The Effects of Divalent Cation-Chelated Prion Fibrils on the Immune Response of EOC 13.31 Microglia Cells
Abstract
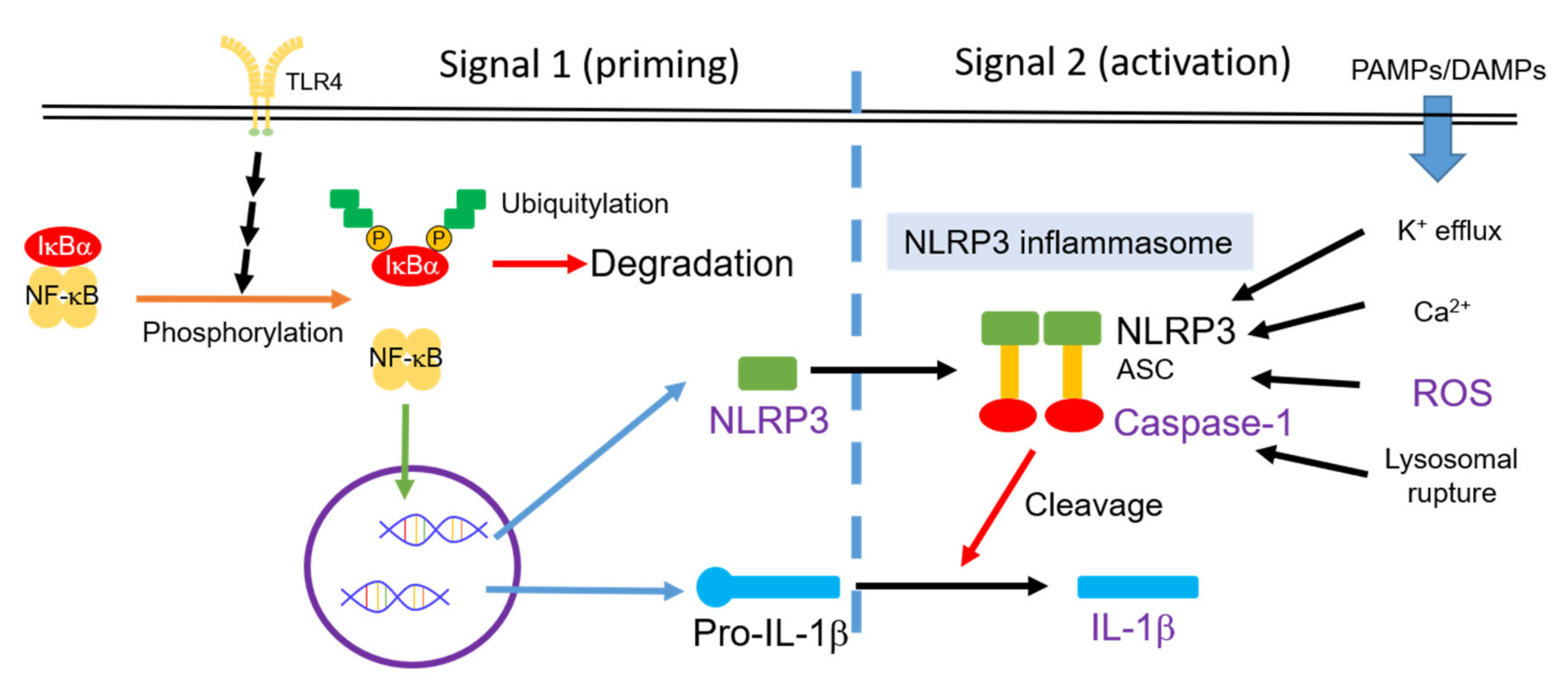
:1. Introduction
2. Materials and Methods
2.1. Prion Purification
2.2. Fibril Conversion
2.3. Cell Culture
2.4. Detection of Cell Viability and Cellular Reactive Oxygen Species
2.5. Western Blot
2.6. Extraction of mRNA
2.7. Quantitative PCR
2.8. Statistical Analysis
3. Results
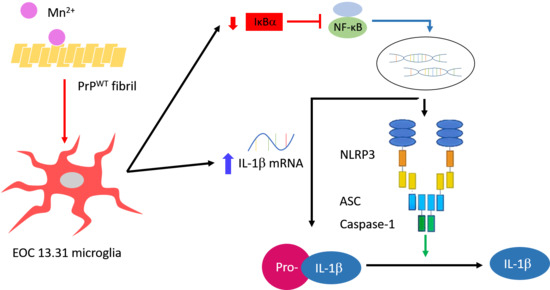
3.1. The Effect of Divalent Cations and Fprpwt on the Production of Cellular Ros in Eoc 13.31 Microglia Cells
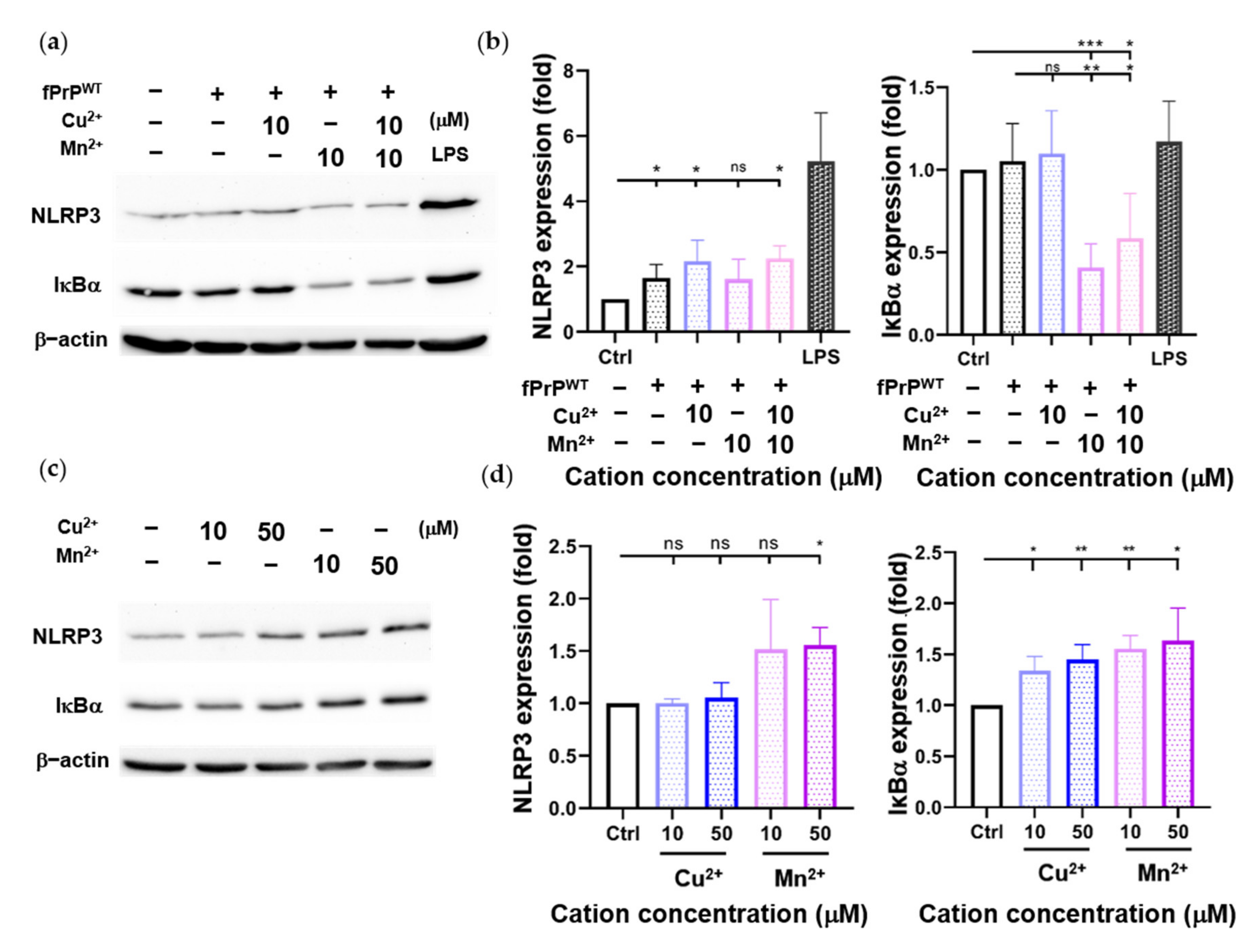
3.2. The Effect of Divalent Cations on the Expression of nlrp3 and IκBα in Fprpwt-Treated Microglia Cells
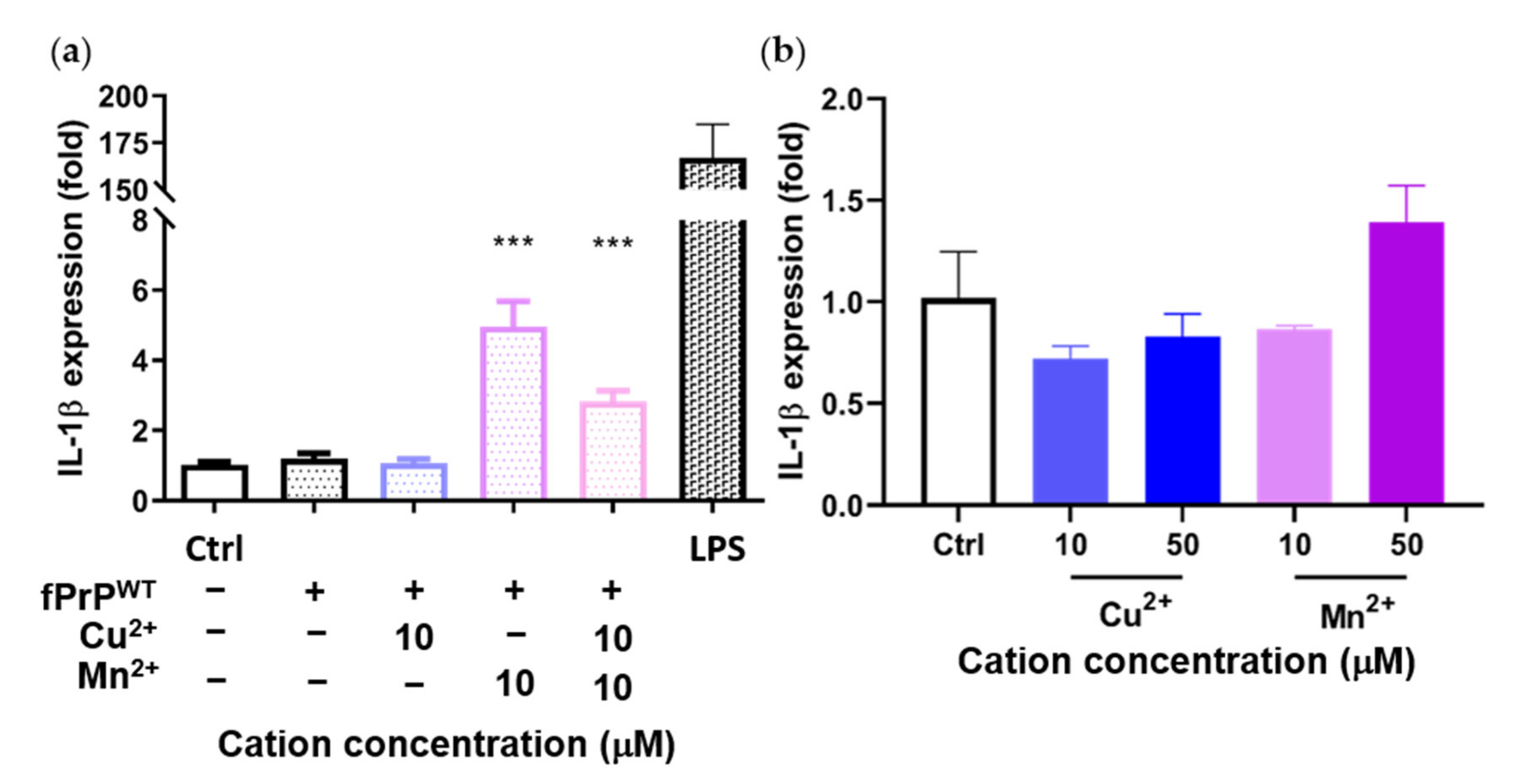
3.3. The Effect of Divalent Cations on the Expression of IL-1β Mrna in Fprpwt-Treated Microglia Cells
3.4. The Effect of Octapeptide Repeat of Prions on the Expression of Inflammatory Cytokines in Microglia Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Soto, C.; Satani, N. The intricate mechanisms of neurodegeneration in prion diseases. Trends Mol. Med. 2011, 17, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Gambetti, P.; Parchi, P.; Chen, S.G. Hereditary Creutzfeldt-Jakob disease and fatal familial insomnia. Clin. Lab. Med. 2003, 23, 43–64. [Google Scholar] [CrossRef]
- Will, R.G. Acquired prion disease: Iatrogenic CJD, variant CJD, kuru. Br. Med. Bull. 2003, 66, 255–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prusiner, S.B. Cell biology. A unifying role for prions in neurodegenerative diseases. Science 2012, 336, 1511–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wroe, S.J.; Pal, S.; Siddique, D.; Hyare, H.; Macfarlane, R.; Joiner, S.; Linehan, J.M.; Brandner, S.; Wadsworth, J.D.F.; Hewitt, P.; et al. Clinical presentation and pre-mortem diagnosis of variant Creutzfeldt-Jakob disease associated with blood transfusion: A case report. Lancet 2006, 368, 2061–2067. [Google Scholar] [CrossRef]
- Llewelyn, C.A.; Hewitt, P.E.; Knight, R.S.G.; Amar, K.; Cousens, S.; Mackenzie, J.; Will, R.G. Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Lancet 2004, 363, 417–421. [Google Scholar] [CrossRef]
- Prusiner, S.B. Prions. Proc. Natl. Acad. Sci. USA 1998, 95, 13363. [Google Scholar] [CrossRef] [Green Version]
- Bradford, B.M.; Mabbott, N.A. Prion disease and the innate immune system. Viruses 2012, 4, 3389–3419. [Google Scholar] [CrossRef] [Green Version]
- Geschwind, M.D. Prion Diseases. Continuum 2015, 21, 1612–1638. [Google Scholar] [CrossRef]
- Aguzzi, A.; Nuvolone, M.; Zhu, C. The immunobiology of prion diseases. Nat. Rev. Immunol. 2013, 13, 888–902. [Google Scholar] [CrossRef]
- Moore, R.A.; Herzog, C.; Errett, J.; Kocisko, D.A.; Arnold, K.M.; Hayes, S.F.; Priola, S.A. Octapeptide repeat insertions increase the rate of protease-resistant prion protein formation. Protein Sci. 2006, 15, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Croes, E.A.; Theuns, J.; Houwing-Duistermaat, J.J.; Dermaut, B.; Sleegers, K.; Roks, G.; Van den Broeck, M.; van Harten, B.; van Swieten, J.C.; Cruts, M.; et al. Octapeptide repeat insertions in the prion protein gene and early onset dementia. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Deloncle, R.; Fauconneau, B.; Guillard, O.; Delaval, J.; Lesage, G.; Pineau, A. Copper brain protein protection against free radical-induced neuronal death: Survival ratio in SH-SY5Y neuroblastoma cell cultures. J. Trace Elem. Med. Biol. 2017, 39, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.F.; Harischandra, D.S.; Kanthasamy, A.; Sivasankar, S. Copper-induced structural conversion templates prion protein oligomerization and neurotoxicity. Sci. Adv. 2016, 2, e1600014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hesketh, S.; Sassoon, J.; Knight, R.; Brown, D.R. Elevated manganese levels in blood and CNS in human prion disease. Mol. Cell Neurosci. 2008, 37, 590–598. [Google Scholar] [CrossRef]
- Johnson, C.J.; Gilbert, P.U.; Abrecht, M.; Baldwin, K.L.; Russell, R.E.; Pedersen, J.A.; Aiken, J.M.; McKenzie, D. Low copper and high manganese levels in prion protein plaques. Viruses 2013, 5, 654–662. [Google Scholar] [CrossRef] [Green Version]
- Scheiber, I.F.; Mercer, J.F.B.; Dringen, R. Metabolism and functions of copper in brain. Prog. Neurobiol. 2014, 116, 33–57. [Google Scholar] [CrossRef]
- Singh, N.; Singh, A.; Das, D.; Mohan, M.L. Redox control of prion and disease pathogenesis. Antioxid. Redox Signal. 2010, 12, 1271–1294. [Google Scholar] [CrossRef] [Green Version]
- Salter, M.W.; Stevens, B. Microglia emerge as central players in brain disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef]
- Davalos, D.; Grutzendler, J.; Yang, G.; Kim, J.V.; Zuo, Y.; Jung, S.; Littman, D.R.; Dustin, M.L.; Gan, W.B. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005, 8, 752–758. [Google Scholar] [CrossRef]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflammation 2014, 11, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colton, C.; Wilcock, D.M. Assessing activation states in microglia. Cns Neurol. Disord. Drug Targets 2010, 9, 174–191. [Google Scholar] [CrossRef] [PubMed]
- Colton, C.A. Heterogeneity of microglial activation in the innate immune response in the brain. J. Neuroimmune Pharm. 2009, 4, 399–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, W.; Rowe, D.; Xie, W.; Ortiz, I.; He, Y.; Appel, S.H. Microglial activation and dopaminergic cell injury: An in vitro model relevant to Parkinson’s disease. J. Neurosci. 2001, 21, 8447–8455. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Huang, Y.G.; Fang, D.; Le, W.D. (-)-Epigallocatechin gallate inhibits lipopolysaccharide-induced microglial activation and protects against inflammation-mediated dopaminergic neuronal injury. J. Neurosci. Res. 2004, 78, 723–731. [Google Scholar] [CrossRef]
- Sawada, M.; Suzumura, A.; Hosoya, H.; Marunouchi, T.; Nagatsu, T. Interleukin-10 inhibits both production of cytokines and expression of cytokine receptors in microglia. J. Neurochem. 1999, 72, 1466–1471. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [Green Version]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Duncan, B.; Li, X.; Shi, J. The role of NLRP3 inflammasome in infection-related, immune-mediated and autoimmune skin diseases. J. Derm. Sci. 2020, 98, 146–151. [Google Scholar] [CrossRef]
- Sarkar, S.; Rokad, D.; Malovic, E.; Luo, J.; Harischandra, D.S.; Jin, H.; Anantharam, V.; Huang, X.; Lewis, M.; Kanthasamy, A.; et al. Manganese activates NLRP3 inflammasome signaling and propagates exosomal release of ASC in microglial cells. Sci. Signal. 2019, 12, 563. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.H.; Huang, M.Y.; Lee, Y.R.; Lin, Y.K.; Chen, H.R.; Lee, C.I. The effect of octapeptid repeats on prion folding and misfolding. 2020. in preparation. [Google Scholar]
- Bocharova, O.V.; Breydo, L.; Parfenov, A.S.; Salnikov, V.V.; Baskakov, I.V. In vitro conversion of full-length mammalian prion protein produces amyloid form with physical properties of PrP(Sc). J. Mol. Biol. 2005, 346, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.J.; Yu, K.H.; Wu, J.R.; Lee, C.F.; Jheng, C.P.; Chen, H.R.; Lee, C.I. Liberation of GPI-anchored prion from phospholipids accelerates amyloidogenic conversion. Int. J. Mol. Sci. 2013, 14, 17943–17957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, C.J.; Anantharam, V.; Saetveit, N.J.; Houk, R.S.; Kanthasamy, A.; Kanthasamy, A.G. Normal cellular prion protein protects against manganese-induced oxidative stress and apoptotic cell death. Toxicol. Sci. 2007, 98, 495–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minutoli, L.; Puzzolo, D.; Rinaldi, M.; Irrera, N.; Marini, H.; Arcoraci, V.; Bitto, A.; Crea, G.; Pisani, A.; Squadrito, F.; et al. ROS-Mediated NLRP3 Inflammasome Activation in Brain, Heart, Kidney, and Testis Ischemia/Reperfusion Injury. Oxid. Med. Cell Longev. 2016, 2016, 2183026. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.R. Metallic prions. Biochem. Soc. Symp. 2004, 193–202. [Google Scholar]
- Brazier, M.W.; Davies, P.; Player, E.; Marken, F.; Viles, J.H.; Brown, D.R. Manganese binding to the prion protein. J. Biol. Chem. 2008, 283, 12831–12839. [Google Scholar] [CrossRef] [Green Version]
- Peres, T.V.; Schettinger, M.R.C.; Chen, P.; Carvalho, F.; Avila, D.S.; Bowman, A.B.; Aschner, M. Manganese-induced neurotoxicity: A review of its behavioral consequences and neuroprotective strategies. BMC Pharm. Toxicol. 2016, 17, 57. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Asthana, A.; Baksi, S.; Desai, V.; Haldar, S.; Hari, S.; Tripathi, A.K. The prion-ZIP connection: From cousins to partners in iron uptake. Prion 2015, 9, 420–428. [Google Scholar] [CrossRef] [Green Version]
- Stahl, J.L.; Greger, J.L.; Cook, M.E. Zinc, copper and iron utilisation by chicks fed various concentrations of zinc. Br. Poult. Sci. 1989, 30, 123–134. [Google Scholar] [CrossRef]
- Watt, N.T.; Taylor, D.R.; Kerrigan, T.L.; Griffiths, H.H.; Rushworth, J.V.; Whitehouse, I.J.; Hooper, N.M. Prion protein facilitates uptake of zinc into neuronal cells. Nat. Commun. 2012, 3, 1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Summersgill, H.; England, H.; Lopez-Castejon, G.; Lawrence, C.B.; Luheshi, N.M.; Pahle, J.; Mendes, P.; Brough, D. Zinc depletion regulates the processing and secretion of IL-1β. Cell Death Dis. 2014, 5, e1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muroi, M.; Tanamoto, K. Zinc- and oxidative property-dependent degradation of pro-caspase-1 and NLRP3 by ziram in mouse macrophages. Toxicol. Lett. 2015, 235, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Hafner-Bratkovič, I.; Benčina, M.; Fitzgerald, K.A.; Golenbock, D.; Jerala, R. NLRP3 inflammasome activation in macrophage cell lines by prion protein fibrils as the source of IL-1β and neuronal toxicity. Cell Mol. Life Sci. 2012, 69, 4215–4228. [Google Scholar]
- Stoll, G.; Jander, S.; Schroeter, M. Cytokines in CNS disorders: Neurotoxicity versus neuroprotection. J. Neural. Transm. Suppl. 2000, 59, 81–89. [Google Scholar]
- Beringue, V.; Demoy, M.; Lasmézas, C.I.; Gouritin, B.; Weingarten, C.; Deslys, J.P.; Andreux, J.P.; Couvreur, P.; Dormont, D. Role of spleen macrophages in the clearance of scrapie agent early in pathogenesis. J. Pathol. 2000, 190, 495–502. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jen, H.-I.; Lin, Z.-Y.; Guo, J.-X.; Lee, C.-I. The Effects of Divalent Cation-Chelated Prion Fibrils on the Immune Response of EOC 13.31 Microglia Cells. Cells 2020, 9, 2285. https://doi.org/10.3390/cells9102285
Jen H-I, Lin Z-Y, Guo J-X, Lee C-I. The Effects of Divalent Cation-Chelated Prion Fibrils on the Immune Response of EOC 13.31 Microglia Cells. Cells. 2020; 9(10):2285. https://doi.org/10.3390/cells9102285
Chicago/Turabian StyleJen, Huan-I, Zih-You Lin, Jin-Xun Guo, and Cheng-I Lee. 2020. "The Effects of Divalent Cation-Chelated Prion Fibrils on the Immune Response of EOC 13.31 Microglia Cells" Cells 9, no. 10: 2285. https://doi.org/10.3390/cells9102285