SSH-Aerosol v1.1: A Modular Box Model to Simulate the Evolution of Primary and Secondary Aerosols
Abstract
:1. Introduction
1.1. Aerosol Dynamics
1.2. Formation of Condensable
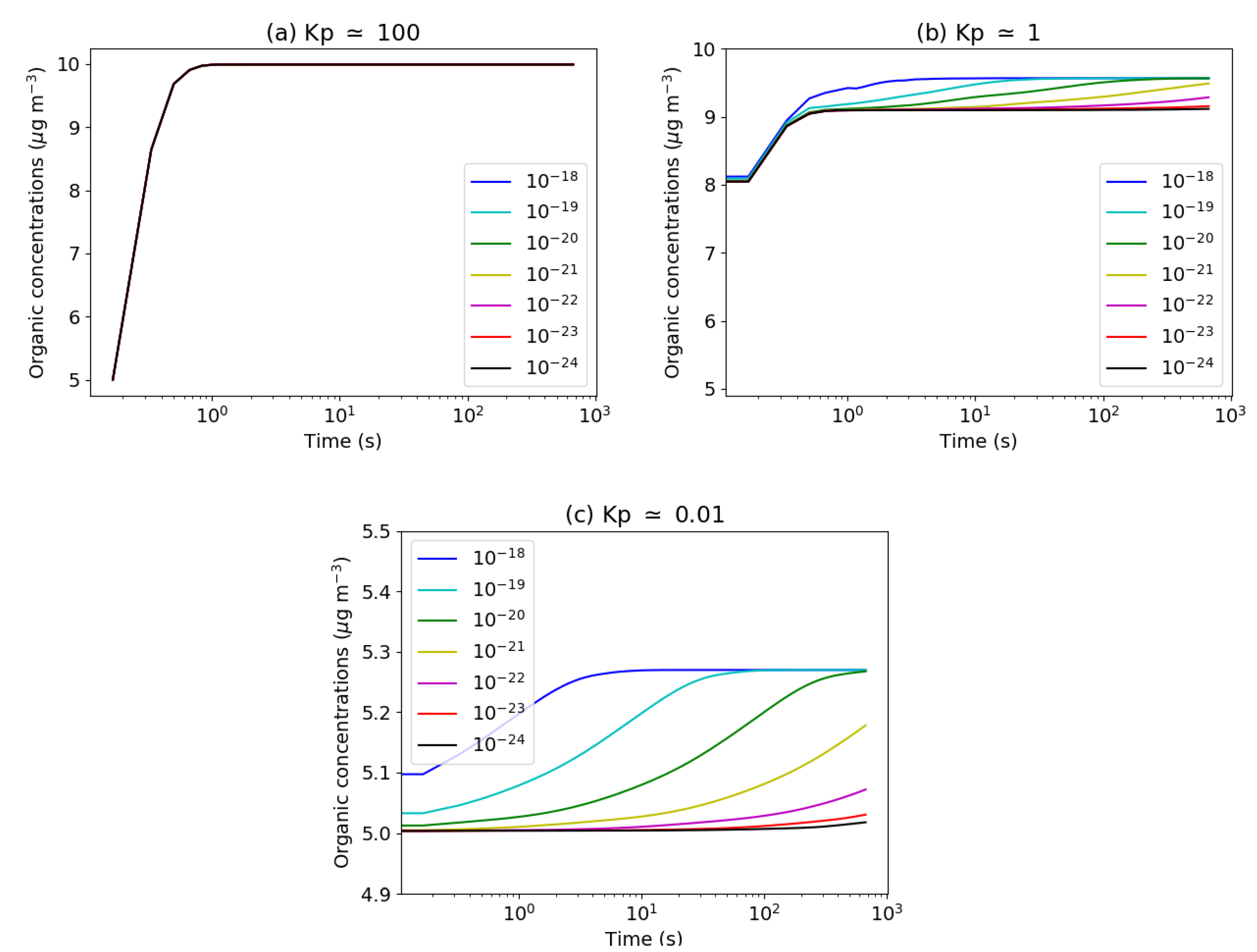
1.3. Gas/Particle Partitioning of Organics
2. Description of the Model
2.1. Input Data and Model Results
2.2. Structure of the Model
2.3. Coupling to 3D Models
3. Aerosol Processes
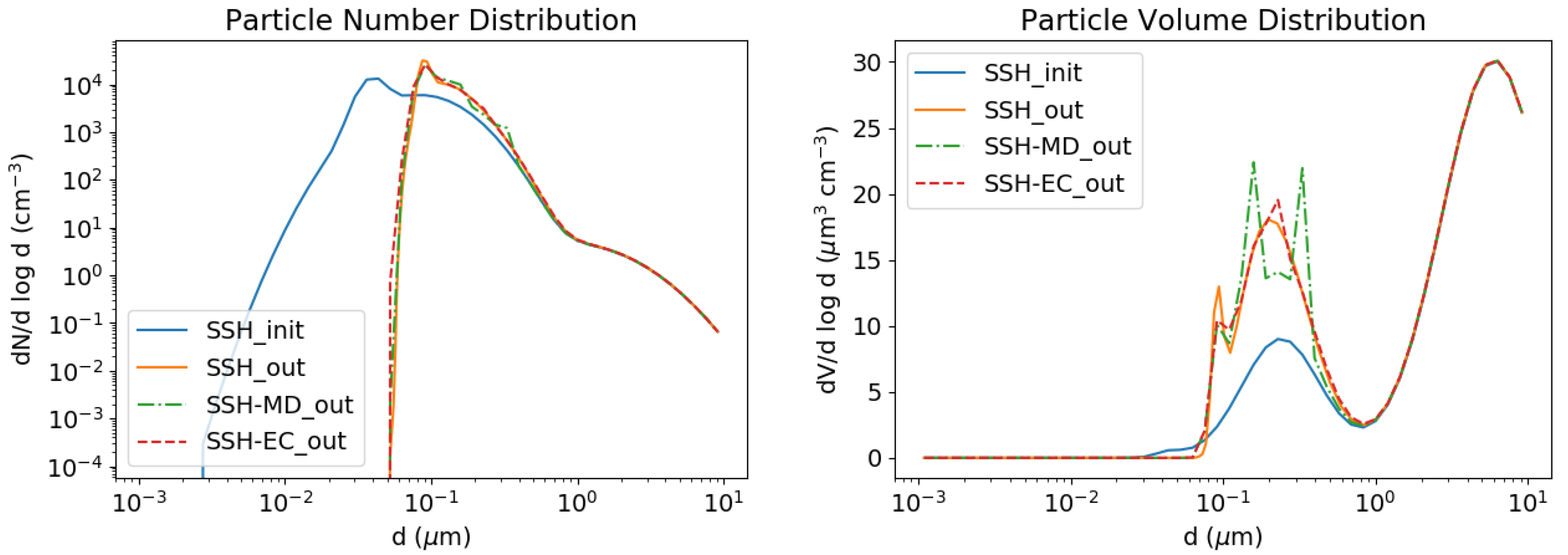
3.1. Coagulation and Condensation of Extremely Low-Volatility Compounds
3.2. Condensation/Evaporation
3.2.1. Redistribution
3.2.2. Thermodynamic Equilibrium
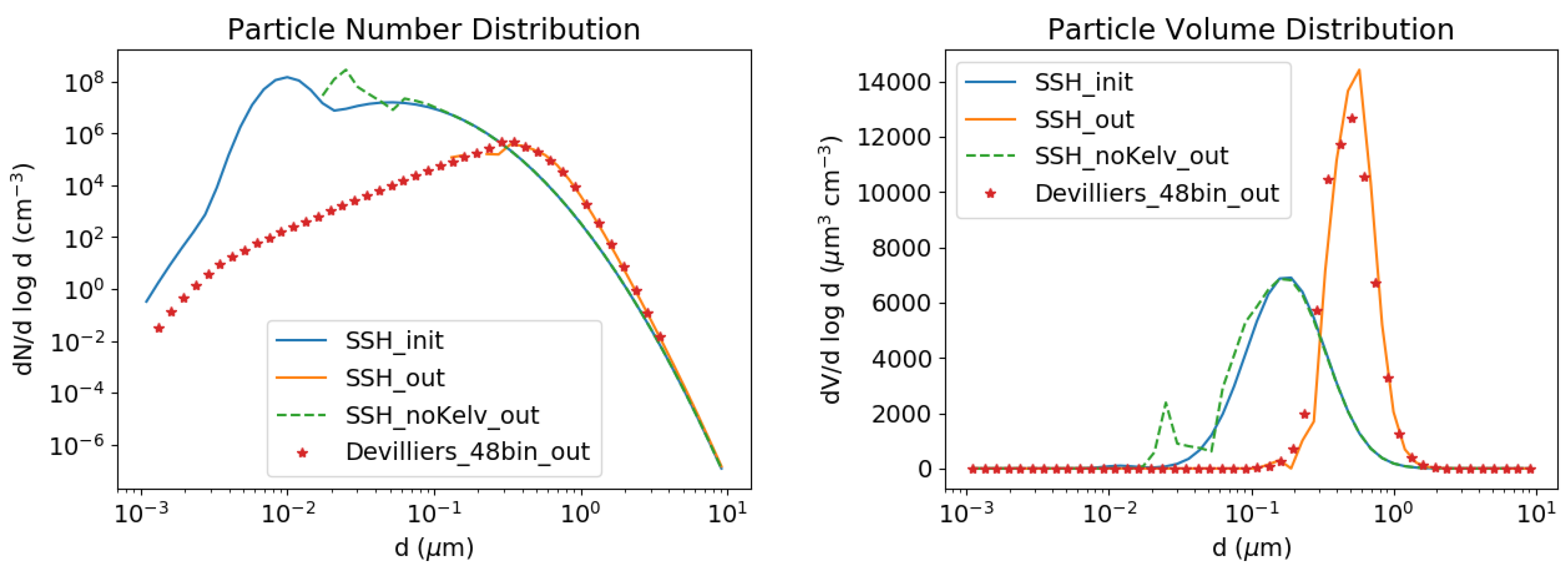
3.2.3. Kelvin Effect
3.2.4. Particle Viscosity
3.3. Mixing State
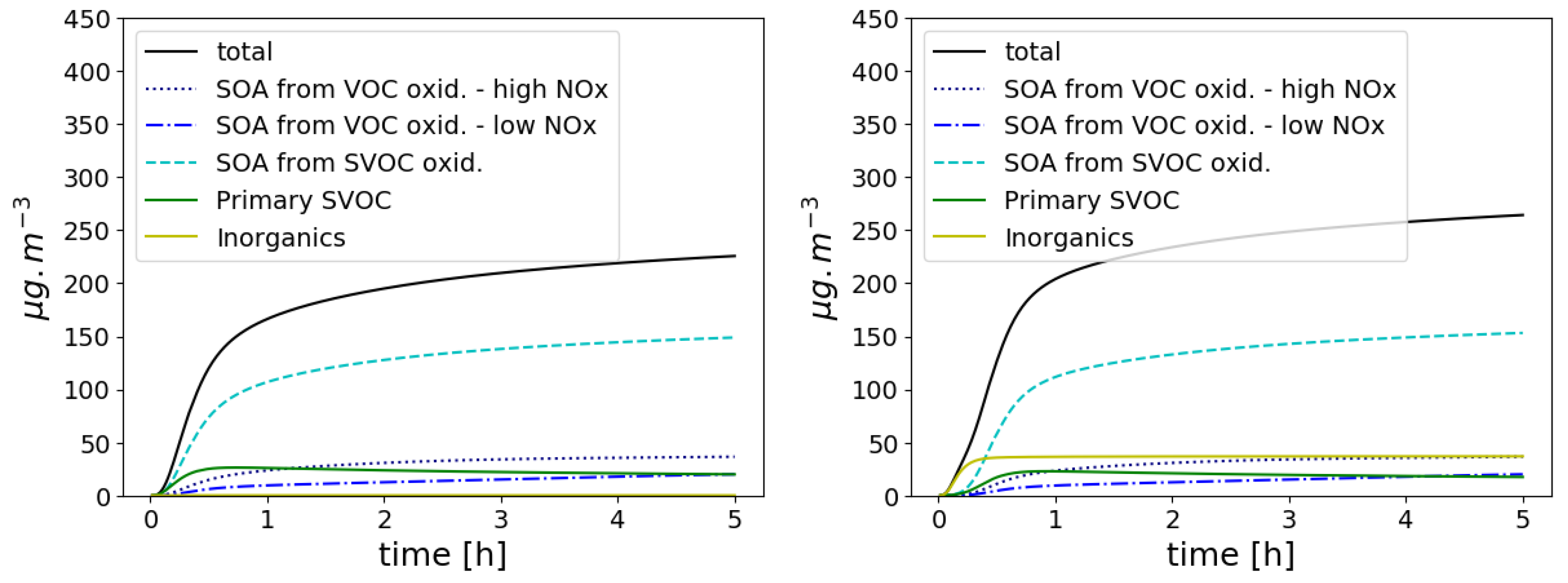
3.4. Organic Aerosol Formation
3.4.1. Formation of Condensable
3.4.2. Absorption into an Aqueous or an Organic Phase
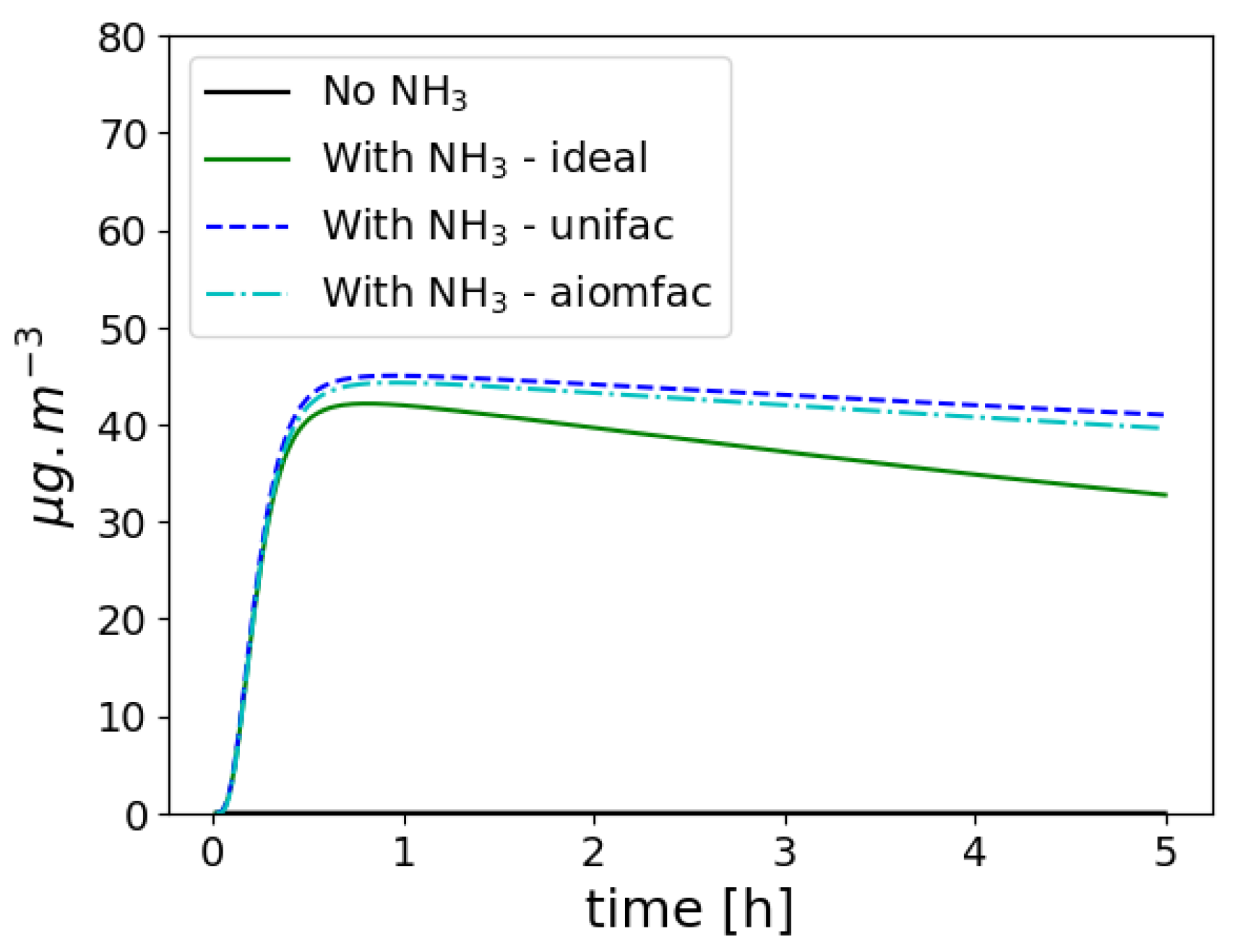
3.4.3. Ideality
3.5. Nucleation and Coupling between Processes
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Appendix A. Code Metadata
| Current code version | v1.1 |
| Link to code/repository | https://github.com/sshaerosol/ssh-aerosol |
| Legal Code License | GNU license v3 |
| Software code languages | C++, FORTRAN and python for visualisation |
| Link to manual | https://github.com/sshaerosol/ssh-aerosol/blob/master/user_manual |
| Support email for questions | [email protected] |
Appendix B. Aerosol Surrogates
| Surrogate | Type | Molecular Structure | MW | r | H | Kp | ΔHvap | Precursor |
|---|---|---|---|---|---|---|---|---|
| BiMT | A | methyl tetrol | 136 | 2.3 | 330 | 0.064 | 38 | isoprene |
| BiPER | A | methyl dihydroxy dihydroperoxide | 168 | 2.8 | 81 | 0.036 | 38 | |
| BiDER | A | methyl tetrol | 136 | 2.3 | 891 | 0.227 | 38 | |
| BiMGA | A | methyl glyceric acid (MGA) | 120 | 2.5 | 5.3 | 0.007 | 43 | |
| BiNGA | B | nitrate derivative of MGA | 165 | 3.5 | 0.4 | 0.007 | 43 | |
| BiNIT3 | B | methyl hydroxy trinitrate butane | 272 | 4.6 | 0.4 | 0.064 | 38 | |
| BiA0D | A | pinonaldehyde | 168 | 1.4 | 0.02 | 0.0003 | 50 | mono-terpenes |
| BiA1D | A | norpinic acid | 170 | 1.5 | 1.12 | 0.428 | 50 | |
| BiA2D | A | pinic acid | 186 | 1.7 | 2.7 | 0.650 | 109 | |
| BiA3D | B | 3-methyl-1,2,3-butane tricarboxylic acid | 204 | 2.1 | - | n.v. | - | |
| BiNIT | B | Nitrooxy-limonene-ol | 215 | 1.8 | 0.02 | 0.037 | 50 | |
| Monomer | C | C10H14O9 | 278 | 2.3 | - | n. v. | - | |
| Dimer | C | C19H28O11 | 432 | 1.9 | - | n. v. | - | |
| BiBlP | B | C15 hydroxy nitrate aldehyde | 298 | 1.7 | 2.34 | 154.9 | 175 | sesqui-terpenes |
| BiBmP | B | C15 oxo aldehyde | 236 | 1.3 | - | 0.309 | 175 | |
| AnBlP | B | methyl nitro benzoic acid | 167 | 2 | 2.48 | 1.37 | 50 | xylenes and toluene |
| AnBmP | B | methyl hydroxy benzoic acid | 152 | 1.6 | 12.4 | 0.011 | 50 | |
| AnClP | C | No structure | 167 | 2 | - | n. v. | - | |
| ACIDMAL | A | maleylacetic acid | 158 | 2.2 | 8685 | 2.02 | 50 | phenol catechol benzene |
| DHMB | A | dihydroxymethyl benzoquinone | 154 | 1.8 | 36.2 | 0.026 | 50 | methyl-catechol cresol |
| PSYR | A | C8H10O5 | 186 | 1.9 | 14.5 | 0.0123 | 50 | syringol |
| GHDPerox | A | SOA (hydroperoxide) | 174 | 2.1 | 63.3 | 0.171 | 50 | guaiacol |
| PAHlN | A | dihydroxytere | 198 | 2.1 | n. v. | - | - | naphtalene |
| phtalic acid | methyl-PAHhN | |||||||
| A | phthalic acid | 166 | 1.7 | 14.9 | 0.093 | 50 | naphtalene | |
| POAlP | B | primary OA of low volatility | 280 | 1.3 | - | 1.1 | 106 | - |
| POAmP | B | primary OA of medium volatility | 280 | 1.3 | - | 0.0116 | 91 | - |
| POAhP | B | primary OA of high volatility | 280 | 1.3 | - | 0.00031 | 79 | - |
| SOAlP | C | secondary OA of low volatility | 392 | 1.8 | - | 110 | 106 | POAlP |
| SOAmP | B | secondary OA of medium volatility | 392 | 1.8 | - | 1.16 | 91 | POAmP |
| SOAhP | B | secondary OA of high volatility | 392 | 1.8 | - | 0.031 | 79 | POAhP |
Appendix C. Example of a 3D Simulation by Coupling to a CTM
| OC2.5 | OM1 | |||||||
|---|---|---|---|---|---|---|---|---|
| MFE | MFB | MFE | MFB | |||||
| Montseny | 2.4 | 3.1 | 33 | 22 | 7.6 | 4.9 | 46 | −41 |
| Melpitz | 1.4 | 1.9 | 34 | 26 | 3.8 | 2.9 | 33 | −24 |
| Cabauw | – | – | – | – | 2.9 | 2.7 | 21 | 0 |
| Košetice | 2.4 | 2.6 | 30 | −5 | – | – | – | – |
| Ispra | 2.8 | 4.6 | 50 | 49 | – | – | – | – |
| Aspvreten | 2.2 | 1.6 | 40 | −36 | – | – | – | – |
| Average | 2.2 | 2.8 | 38 | 11 | 4.8 | 3.5 | 33 | −22 |
| SO4,1 | NH4,1 | NO3,1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MFE | MFB | MFE | MFB | MFE | MFB | |||||||
| Montseny | 2.5 | 1.7 | 41 | −35 | 1.1 | 0.8 | 36 | −30 | 0.6 | 0.6 | 51 | 0 |
| Melpitz | 1.6 | 1.1 | 46 | −43 | 0.7 | 0.6 | 30 | −11 | 0.8 | 0.7 | 47 | −16 |
| Cabauw | 0.9 | 1.1 | 26 | 19 | 1.0 | 1.3 | 36 | 24 | 2.9 | 3.2 | 51 | 27 |
| Average | 1.7 | 1.3 | 38 | −20 | 0.9 | 0.9 | 34 | −6 | 1.4 | 1.5 | 49 | 4 |

References
- Pope, C.; Thun, M.; Namboodiri, M.; Dockery, D.; Evans, J.; Speizer, F.; Heath, C. Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. Am. J. Respir. Crit. Care Med. 1995, 151, 669–674. [Google Scholar] [CrossRef]
- Haywood, J.; Boucher, O. Estimates of the direct and indirect radiative forcing due to tropospheric aerosols: A review. Rev. Geophys. 2000, 38, 513–543. [Google Scholar] [CrossRef]
- Meng, Z.; Dabdub, D.; Seinfeld, J.H. Size-resolved and chemically resolved model of atmospheric aerosol dynamics. J. Geophys. Res. 1998, 103, 3419–3435. [Google Scholar] [CrossRef]
- Korhonen, H.; Lehtinen, K.; Kulmala, M. Multicomponent aerosol dynamics model UHMA: Model development and validation. Atmos. Chem. Phys. 2004, 4, 757–771. [Google Scholar] [CrossRef] [Green Version]
- Bessagnet, B.; Hodzic, A.; Vautard, R.; Beekmann, M.; Cheinet, S.; Honoré, C.; Liousse, C.; Rouil, L. Aerosol modeling with CHIMERE-preliminary evaluation at the continental scale. Atmos. Environ. 2004, 38, 2803–2817. [Google Scholar] [CrossRef]
- Zhou, C.; Sun, M.; Zhao, T.; Gong, S. Mesoscale aerosol numerical system developed in NMC, China. In Proceedings of the International Symposium on Environmental Management—Air Pollution and Urban Solid Waste Management and Related Policy Issues, Kanazawa, Japan; 2004; pp. 47–52. [Google Scholar]
- Grini, A.; Korhonen, H.; Lehtinen, K.E.; Isaksen, I.S.; Kulmala, M. A combined photochemistry/aerosol dynamics model: Model development and a study of new particle formation. Boreal Environ. Res. 2005, 10, 525–541. [Google Scholar]
- Debry, É.; Fahey, K.; Sartelet, K.; Sportisse, B.; Tombette, M. Technical Note: A new SIze REsolved Aerosol Model (SIREAM). Atmos. Chem. Phys. 2007, 7, 1537–1547. [Google Scholar] [CrossRef] [Green Version]
- Zaveri, R.A.; Easter, R.C.; Fast, J.D.; Peters, L.K. Model for Simulating Aerosol Interactions and Chemistry (MOSAIC). J. Geophys. Res. 2008, 113. [Google Scholar] [CrossRef]
- Matsui, H. Development of a global aerosol model using a two-dimensional sectional method: 1. Model design. J. Adv. Model. Earth Syst. 2017, 9, 1921–1947. [Google Scholar] [CrossRef]
- Binkowski, F.S.; Roselle, S.J. Models-3 Community Multiscale Air Quality (CMAQ) model aerosol component 1. Model description. J. Geophys. Res. Atmos. 2003, 108. [Google Scholar] [CrossRef]
- Vignati, E.; Wilson, J.; Stier, P. M7: An efficient size-resolved aerosol microphysics module for large-scale aerosol transport models. J. Geophys. Res. 2004, 109. [Google Scholar] [CrossRef] [Green Version]
- Tulet, P.; Grini, A.; Griffin, R.J.; Petitcol, S. ORILAM-SOA: A computationally efficient model for predicting secondary organic aerosols in three-dimensional atmospheric models. J. Geophys. Res. 2006, 111. [Google Scholar] [CrossRef]
- Sartelet, K.; Hayami, H.; Albriet, B.; Sportisse, B. Development and preliminary validation of a modal aerosol model for tropospheric chemistry: MAM. Aerosp. Sci. Technol. 2006, 40, 118–127. [Google Scholar] [CrossRef]
- Bergström, R.; Denier van der Gon, H.; Prévôt, A.; Yttri, K.; Simpson, D. Modelling of organic aerosols over Europe (2002–2007) using a volatility basis set (VBS) framework: Application of different assumptions regarding the formation of secondary organic aerosol. Atmos. Chem. Phys. 2012, 12, 8499–8527. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Aksoyoglu, S.; El-Haddad, I.; Ciarelli, G.; DeniervanderGon, H.; Canonaco, F.; Gilardoni, S.; Paglione, M.; Minguillón, M.; Favez, O.; et al. Sources of organic aerosols in Europe: A modeling study using CAMx with modified volatility basis set scheme. Atmos. Chem. Phys. 2019, 19, 15247–15270. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Sartelet, K.N.; Seigneur, C. A size-composition resolved aerosol model for simulating the dynamics of externally mixed particles: SCRAM (v 1.0). Geosci. Model Dev. 2015, 8, 1595–1612. [Google Scholar] [CrossRef] [Green Version]
- Couvidat, F.; Sartelet, K. The Secondary Organic Aerosol Processor (SOAP v1. 0) model: A unified model with different ranges of complexity based on the molecular surrogate approach. Geosci. Model Dev. 2015, 8, 1111–1138. [Google Scholar] [CrossRef] [Green Version]
- Couvidat, F.; Debry, E.; Sartelet, K.; Seigneur, C. A hydrophilic/hydrophobic organic (H2O) aerosol model: Development, evaluation and sensitivity analysis. J. Geophys. Res. Atmos. 2012, 117. [Google Scholar] [CrossRef]
- Chrit, M.; Sartelet, K.; Sciare, J.; Pey, J.; Marchand, N.; Couvidat, F.; Sellegri, K.; Beekmann, M. Modelling organic aerosol concentrations and properties during ChArMEx summer campaigns of 2012 and 2013 in the western Mediterranean region. Atmos. Chem. Phys. 2017, 17, 12509–12531. [Google Scholar] [CrossRef] [Green Version]
- Majdi, M.; Sartelet, K.; Lanzafame, G.M.; Couvidat, F.; Kim, Y.; Chrit, M.; Turquety, S. Precursors and formation of secondary organic aerosols from wildfires in the Euro-Mediterranean region. Atmos. Chem. Phys. 2019, 19, 5543–5569. [Google Scholar] [CrossRef] [Green Version]
- Devilliers, M.; Debry, E.; Sartelet, K.; Seigneur, C. A new algorithm to solve condensation/evaporation for ultra fine, fine, and coarse particles. J. Atmos. Sci. 2013, 55, 116–136. [Google Scholar] [CrossRef]
- Sartelet, K.; Couvidat, F.; Seigneur, C.; Roustan, Y. Impact of biogenic emissions on air quality over Europe and North America. Atmos. Environ. 2012, 53, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Woody, M.; Baker, K.; Hayes, P.; Jimenez, J.; Koo, B.; Pye, H. Understanding sources of organic aerosol during CalNex-2010 using the CMAQ-VBS. Atmos. Chem. Phys. 2016, 16, 4081–4100. [Google Scholar] [CrossRef] [Green Version]
- Couvidat, F.; Bessagnet, B.; Garcia-Vivanco, M.; Real, E.; Menut, L.; Colette, A. Development of an inorganic and organic aerosol model (CHIMERE 2017β v1.0): Seasonal and spatial evaluation over Europe. Geosci. Model Dev. 2018, 11, 165–194. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Bowman, F.M. A detailed aerosol mixing state model for investigating interactions between mixing state, semivolatile partitioning, and coagulation. Atmos. Chem. Phys. 2010, 10, 4033–4046. [Google Scholar] [CrossRef] [Green Version]
- Stier, P.; Feichter, J.; Kinne, S.; Kloster, S.; Vignati, E.; Wilson, J.; Ganzeveld, L.; Tegen, I.; Werner, M.; Balkanski, Y.; et al. The aerosol-climate model ECHAM5-HAM. Atmos. Chem. Phys. 2005, 5, 1125–1156. [Google Scholar] [CrossRef] [Green Version]
- Dergaoui, H.; Sartelet, K.; Debry, É.; Seigneur, C. Modeling coagulation of externally mixed particles: Sectional approach for both size and chemical composition. J. Aerosol Sci. 2013, 58, 17–32. [Google Scholar] [CrossRef]
- Oshima, N.; Koike, M.; Zhang, Y.; Kondo, Y.; Moteki, N.; Takegawa, N.; Miyazaki, Y. Aging of black carbon in outflow from anthropogenic sources using a mixing state resolved model: Model development and evaluation. J. Geophys. Res. 2009, 114, D06210. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Sartelet, K.; Zhang, Y.; Nenes, A. Three-dimensional modelling of the mixing state of particles over Greater Paris. J. Geophys. Res. 2016, 121. [Google Scholar] [CrossRef] [Green Version]
- Yarwood, G.; Rao, S.; Yocke, M.; Whitten, G. Updates to the Carbon Bond Chemical Mechanism: CB05 Final Report to the US EPA, RT-04006752005. Available online: http://www.camx.com/publ/pdfs/CB05_Final_Report_120805.pdf (accessed on 19 May 2020).
- Goliff, W.; Stockwell, W.; Lawson, C. The Regional Atmospheric Chemistry Mechanism, version 2. Atmos. Environ. 2013, 68, 174–185. [Google Scholar] [CrossRef]
- Carter, W. Development of the SAPRC-07 chemical mechanism. Atmos. Environ. 2010, 44, 5324–5335. [Google Scholar] [CrossRef]
- Schell, B.; Ackermann, I.; Hass, H.; Binkowski, F.; Ebel, A. Modeling the formation of secondary organic aerosol within a comprehensive air quality model system. J. Geophys. Res. 2001, 106, 28275–28294. [Google Scholar] [CrossRef]
- Donahue, N.; Robinson, A.; Stanier, C.; Pandis, S. Coupled partitioning, dilution, and chemical aging of semi-volatile organics. Environ. Sci. Technol. 2006, 40, 2635–2643. [Google Scholar] [CrossRef] [PubMed]
- Odum, J.; Hoffmann, T.; Bowman, F.; Collins, D.; Flagan, R.; Seinfeld, J. Gas/particle partitioning and secondary organic aerosol yields. Environ. Sci. Technol. 1996, 30, 2580–2585. [Google Scholar] [CrossRef]
- Donahue, N.; Epstein, S.; Pandis, S.; Robinson, A. A two-dimensional volatility basis set: 1. organic-aerosol mixing thermodynamics. Atmos. Chem. Phys. 2011, 11, 3303–3318. [Google Scholar] [CrossRef] [Green Version]
- Lannuque, V.; Camredon, M.; Couvidat, F.; Hodzic, A.; Valorso, R.; Madronich, S.; Bessagnet, B.; Aumont, B. Exploration of the influence of environmental conditions on secondary organic aerosol formation and organic species properties using explicit simulations: Development of the VBS-GECKO parameterization. Atmos. Chem. Phys. 2018, 18, 13411–13428. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Couvidat, F.; Sartelet, K.; Seigneur, C. Comparison of different gas-phase mechanisms and aerosol modules for simulating particulate matter formation. J. Air Waste Manag. Assoc. 2011, 61, 1218–1226. [Google Scholar] [CrossRef] [Green Version]
- Freney, E.; Sellegri, K.; Chrit, M.; Adachi, K.; Brito, J.; Waked, A.; Borbon, A.; Colomb, A.; Dupuy, R.; Pichon, J.M.; et al. Aerosol composition and the contribution of SOA formation over Mediterranean forests. Atmos. Chem. Phys. 2018, 18, 7041–7056. [Google Scholar] [CrossRef] [Green Version]
- Chrit, M.; Sartelet, K.; Sciare, J.; Majdi, M.; Nicolas, J.; Petit, J.E.; Dulac, F. Modeling organic aerosol concentrations and properties during winter 2014 in the northwestern Mediterranean region. Atmos. Chem. Phys. 2018, 18, 18079–18100. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Sartelet, K.; Couvidat, F. Modeling the effect of non-ideality, dynamic mass transfer and viscosity on SOA formation in a 3-D air quality model. Atmos. Chem. Phys. 2019, 19, 1241–1261. [Google Scholar] [CrossRef] [Green Version]
- Couvidat, F.; Seigneur, C. Modeling secondary organic aerosol formation from isoprene oxidation under dry and humid conditions. Atmos. Chem. Phys. 2011, 11, 893–909. [Google Scholar] [CrossRef] [Green Version]
- Pun, B.K.; Seigneur, C. Investigative modeling of new pathways for secondary organic aerosol formation. Atmos. Chem. Phys. 2007, 7, 2199–2216. [Google Scholar] [CrossRef] [Green Version]
- Verwer, J.; Spee, E.; Blom, J.; Hundsdorfer, W. A Second-Order Rosenbrock Method Applied to Photochemical Dispersion Problems. SIAM J. Sci. Comput. 1999, 20, 1456–1480. [Google Scholar] [CrossRef] [Green Version]
- Ascher, U.; Petzold, L. Computer Methods for Ordinary Differential Equations and Differential-Algebraic Equations; Society for Industrial and Applied Mathematics: Philadelphia, PA, USA, 1998; ISBN 978-0-89871-412-8. [Google Scholar]
- Nenes, A.; Pandis, S.; Pilinis, C. ISORROPIA: A new thermodynamic equilibrium model for multiphase multicomponent inorganic aerosols. Aquat. Geochem. 1998, 4, 123–152. [Google Scholar] [CrossRef]
- Blom, J.; Verwer, J. A comparison of integration methods for atmospheric transport-chemistry problems. J. Comput. Appl. Math. 2000, 126, 381–396. [Google Scholar] [CrossRef] [Green Version]
- Seigneur, C.; Hudischewskyj, A.B.; Seinfeld, J.H.; Whitby, K.T.; Whitby, E.R.; Brock, J.R.; Barnes, H.M. Simulation of aerosol dynamics: A comparative review of mathematical models. Aerosp. Sci. Technol. 1986, 5, 205–222. [Google Scholar] [CrossRef]
- Zhang, Y.; Seigneur, C.; Seinfeld, J.H.; Jacobson, M.Z.; Binkowski, F.S. Simulation of aerosol dynamics: A comparative review of algorithms used in air quality models. Aerosp. Sci. Technol. 1999, 31, 487–514. [Google Scholar] [CrossRef]
- Allen, A.; Harrison, R.; Erisman, J.W. Field measurements of the dissociation of ammonium nitrate and ammonium chloride aerosols. Atmos. Environ. 1989, 23, 1591–1599. [Google Scholar] [CrossRef]
- Wexler, A.; Seinfeld, J. The distribution of ammonium salts among a size and composition dispersed aerosol. Atmos. Environ. 1990, 24A, 1231–1246. [Google Scholar] [CrossRef]
- Pilinis, C.; Capaldo, K.; Nenes, A.; Pandis, S.N. MADM—A New Multicomponent Aerosol Dynamics Model. Aerosp. Sci. Technol. 2000, 32, 482–502. [Google Scholar] [CrossRef]
- Kittelson, D.; Watts, W.; Johnson, J. On-road and laboratory evaluation of combustion aerosols—Part 1: Summary of diesel engine results. J. Atmos. Sci. 2006, 37, 913–930. [Google Scholar] [CrossRef]
- Shiraiwa, M.; Seinfeld, J. Equilibration timescale of atmospheric secondary organic aerosol partitioning. Geophys. Res. Lett. 2012, 39. [Google Scholar] [CrossRef] [Green Version]
- Abramson, E.; Imre, D.; Beránek, J.; Wilson, J.; Zelenyu, A. Experimental determination of chemical diffusion within secondary organic aerosol particles. Phys. Chem. Chem. Phys. 2013, 15, 2983–2991. [Google Scholar] [CrossRef] [PubMed]
- Hosny, N.; Fitzgerald, C.; Vyšniauska, A.; Athanasiadi, A.; Berkemeie, T.; Uygu, N.; Pösch, U.; Shiraiwa, M.; Kalberer, M.; Pope, F.; et al. Direct imaging of changes in aerosol particle viscosity upon hydration and chemical aging. Chem. Sci. 2016, 7, 1357–1367. [Google Scholar] [CrossRef] [Green Version]
- Platt, S.M.; Haddad, I.E.; Zardini, A.; Clairotte, M.; Astorga, C.; Wolf, R.; Slowik, J.; Temime-Roussel, B.; Marchand, N.; Ježek, I.; et al. Secondary organic aerosol formation from gasoline vehicle emissions in a new mobile environmental reaction chamber. Atmos. Chem. Phys. 2013, 13, 9141–9158. [Google Scholar] [CrossRef] [Green Version]
- Sartelet, K.; Zhu, S.; Moukhtar, S.; André, M.; André, J.; Gros, V.; Favez, O.; Brasseur, A.; Redaelli, M. Emission of intermediate, semi and low volatile organic compounds from traffic and their impact on secondary organic aerosol concentrations over Greater Paris. Atmos. Environ. 2018, 180, 126–137. [Google Scholar] [CrossRef]
- Zhao, Y.; Nguyen, N.T.; Presto, A.A.; Hennigan, C.J.; May, A.A.; Robinson, A.L. Intermediate volatility organic compound emissions from on-road gasoline vehicles and small off-road gasoline engines. Environ. Sci. Technol. 2016, 50, 4554–4563. [Google Scholar] [CrossRef]
- Theloke, J.; Friedrich, R. Compilation of a database on the composition of anthropogenic VOC emissions for atmospheric modeling in Europe. Atmos. Environ. 2007, 41, 4148–4160. [Google Scholar] [CrossRef]
- Suarez-Bertoa, R.; Mendoza-Villafuerte, P.; Riccobono, F.; Vojtisek, M.; Pechout, M.; Perujo, A.; Astorga, C. On-road measurement of NH3 emissions from gasoline and diesel passenger cars during real world driving conditions. Atmos. Environ. 2017, 166, 488–497. [Google Scholar] [CrossRef]
- Fredenslund, A.; Jones, R.; Prausnitz, J. Group-contribution estimation of activity coefficients in nonideal liquid mixtures. AIChE J. 1975, 21, 1086–1099. [Google Scholar] [CrossRef]
- Zuend, A.; Marcolli, C.; Luo, B.; Peter, T. A thermodynamic model of mixed organic-inorganic aerosols to predict activity coefficients. Atmos. Chem. Phys. 2008, 8, 4559–4593. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; McMurry, P.; Yu, F.; Jacobson, M. A comparative study of nucleation parameterizations: 1. Examination and evaluation of the formulations. J. Geophys. Res. 2010, 115. [Google Scholar] [CrossRef] [Green Version]
- Vehkamaki, H.; Kulmala, M.; Napari, I.; Lehtinen, K.; Timmreck, C.; Noppel, M.; Laaksonen, A. An improved parameterization for sulfuric acid-water nucleation rates for tropospheric and stratospheric conditions. J. Geophys. Res. 2002, 107, 4622. [Google Scholar] [CrossRef]
- Napari, I.; Noppel, M.; Vehkamaki, H.; Kulmala, M. Parametrization of ternary nucleation rates for H2SO4-NH3-H2O vapors. J. Geophys. Res. 2002, 107. [Google Scholar] [CrossRef]
- Merikanto, J.; Napari, I.; Vehkamäki, H.; Anttila, T.; Kulmala, M. New parameterization of sulfuric acid-ammonia-water ternary nucleation rates at tropospheric conditions. J. Geophys. Res. 2007, 112. [Google Scholar] [CrossRef] [Green Version]
- Merikanto, J.; Napari, I.; Vehkamäki, H.; Anttila, T.; Kulmala, M. Correction to “New parameterization of sulfuric acid-ammonia-water ternary nucleation rates at tropospheric conditions”. J. Geophys. Res. 2009, 114. [Google Scholar] [CrossRef] [Green Version]
- Patoulias, D.; Fountoukis, C.; Riipinen, I.; Asmi, A.; Kulmala, M.; Pandis, S. Simulation of the size-composition distribution of atmospheric nanoparticles over Europe. Atmos. Chem. Phys. 2018, 18, 13639–13654. [Google Scholar] [CrossRef] [Green Version]
- Mallet, V.; Quélo, D.; Sportisse, B.; Ahmed de Biasi, M.; Debry, É.; Korsakissok, I.; Wu, L.; Roustan, Y.; Sartelet, K.; Tombette, M.; et al. Technical Note: The air quality modeling system Polyphemus. Atmos. Chem. Phys. 2007, 7, 5479–5487. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Sartelet, K.; Healy, R.; Wenger, J. Simulation of particle diversity and mixing state over Greater Paris: A model-measurement inter-comparison. Faraday Discuss. 2016, 189, 547–566. [Google Scholar] [CrossRef]
- Couvidat, F.; Kim, Y.; Sartelet, K.; Seigneur, C.; Marchand, N.; Sciare, J. Modeling secondary organic aerosol in an urban area: Application to Paris. Atmos. Chem. Phys. 2013, 13, 983–996. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, C.; Afif, C.; El Masri, N.; Öztürk, F.; Keleç, M.; Sartelet, K. A first annual assessment of air quality modeling over Lebanon using WRF/Polyphemus. Atmos. Pollut. Res. 2018, 9, 643–654. [Google Scholar] [CrossRef]
- Chrit, M.; Sartelet, K.; Sciare, J.; Pey, J.; Nicolas, J.; Marchand, N.; Freney, E.; Sellegri, K.; Beekmann, M.; Dulac, F. Aerosol sources in the western Mediterranean during summertime: A model-based approach. Atmos. Chem. Phys. 2018, 18, 9631–9659. [Google Scholar] [CrossRef] [Green Version]
- Majdi, M.; Turquety, S.; Sartelet, K.; Legorgeu, C.; Menut, L.; Kim, Y. Impact of wildfires on particulate matter in the Euro-Mediterranean in 2007: Sensitivity to some parameterizations of emissions in air quality models. Atmos. Chem. Phys. 2019, 19, 785–812. [Google Scholar] [CrossRef] [Green Version]
- Cholakian, A.; Beekmann, M.; Colette, A.; Coll, I.; Siour, G.; Sciare, J.; Marchand, N.; Couvidat, F.; Pey, J.; Gros, V.; et al. Simulation of fine organic aerosols in the western Mediterranean area during the ChArMEx 2013 summer campaign. Atmos. Chem. Phys. 2018, 18, 7287–7312. [Google Scholar] [CrossRef] [Green Version]
- Lannuque, V.; Couvidat, F.; Camredon, M.; Aumont, B.; Bessagnet, B. Modeling organic aerosol over Europe in summer conditions with the VBS-GECKO parameterization: Sensitivity to secondary organic compound properties and IVOC (intermediate-volatility organic compound) emissions. Atmos. Chem. Phys. 2020, 20, 4905–4931. [Google Scholar] [CrossRef]
- Torseth, K.; Aas, W.; Breivik, K.; Faeraa, A.; Fiebig, M.; Hjellbrekke, A.; LundMyhre, C.; Solberg, S.; Yttri, K. Introduction to the European Monitoring and Evaluation Programme (EMEP) and observed atmospheric composition change during 1972–2009. Atmos. Chem. Phys. 2012, 12, 5447–5481. [Google Scholar] [CrossRef] [Green Version]
- Boylan, J.; Russell, A. PM and light extinction model performance metrics, goals, and criteria for three-dimensional air quality models. Atmos. Environ. 2006, 40, 4946–4959. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sartelet, K.; Couvidat, F.; Wang, Z.; Flageul, C.; Kim, Y. SSH-Aerosol v1.1: A Modular Box Model to Simulate the Evolution of Primary and Secondary Aerosols. Atmosphere 2020, 11, 525. https://doi.org/10.3390/atmos11050525
Sartelet K, Couvidat F, Wang Z, Flageul C, Kim Y. SSH-Aerosol v1.1: A Modular Box Model to Simulate the Evolution of Primary and Secondary Aerosols. Atmosphere. 2020; 11(5):525. https://doi.org/10.3390/atmos11050525
Chicago/Turabian StyleSartelet, Karine, Florian Couvidat, Zhizhao Wang, Cédric Flageul, and Youngseob Kim. 2020. "SSH-Aerosol v1.1: A Modular Box Model to Simulate the Evolution of Primary and Secondary Aerosols" Atmosphere 11, no. 5: 525. https://doi.org/10.3390/atmos11050525





