, Y.S.; Kokhanenko, G.P.; Penner, I.E.; Nasonov, S.V. Features of the transport and transformation of aerosol and gas impurities in the atmosphere in the coastal zone of the Lake Baikal. Opt. Atmos. Okeana 2018, 31, 968–973. (In Russian) [Google Scholar] [CrossRef]
- Fisher, J.A.; Jacob, D.J.; Wang, Q.Q.; Bahreini, R.; Carouge, C.C.; Cubison, M.J.; Dibb, J.E.; Diehl, T.; Jimenez, J.L.; Leibensperger, E.M.; et al. Sources, distribution, and acidity of sulfate-ammonium aerosol in the Arctic in winter-spring. Atmos. Environ. 2011, 45, 7301–7318. [Google Scholar] [CrossRef] [Green Version]
- Qi, L.; Li, Q.B.; Henze, D.K.; Tseng, H.L.; He, C.L. Sources of springtime surface black carbon in the Arctic: An adjoint analysis for April 2008. Atmos. Chem. Phys. 2017, 17, 9697–9716. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Ishizawa, M.; Chan, D.; Lavoue, D.; Andrews, E.; Eleftheriadis, K.; Maksyutov, S. 16-year simulation of Arctic black carbon: Transport, source contribution, and sensitivity analysis on deposition. J. Geophys. Res.-Atmos. 2013, 118, 943–964. [Google Scholar] [CrossRef]
- Stohl, A. Characteristics of atmospheric transport into the Arctic troposphere. J. Geophys. Res.-Atmos. 2006, 111, 17. [Google Scholar] [CrossRef]
- Zayakhanov, A.S.; Zhamsueva, G.S.; Tcydypov, V.V.; Balzhanov, T.S.; Dementeva, A.L.; Khodzher, T.V. Investigation of Transport and Transformation of Tropospheric Ozone in Terrestrial Ecosystems of the Coastal Zone of Lake Baikal. Atmosphere 2019, 10, 739. [Google Scholar] [CrossRef] [Green Version]
- Khodzher, T.V.; Zagaynov, V.A.; Lushnikov, A.A.; Chausov, V.D.; Zhamsueva, G.S.; Zayakhanov, A.S.; Tsydypov, V.V.; Potemkin, V.L.; Marinaite, I.I.; Maksimenko, V.V.; et al. Study of Aerosol Nano- and Submicron Particle Compositions in the Atmosphere of Lake Baikal During Natural Fire Events and Their Interaction with Water Surface. Water Air Soil Pollut. 2021, 232, 266. [Google Scholar] [CrossRef]
- Kravtsova, L.S.; Izhboldina, L.A.; Khanaev, I.V.; Pomazkina, G.V.; Rodionova, E.V.; Domysheva, V.M.; Sakirko, M.V.; Tomberg, I.V.; Kostornova, T.Y.; Kravchenko, O.S.; et al. Nearshore benthic blooms of filamentous green algae in Lake Baikal. J. Great Lakes Res. 2014, 40, 441–448. [Google Scholar] [CrossRef]
- Timoshkin, O.; Bondarenko, N.; Volkova, Y.A.; Tomberg, I.; Vishnyakov, V.; Malnik, V. Mass development of green filamentous algae of the genera Spirogyra and Stigeoclonium (Chlorophyta) in the littoral zone of the southern part of Lake Baikal. Hydrobiol. J. 2015, 51, 13–23. [Google Scholar] [CrossRef]
- Timoshkin, O.A.; Moore, M.V.; Kulikova, N.N.; Tomberg, I.V.; Malnik, V.V.; Shimaraev, M.N.; Troitskaya, E.S.; Shirokaya, A.A.; Sinyukovich, V.N.; Zaitseva, E.P.; et al. Groundwater contamination by sewage causes benthic algal outbreaks in the littoral zone of Lake Baikal (East Siberia). J. Great Lakes Res. 2018, 44, 230–244. [Google Scholar] [CrossRef]
- Khuriganova, O.I.; Obolkin, V.A.; Golobokova, L.P.; Khodzher, T.V. Variability of gas impurities in the ground atmosphere of South-Eastern Siberia. In Proceedings of the 24th International Symposium on Atmospheric and Ocean Optics—Atmospheric Physics, Tomsk, Russia, 2–5 July 2018; Spie-Int Soc Optical Engineering: Bellingham, WA, USA, 2018; Volume 10833. [Google Scholar] [CrossRef]
- Obolkin, V.A.; Maysyuk, E.P.; Ivanova, I.Y.; Khodzher, T.V. Nitrogen oxides in the atmosphere of coastal areas of Lake Baikal. Sources and possible impact on the ecosystem of the lake. In Proceedings of the 24th International Symposium on Atmospheric and Ocean Optics—Atmospheric Physics, Tomsk, Russia, 2–5 July 2018; Spie-Int Soc Optical Engineering: Bellingham, WA, USA, 2018; Volume 10833. [Google Scholar] [CrossRef]
- Sakirko, M.V.; Panchenko, M.V.; Domysheva, V.M.; Pestunov, D.A. Diurnal Rhythms of Carbon Dioxide Concentration in the Sea-level Air Layer and in the Surface Water of Lake Baikal in Different Hydrological Seasons. Russ. Meteorol. Hydrol. 2008, 33, 112–116. [Google Scholar] [CrossRef]
- Julanov, Y.V.; Lushnikov, A.A.; Zagaynov, V.A. Diffusion aerosol spectrometer. Atmos. Res. 2002, 62, 295–302. [Google Scholar] [CrossRef]
- Zayakhanov, A.S.; Zhamsueva, G.S.; Tsydypov, V.V.; Ayurzhanaev, A.A. Automated system for monitoring atmospheric pollution. Meas. Tech. 2008, 51, 1342–1346. [Google Scholar] [CrossRef]
- Quality Management. Available online: https://www.iso.org/ru/iso-9001-quality-management.html (accessed on 14 December 2021).
- Zayakhanov, A.S.; Zhamsueva, G.S.; Tsydypov, V.V. A Hardware-Software System for Monitoring the Content of Atmospheric Impurities. Meas. Tech. 2015, 58, 355–361. [Google Scholar] [CrossRef]
- Azbukin, A.A.; Bogushevich, A.Y.; Korolkov, V.A.; Tikhomirov, A.A.; Shelevoi, V.D. A field version of the AMK-03 automated ultrasonic meteorological complex. Russ. Meteorol. Hydrol. 2009, 34, 133–136. [Google Scholar] [CrossRef]
- Golobokova, L.; Khodzher, T.; Obolkin, V.; Potemkin, V.; Khuriganova, O.; Onischuk, N. Aerosol in the atmosphere of the Baikal region: History and contemporary researches. Limnol. Freshw. Biol. 2018, 1, 49–57. [Google Scholar] [CrossRef] [Green Version]
- EMEP. Manual for Sampling and Chemical Analysis. EMEP Cooperative Programme for Monitoring and Evaluation of the Long-range Transmission of Air Pollutant in Europe. Available online: http://www.itm.su.se (accessed on 11 October 2020).
- NILU. The European Monitoring and Evaluation Programme/CCC-Report 1/95. Reference: 0-7726. Available online: http://www.nilu.no (accessed on 11 October 2020).
- The Quality Assurance/Science Activity Centre—Americas (QA/SAC-Americas). Available online: https://www.qasac-americas.org/ (accessed on 14 November 2021).
- Asia Center for Air Pollution Research (ACAP). Available online: https://www.acap.asia/ (accessed on 14 November 2021).
- Norwegian Institute for Air Research. Available online: https://www.nilu.com/ (accessed on 14 November 2021).
- Allan, M.A. Manual for the GAW Precipitation Chemistry Programme: Guidelines, Data Quality Objectives and Standard Operating Procedures; World Meteorological Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Arctic and Antarctic Research Institute. Available online: https://www.aari.ru/ (accessed on 14 November 2021).
- ARL NOAA. Atmospheric Resource Laboratory NOAA. Available online: http://www.arl.noaa.gov (accessed on 14 November 2021).
- Hanaoka, T.; Masui, T. Exploring effective short-lived climate pollutant mitigation scenarios by considering synergies and trade-offs of combinations of air pollutant measures and low carbon measures towards the level of the 2 degrees C target in Asia. Environ. Pollut. 2020, 261, 9. [Google Scholar] [CrossRef]
- Vadrevu, K.P.; Ohara, T. Greenhouse gases, Short-Lived Climate Pollutants and aerosol pollution in South/Southeast Asia–Drivers, states and impacts. Environ. Pollut. 2021, 277, 3. [Google Scholar] [CrossRef]
- Obolkin, V.A.; Potemkin, V.L.; Makukhin, V.L.; Khodzher, T.V.; Chipanina, E.V. Long-distance transport of plumes of atmospheric emissions from regional coal-fired CHPPs to the water area of South Baikal. Atmos. Ocean. Opt. 2009, 22, 853–858. [Google Scholar]
- Zayakhanov, A.S.; Zhamsueva, G.S.; Tsydypov, V.V.; Balzhanov, T.S. Daily dynamics of ozone and other small gas impurities in the coastal zone of Lake Baikal (station Boyarsky). Russ. Meteorol. Hydrol. 2017, 8, 85–92. [Google Scholar]
- Martin, R.V.; Jacob, D.J.; Yantosca, R.M.; Chin, M.; Ginoux, P. Global and regional decreases in tropospheric oxidants from photochemical effects of aerosols. J. Geophys. Res. 2003, 108, 4097. [Google Scholar] [CrossRef]
- Arshinov, M.Y.; Belan, B.D. Investigation of the dispersed aerosol composition during spring haze and forest fires. Opt. Atmos. Okeana J. 2011, 24, 468–477. (In Russian) [Google Scholar]
- Ivlev, L.S. Mechanisms of formation and decomposition of atmospheric aerosols and clouds and their ecological significance. Interdiscip. Sci. Appl. J. Biosph. 2013, 5, 82–210. (In Russian) [Google Scholar]
- Reichstein, M.; Carvalhais, N. Aspects of Forest Biomass in the Earth System: Its Role and Major Unknowns. Surv. Geophys. 2019, 40, 693–707. [Google Scholar] [CrossRef] [Green Version]
- Keywood, M.; Kanakidou, M.; Stohl, A.; Dentener, F.; Grassi, G.; Meyer, C.P.; Torseth, K.; Edwards, D.; Thompson, A.M.; Lohmann, U.; et al. Fire in the Air: Biomass Burning Impacts in a Changing Climate. Crit. Rev. Environ. Sci. Technol. 2013, 43, 40–83. [Google Scholar] [CrossRef]
- Maccracken, M.C.; Cess, R.D.; Potter, G.L. Climatic effects of anthropogenic arctic aerosols—An illustration of climate feedback mechanisms with one-dimensional and two-dimensional climate models. J. Geophys. Res.-Atmos. 1986, 91, 14445–14450. [Google Scholar] [CrossRef]
- Penner, J.E.; Ghan, S.J.; Walton, J.J. The role of biomass burning in the budget and cycle of carbonaceous soot aerosols and their climate impact. In Global Biomass Burning: Atmospheric, Climatic, and Biospheric Implications; Massachusetts Inst of Tech Press: Cambridge, MA, USA, 1991; pp. 387–393. [Google Scholar]
- Stohl, A.; Berg, T.; Burkhart, J.F.; Fjaeraa, A.M.; Forster, C.; Herber, A.; Hov, O.; Lunder, C.; McMillan, W.W.; Oltmans, S.; et al. Arctic smoke—Record high air pollution levels in the European Arctic due to agricultural fires in Eastern Europe in spring 2006. Atmos. Chem. Phys. 2007, 7, 511–534. [Google Scholar] [CrossRef] [Green Version]
- Kobziar, L.N.; Thompson, G.R. Wildfire smoke, a potential infectious agent. Science 2020, 370, 1408–1410. [Google Scholar] [CrossRef] [PubMed]
- Satellite Monitoring of Fires in the Far East. Available online: http://fires-dv.kosmosnimki.ru/ (accessed on 14 November 2021).
- Vardy, M.; Oppenheimer, M.; Dubash, N.K.; O’Reilly, J.; Jamieson, D. The Intergovernmental Panel on Climate Change: Challenges and Opportunities. Annu. Rev. Environ. Resour. 2017, 42, 55–75. [Google Scholar] [CrossRef] [Green Version]
- Andreae, M.O. Emission of trace gases and aerosols from biomass burning—An updated assessment. Atmos. Chem. Phys. 2019, 19, 8523–8546. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.H.; Kim, J.E.; Kim, Y.J.; Kim, J.; von Hoyningen-Huene, W. Impact of the smoke aerosol from Russian forest fires on the atmospheric environment over Korea during May 2003. Atmos. Environ. 2005, 39, 85–99. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhamsueva, G.; Zayakhanov, A.; Khodzher, T.; Tcydypov, V.; Balzhanov, T.; Dementeva, A. Studies of the Dispersed Composition of Atmospheric Aerosol and Its Relationship with Small Gas Impurities in the Near-Water Layer of Lake Baikal Based on the Results of Ship Measurements in the Summer of 2020. Atmosphere 2022, 13, 139. https://doi.org/10.3390/atmos13010139
Zhamsueva G, Zayakhanov A, Khodzher T, Tcydypov V, Balzhanov T, Dementeva A. Studies of the Dispersed Composition of Atmospheric Aerosol and Its Relationship with Small Gas Impurities in the Near-Water Layer of Lake Baikal Based on the Results of Ship Measurements in the Summer of 2020. Atmosphere. 2022; 13(1):139. https://doi.org/10.3390/atmos13010139
Chicago/Turabian StyleZhamsueva, Galina, Alexander Zayakhanov, Tamara Khodzher, Vadim Tcydypov, Tumen Balzhanov, and Ayuna Dementeva. 2022. "Studies of the Dispersed Composition of Atmospheric Aerosol and Its Relationship with Small Gas Impurities in the Near-Water Layer of Lake Baikal Based on the Results of Ship Measurements in the Summer of 2020" Atmosphere 13, no. 1: 139. https://doi.org/10.3390/atmos13010139





