Chromosome Evolution in the Free-Living Flatworms: First Evidence of Intrachromosomal Rearrangements in Karyotype Evolution of Macrostomum lignano (Platyhelminthes, Macrostomida)
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Samples of M. lignano
2.2. Chromosome Slide Preparation
2.3. Chromosome-Specific DNA Probes
2.4. Generation of Region-Specific Microdissected DNA Probes
2.5. Generation of Labeled Unique DNA Fragments for Fluorescent in situ Hybridization Detection of Homologous Sites in the Chromosome of M. lignano
2.6. Generation of 5S and 28S rDNA Probes
2.7. Fluorescence In Situ Hybridization
2.8. Microscopic Analysis
3. Results
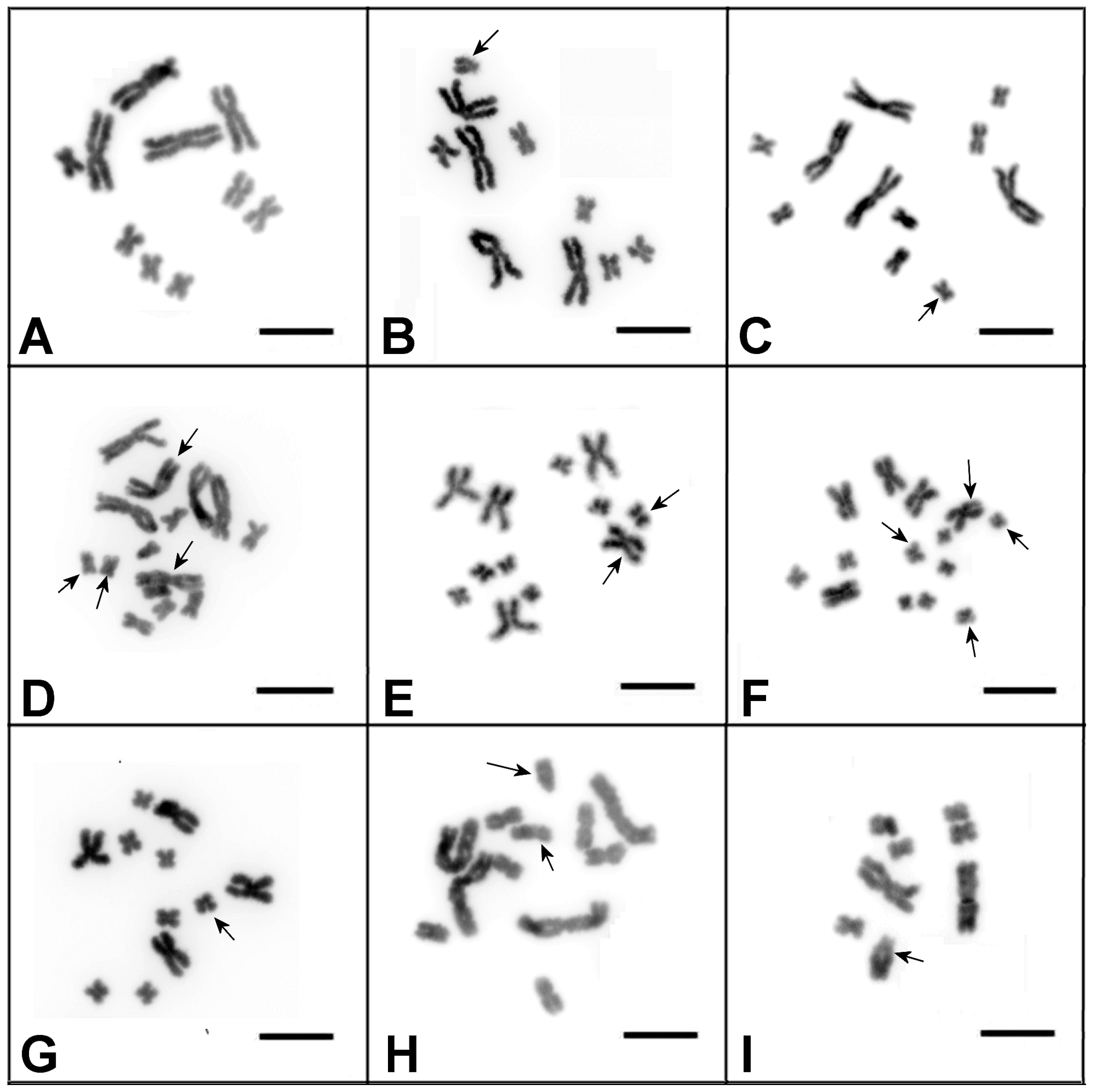
3.1. Characterization of the DV1/10 Subline before the Microdissected DNA Probes’ Generation and Fluorescent in situ Hybridization Experiments
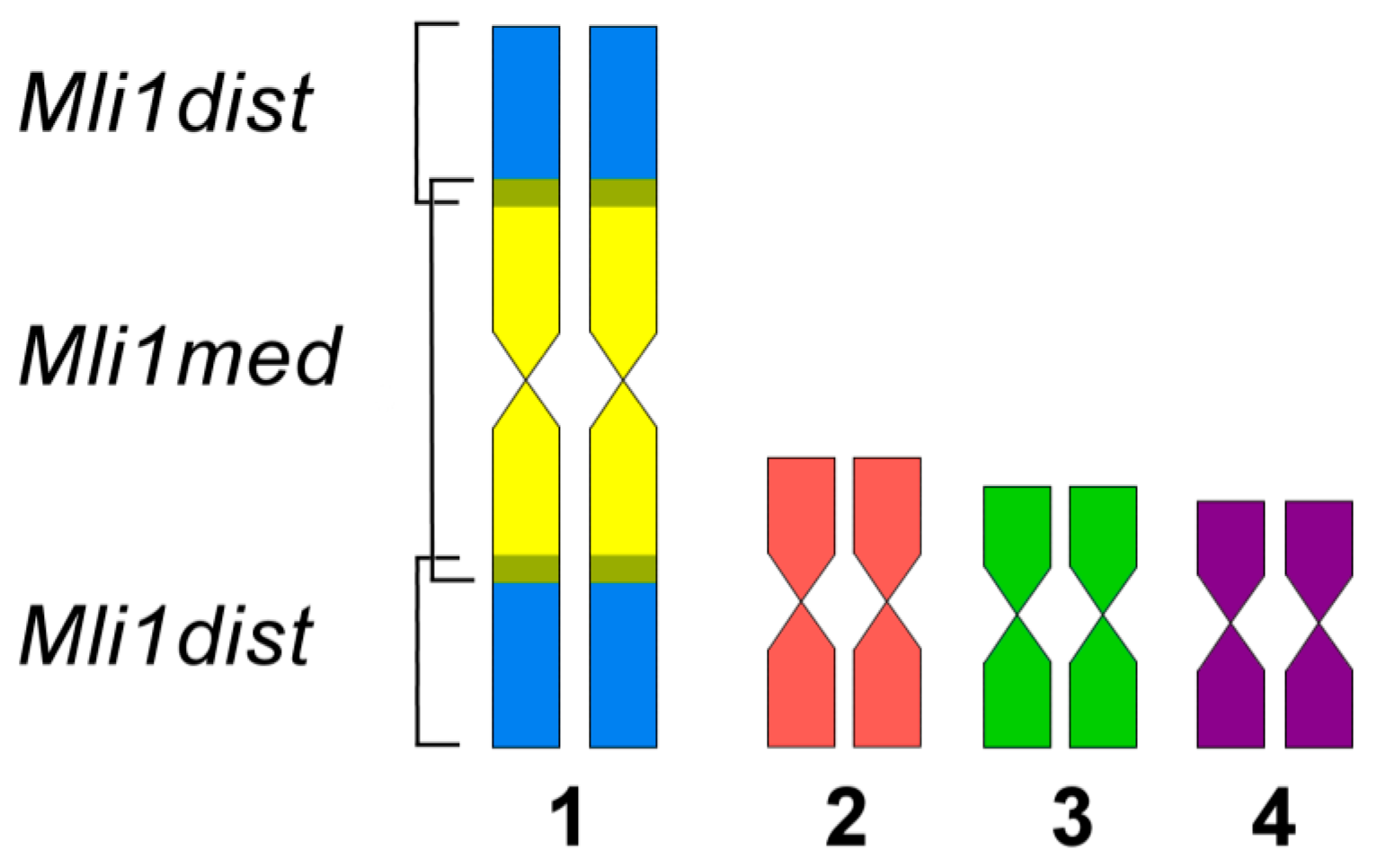
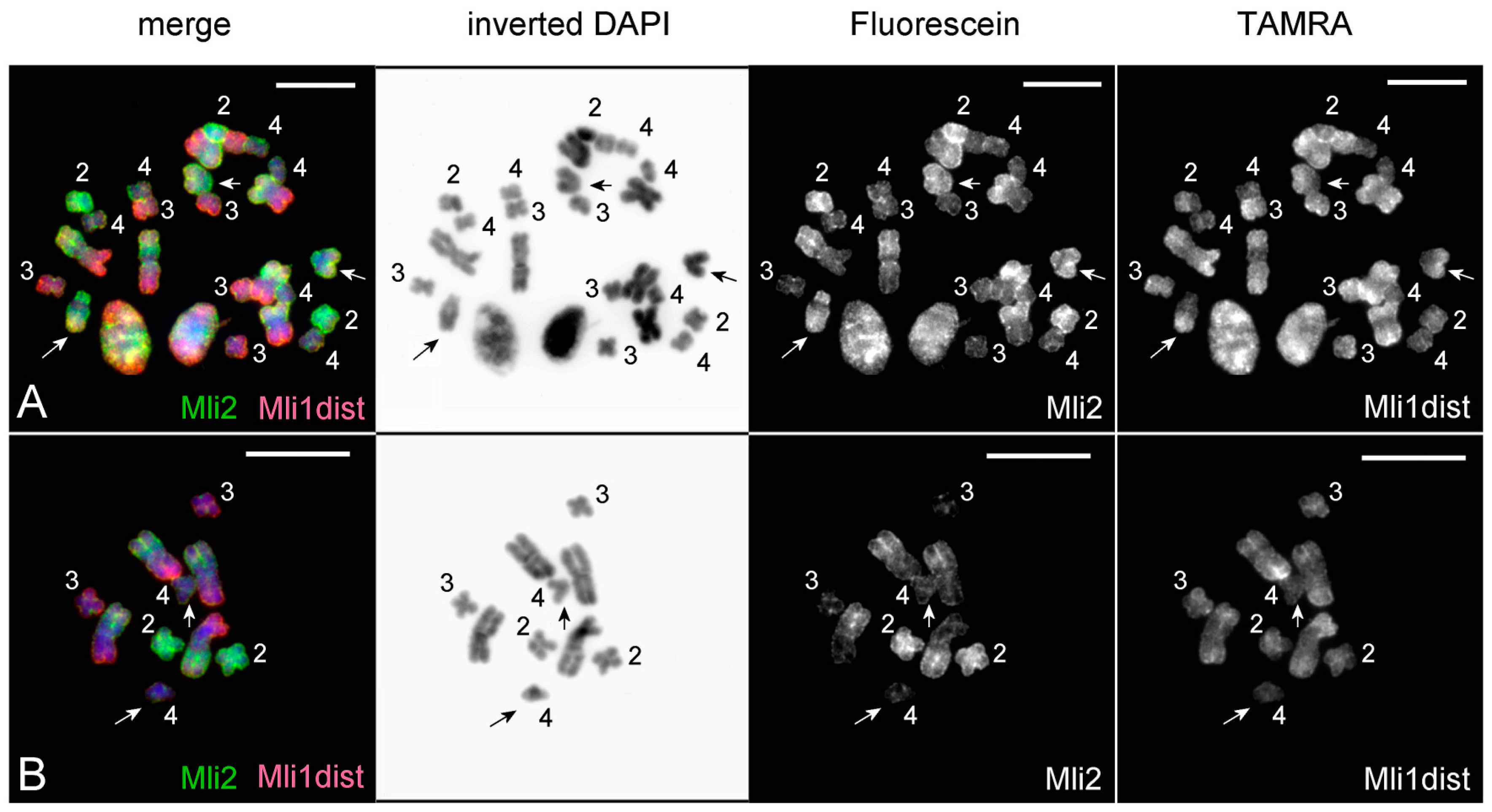
3.2. Generation of Region-Specific Microdissected DNA Probes and Chromosome Painting
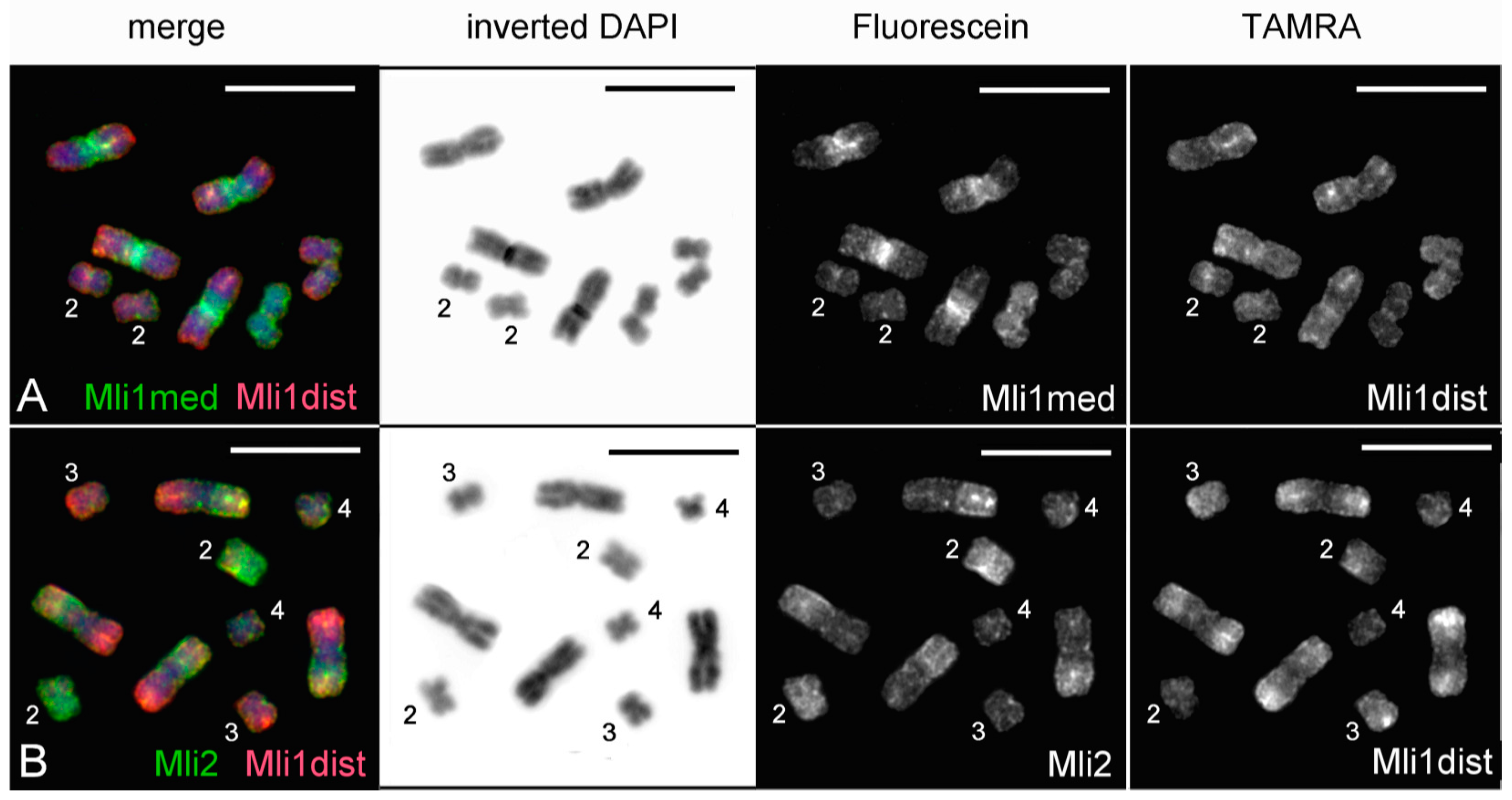
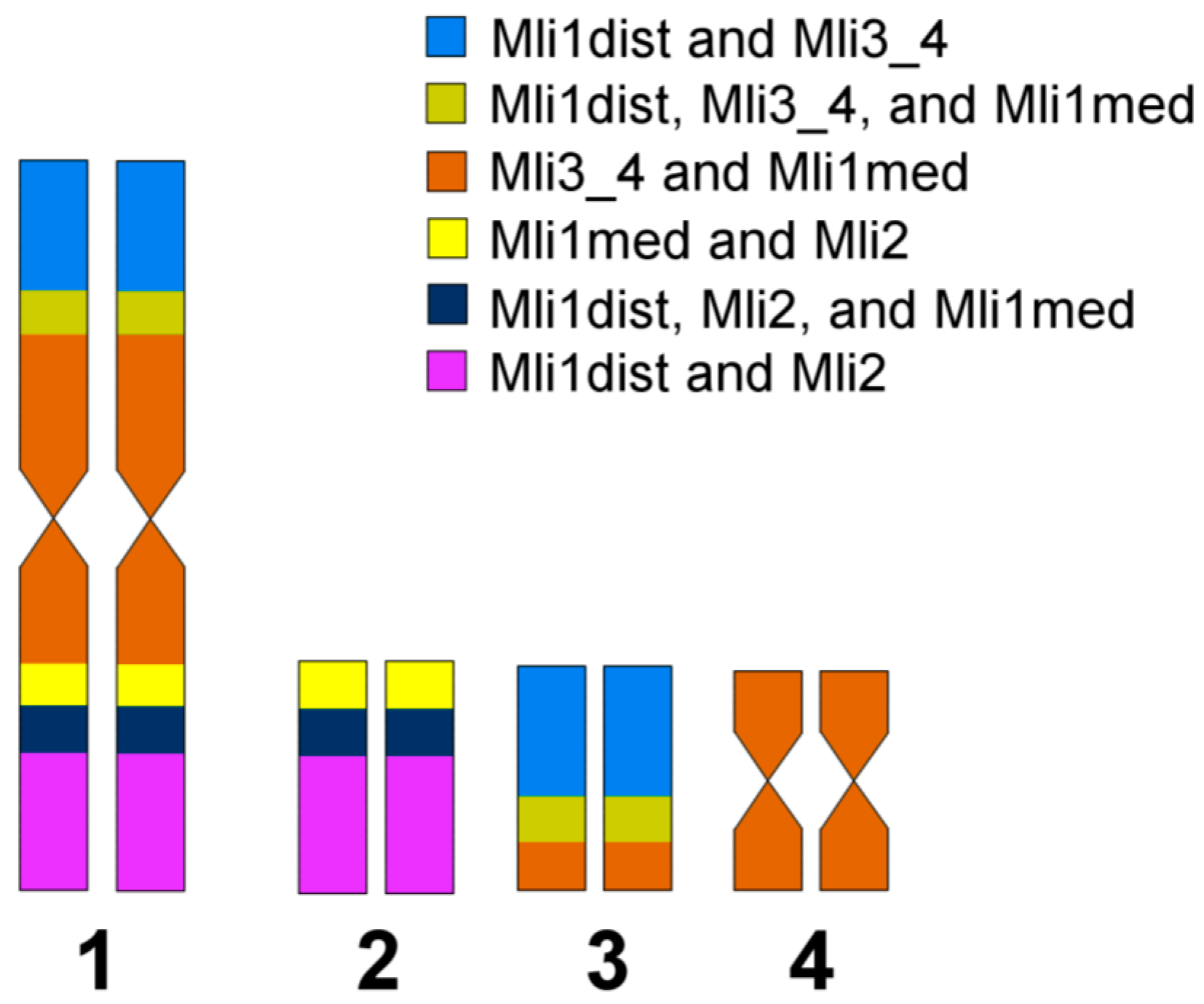
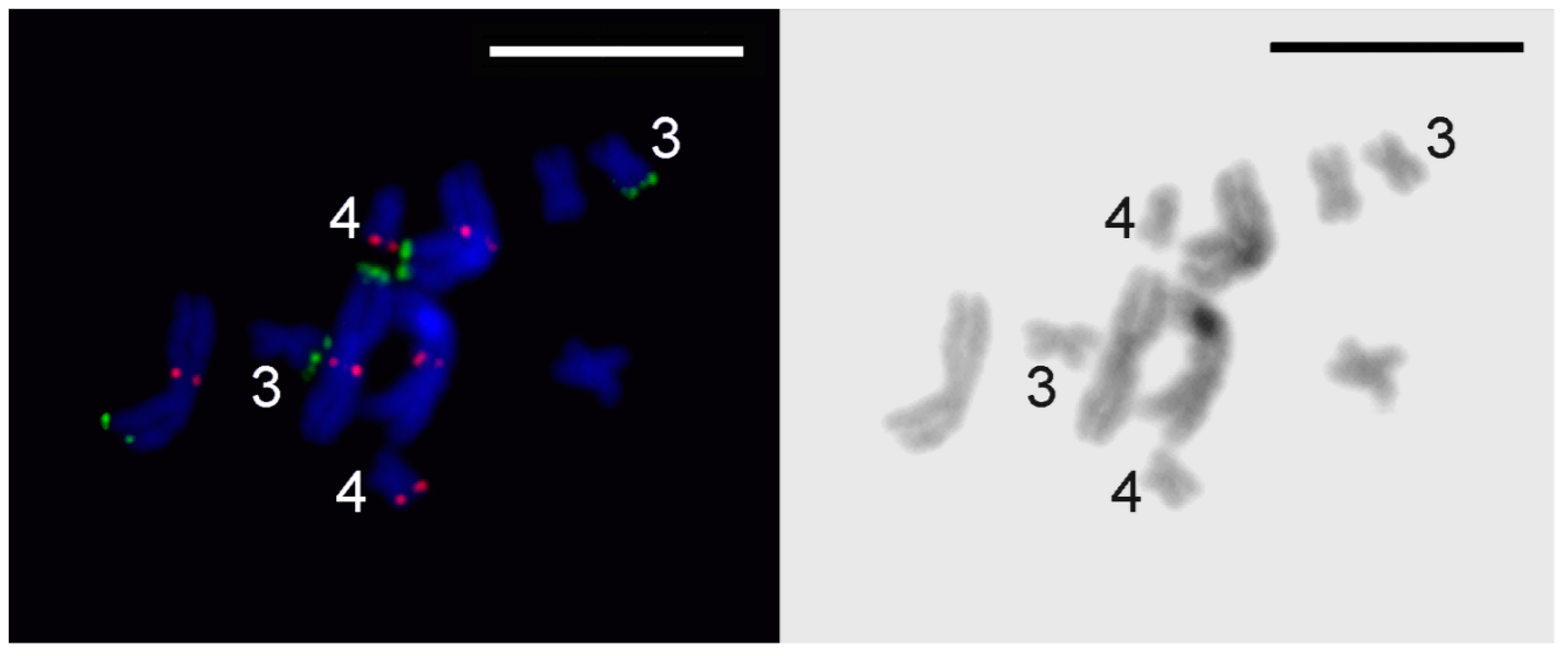
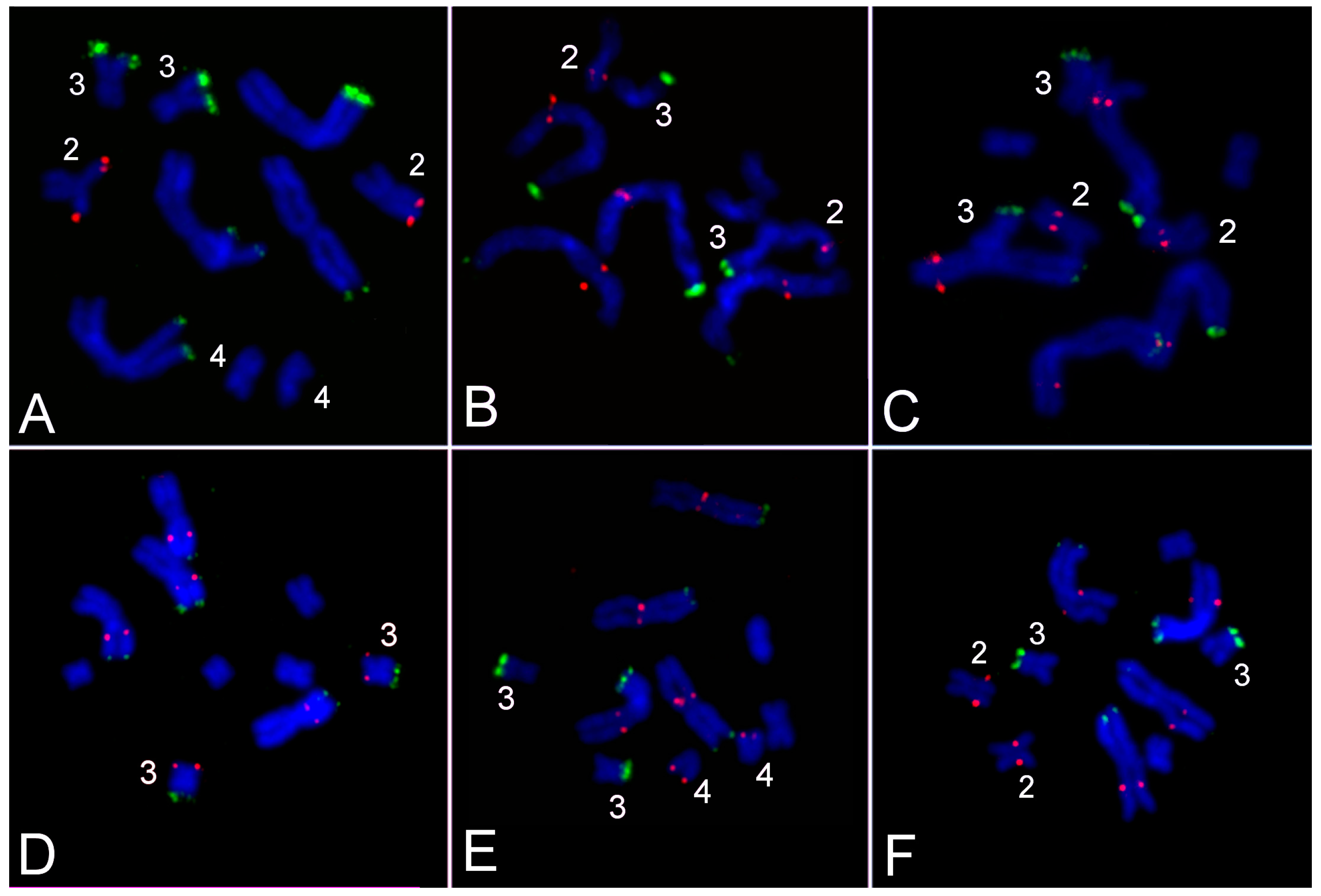
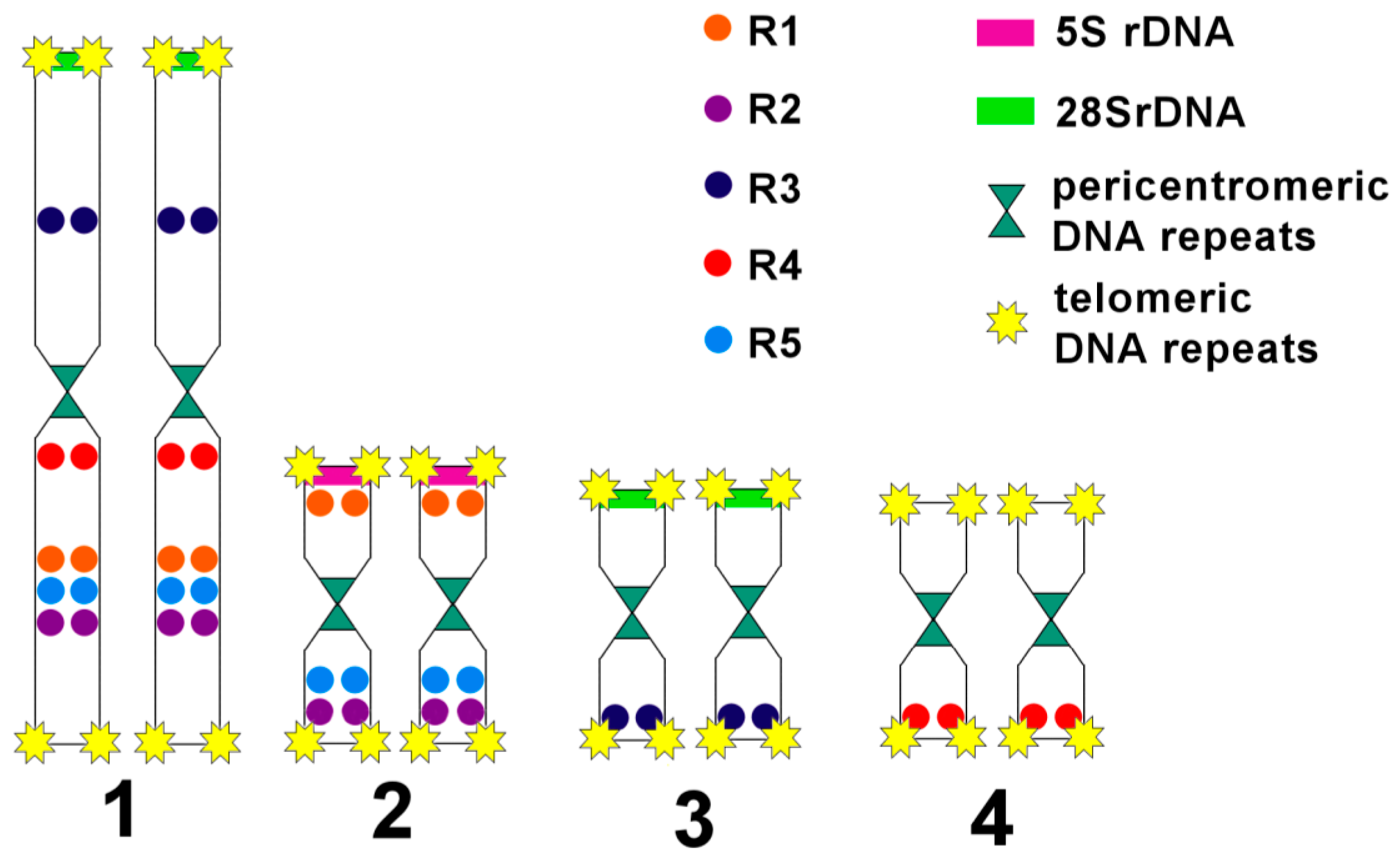
3.3. Comparative Analysis of Small Metacentrics and Homologous Regions in MLI1 with Fluorescent In Situ Hybridization of Unique Labeled DNA Fragments and rDNA Repeats
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zadesenets, K.S.; Schärer, L.; Rubtsov, N.B. New insights into the karyotype evolution of the free-living flatworm Macrostomum lignano (Platyhelminthes, Turbellaria). Sci. Rep. 2017, 7, 6066. [Google Scholar] [CrossRef] [PubMed]
- Zadesenets, K.S.; Vizoso, D.B.; Schlatter, A.; Konopatskaia, I.D.; Berezikov, E.; Schärer, L.; Rubtsov, N.B. Evidence for karyotype polymorphism in the free-living flatworm, Macrostomum lignano, a model organism for evolutionary and developmental biology. PLoS ONE 2016, 11, e0164915. [Google Scholar] [CrossRef] [PubMed]
- Hellsten, U.; Khokha, M.K.; Grammer, T.C.; Harland, R.M.; Richardson, P.; Rokhsar, D.S. Accelerated gene evolution and subfunctionalization in the pseudotetraploid frog Xenopus laevis. BMC Biol. 2007, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-T.; Hou, G.-Y.; Kong, X.-F.; Li, C.-Y.; Zeng, J.-M.; Li, H.-D.; Xiao, G.-B.; Li, X.-M.; Sun, X.-W. The fate of recent duplicated genes following a fourth-round whole genome duplication in a tetraploid fish, common carp (Cyprinus carpio). Sci. Rep. 2015, 5, 8199. [Google Scholar] [CrossRef] [PubMed]
- Wasik, K.; Gurtowski, J.; Zhou, X.; Ramos, O.M.; Delás, M.J.; Battistoni, G.; EI Demerdash, O.; Falciatori, I.; Vizoso, D.B.; Smith, A.D.; et al. Genome and transcriptome of the regeneration-complement flatworm, Macrostomum lignano. Proc. Natl. Acad. Sci. USA 2015, 112, 12462–12467. [Google Scholar] [CrossRef] [PubMed]
- Grudniewska, M.; Mouton, S.; Simanov, D.; Beltman, F.; Grelling, M.; de Mulder, K.; Arindrarto, W.; Weissert, P.M.; van der Elst, S.; Berezikov, E. Transcriptional signatures of somatic neoblasts and germline cells in Macrostomum lignano. eLife 2016, 5, e20607. [Google Scholar] [CrossRef] [PubMed]
- Wudarski, J.; Simanov, D.; Ustyantsev, K.; de Mulder, K.; Grelling, M.; Grudniewska, M.; Beltman, F.; Glazenburg, L.; Demircan, T.; Wunderer, J.; et al. A platform for efficient transgenesis in Macrostomum lignano, a flatworm model organism for stem cell research. bioRxiv 2017, 151654. [Google Scholar] [CrossRef]
- Rieger, R.; Gehlen, M.; Hazprunar, G.; Homlund, M.; Legniti, A.; Salvenmoser, W.; Tyler, S. Laboratory cultures of marine Macrostomida (Turbellaria). Forts Zool. 1988, 36, 525. [Google Scholar]
- Ladurner, P.; Schärer, L.; Salvenmoser, W.; Rieger, R.M. A new model organism among the lower Bilateria and the use of digital microscopy in taxonomy of meiobenthic Platyhelminthes: Macrostomum lignano, n.sp (Rhabditophora, Macrostomorpha). J. Zool. Syst. Evol. Res. 2005, 43, 114–126. [Google Scholar] [CrossRef]
- Egger, B.; Ishida, S. Chromosome fission or duplication in Macrostomum lignano (Macrostomorpha, Plathelminthes)—Remarks on chromosome numbers in ‘archoophoran turbellarians’. J. Zool. Syst. Evol. Res. 2005, 43, 127–132. [Google Scholar] [CrossRef]
- Janicke, T.; Marie-Orleach, L.; De Mulder, K.; Berezikov, E.; Ladurner, P.; Vizoso, D.B.; Schärer, L. Sex allocation adjustment to local sperm competition in a simultaneous hermaphrodite. Evolution 2013, 67, 3233–3242. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3 - new capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed]
- Schärer, L.; Littlewood, D.T.J.; Waeschenbach, A.; Yoshida, W.; Vizoso, D.B. Mating behavior and the evolution of sperm design. Proc. Natl. Acad. Sci. USA 2011, 108, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Zadesenets, K.S.; Katokhin, A.V.; Mordvinov, V.A.; Rubtsov, N.B. Telomeric DNA in chromosomes of five opisthorchid species. Parasitol. Int. 2012, 61, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S. Evolution by Gene Duplication; Springer-Verlag: Berlin, Germany, 1970; ISBN 978-3-642-86661-6. [Google Scholar]
- Kellis, M.; Birren, B.W.; Lander, E.S. Proof and evolutionary analysis of ancient genome duplication in the yeast Saccharomyces cerevisiae. Nature 2004, 428, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.P. The evolutionary consequences of polyploidy. Cell 2007, 131, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Lenormand, T.; Engelstädter, J.; Johnston, S.E.; Wijnker, E.; Haag, C.R. Evolutionary mysteries in meiosis. Philos. Trans. Roy. Soc. B 2016, 371. [Google Scholar] [CrossRef] [PubMed]
- Bomblies, K.; Jones, G.; Franklin, C.; Zickler, D.; Kleckner, N. The challenge of evolving stable polyploidy: Could an increase in “crossover interference distance” play a central role? Chromosoma 2016, 125, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, H.; Levy, A.A.; Feldman, M. Allopolyploidy—Induced rapid genome evolution in the wheat (Aegilops-Triticum) group. Plant Cell 2001, 13, 1735–1748. [Google Scholar] [CrossRef] [PubMed]
- Jenczewski, E.; Alix, K. From diploids to allopolyploids: The emergence of efficient pairing control genes in plants. Crit. Rev. Plant Sci. 2004, 23, 21–45. [Google Scholar] [CrossRef]
- Comai, L. The advantages and disadvantages of being polyploid. Nat. Rev. Genet. 2005, 6, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Semon, M.; Wolfe, K.H. Rearrangement rate following the whole-genome duplication in teleosts. Mol. Biol. Evol. 2007, 24, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Hufton, A.L.; Groth, D.; Vingron, M.; Lehrach, H.; Poustka, A.J.; Panopoulou, G. Early vertebrate whole genome duplications were predated by a period of intense genome rearrangement. Genome Res. 2008, 18, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Inoue, J.; Sato, Y.; Sinclair, R.; Tsukamoto, K.; Nishida, M. Rapid genome reshaping by multiple-gene loss after whole-genome duplication in teleost fish suggested by mathematical modeling. Proc. Natl. Acad. Sci. USA 2015, 112, 14918–14923. [Google Scholar] [CrossRef] [PubMed]
- Selmecki, A.M.; Maruvka, Y.E.; Richmond, P.A.; Guillet, M.; Shoresh, N.; Sorenson, A.L.; De, S.; Kishony, R.; Michor, F.; Dowell, R.; et al. Polyploidy can drive rapid adaptation in yeast. Nature 2015, 519, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Albalat, R.; Canestro, C. Evolution by gene loss. Nat. Rev. Genet. 2016, 17, 179–391. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.; Conery, J.S. The evolutionary fate and consequences of duplicate genes. Science 2000, 290, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.H. Yesterday’s polyploids and the mystery of diploidization. Nat. Rev. Genet. 2001, 2, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Larkin, D.M.; Pape, G.; Donthu, R.; Auvil, L.; Welge, M.; Lewin, H.A. Breakpoint regions and homologous synteny blocks in chromosomes have different evolutionary histories. Genome Res. 2009, 19, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Rubtsov, N.B.; Rubtsova, N.V.; Anopriyenko, O.V.; Karamysheva, T.V.; Shevchenko, A.I.; Mazurok, N.A.; Nesterova, T.B.; Zakian, S.M. Reorganization of the X chromosome in voles of the genus Microtus. Cytogenet. Genome Res. 2002, 99, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Martín-Durán, J.M.; Ryan, J.F.; Vellutini, B.C.; Pang, K.; Hejnol, A. Increased taxon sampling reveals thousands of hidden orthologs in flatworms. Genome Res. 2017, 27, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, S.; Luo, Y.; Xiao, L.; Luo, X.; Gao, S.; Dou, Y.; Zhang, H.; Guo, A.; Meng, Q.; et al. Comparative genomics reveals adaptive evolution of Asian tapeworm in switching to a new intermediate host. Nat. Commun. 2016, 7, 12845. [Google Scholar] [CrossRef] [PubMed]
- Benazzi, M.; Benazzi-Lentati, G. Animal Cytogenetics; Gebrüder Borntraeger: Berlin, Germany, 1976; ISBN 9783443260118. [Google Scholar]
- Garcia-Fernández, J.; Bayascas-Ramírez, J.R.; Marfany, G.; Muñoz-Mármol, A.M.; Casali, A.; Baguñà, J.; Saló, E. High copy number of highly similar mariner-like transposons in planarian (Platyhelminthe): Evidence for a trans-phyla horizontal transfer. Mol. Biol. Evol. 1995, 12, 421–431. [Google Scholar] [PubMed]
- Sperb, F.; Schuck, D.C.; Rodrigues, J.J. Occurrence and abundance of a mariner-like element in freshwater and terrestrial planarians (Platyhelminthes, Tricladida) from southern Brazil. Genet. Mol. Biol. 2009, 32, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K. Evolutionary genomics: Yeasts accelerate beyond BLAST. Curr. Biol. 2004, 14, R392–R394. [Google Scholar] [CrossRef] [PubMed]
| Unique DNA Fragment | Primer Sequence | PCR Product Length | |
|---|---|---|---|
| R1 | F | CGGTACTCCTTCCAGGACATTG | 20,124 bp |
| R | CGCAACAGTGACGCTAACTATC | ||
| R2 | F | CACGTAGACGGTCATACGAGTT | 20,232 bp |
| R | CCGCTAATCCACGTCATGACTA | ||
| R3 | F | CTGACTGGGTGCGAATCCAT | 20,254 bp |
| R | AAACCTATGTCCTAGCTGTGCG | ||
| R4 | F | GTCCTGGAACTGTCTGTACACC | 20,200 bp |
| R | GTTTCGGGCAAACGATAGCTAC | ||
| R5 | F | AAAAATCTGCCGCCATTTGTGA | 20,278 bp |
| R | ATGAATGTTGACTCGGAAGGCT |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zadesenets, K.S.; Ershov, N.I.; Berezikov, E.; Rubtsov, N.B. Chromosome Evolution in the Free-Living Flatworms: First Evidence of Intrachromosomal Rearrangements in Karyotype Evolution of Macrostomum lignano (Platyhelminthes, Macrostomida). Genes 2017, 8, 298. https://doi.org/10.3390/genes8110298
Zadesenets KS, Ershov NI, Berezikov E, Rubtsov NB. Chromosome Evolution in the Free-Living Flatworms: First Evidence of Intrachromosomal Rearrangements in Karyotype Evolution of Macrostomum lignano (Platyhelminthes, Macrostomida). Genes. 2017; 8(11):298. https://doi.org/10.3390/genes8110298
Chicago/Turabian StyleZadesenets, Kira S., Nikita I. Ershov, Eugene Berezikov, and Nikolay B. Rubtsov. 2017. "Chromosome Evolution in the Free-Living Flatworms: First Evidence of Intrachromosomal Rearrangements in Karyotype Evolution of Macrostomum lignano (Platyhelminthes, Macrostomida)" Genes 8, no. 11: 298. https://doi.org/10.3390/genes8110298




