Maternal Protein Restriction Modulates Angiogenesis and AQP9 Expression Leading to a Delay in Postnatal Epididymal Development in Rat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Western Blot
2.3. Morphometrical Analysis
2.4. Immunohistochemistry at PND 14
2.5. Microvascular Density (MVD) Determination at PND14
2.6. Statistical Analysis
3. Results
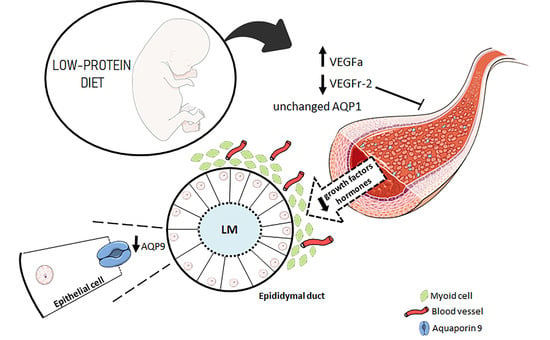
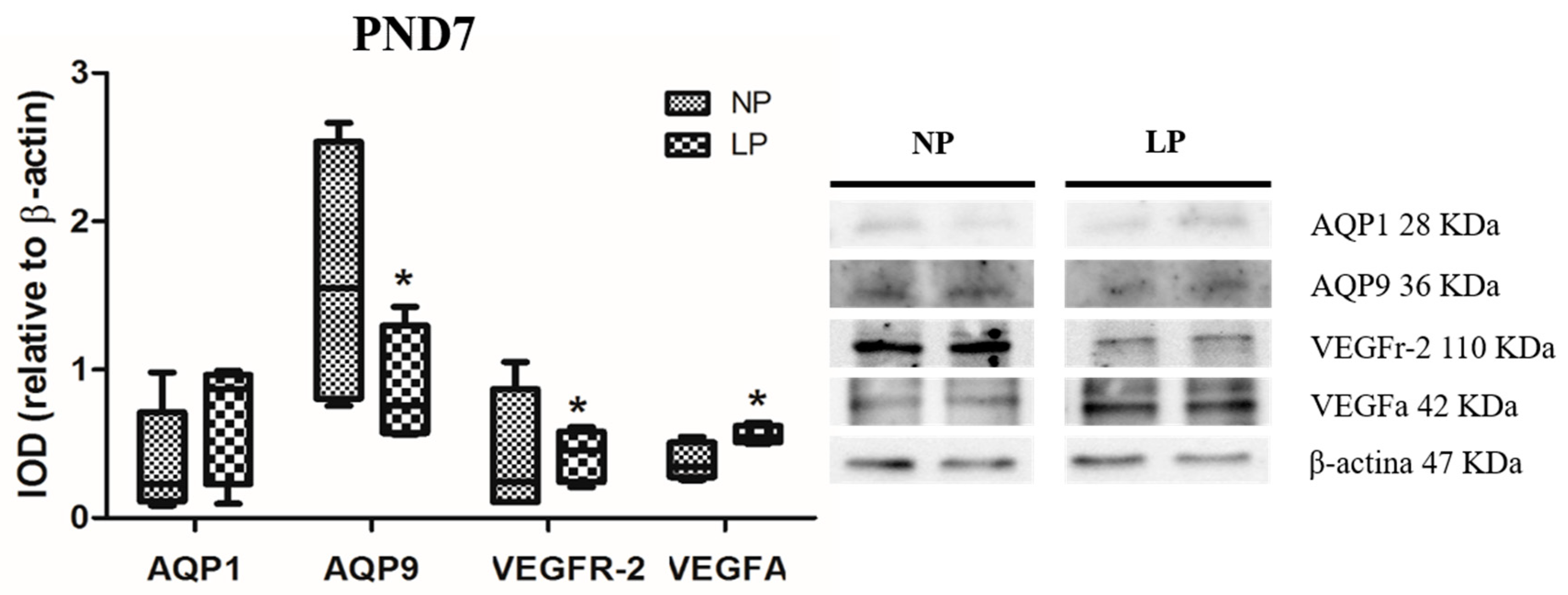
3.1. A Maternal Low-Protein Diet Alters the Protein Levels of VEGFr-2 and VEGFa in the Epididymis of the Offspring in the First Postnatal Week
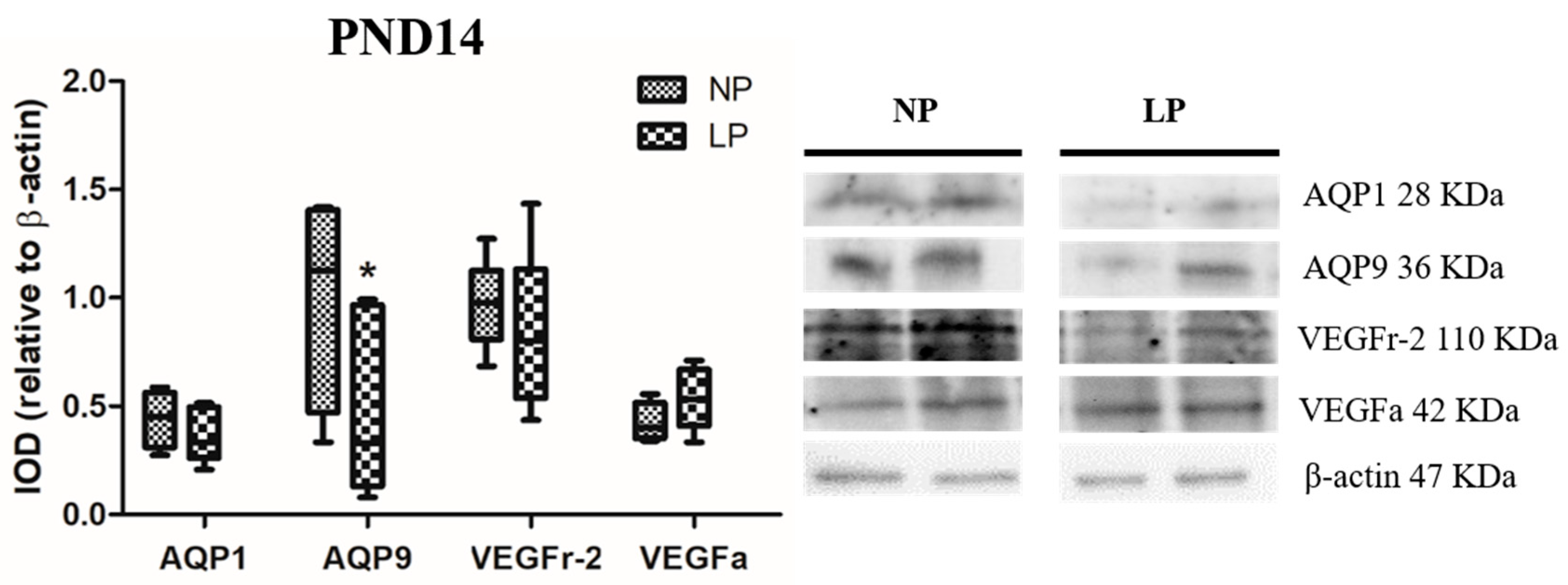
3.2. A Maternal Low-Protein Diet Alters the Protein Levels of AQP9 but Not of AQP1 in the Epididymis of the Offspring at PND7 and PND14
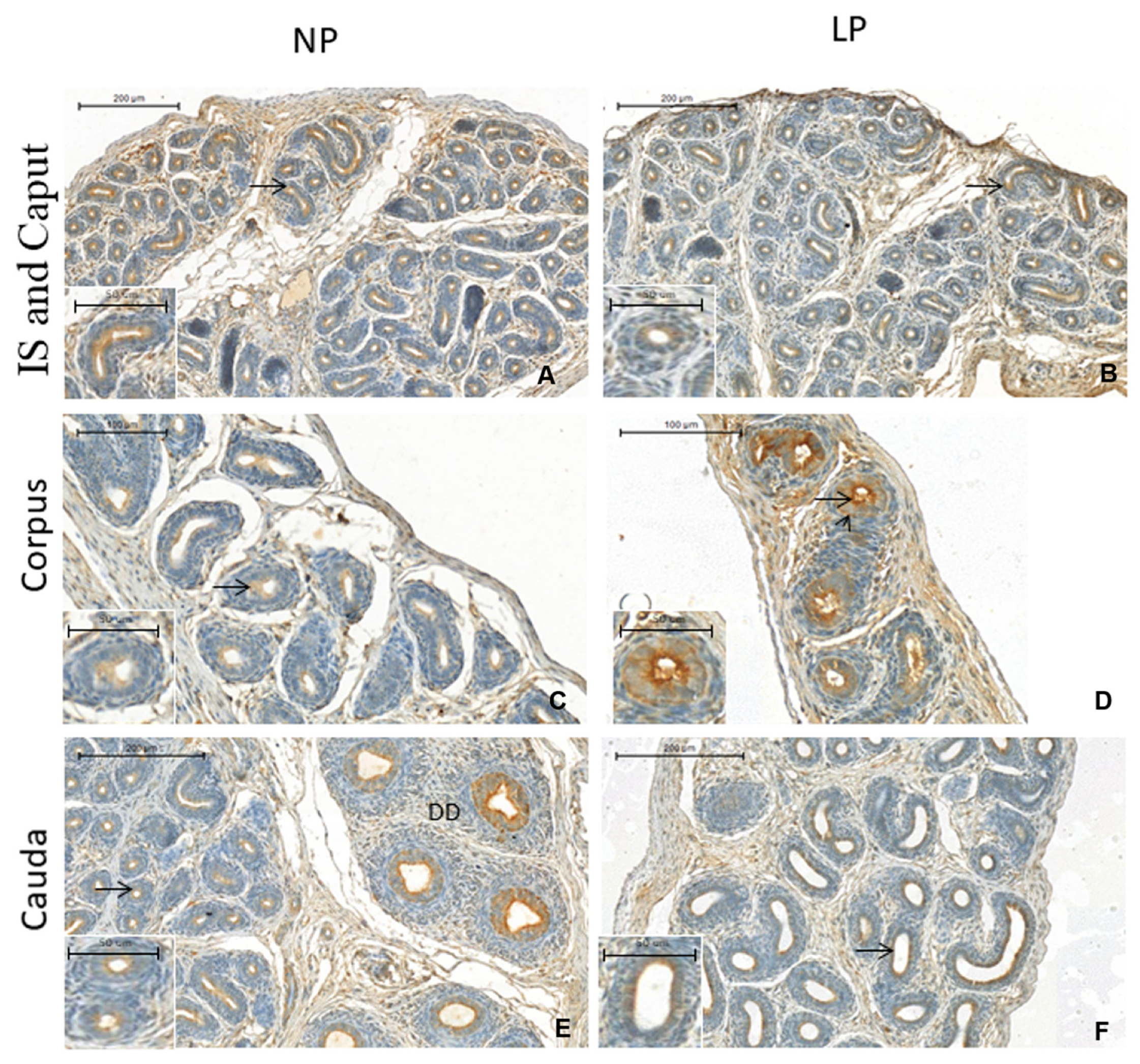
3.3. A Maternal Low-Protein Diet Promotes Changes in the Immunostaining Pattern of AQP9 but Not of AQP1 in the Offspring at PND14
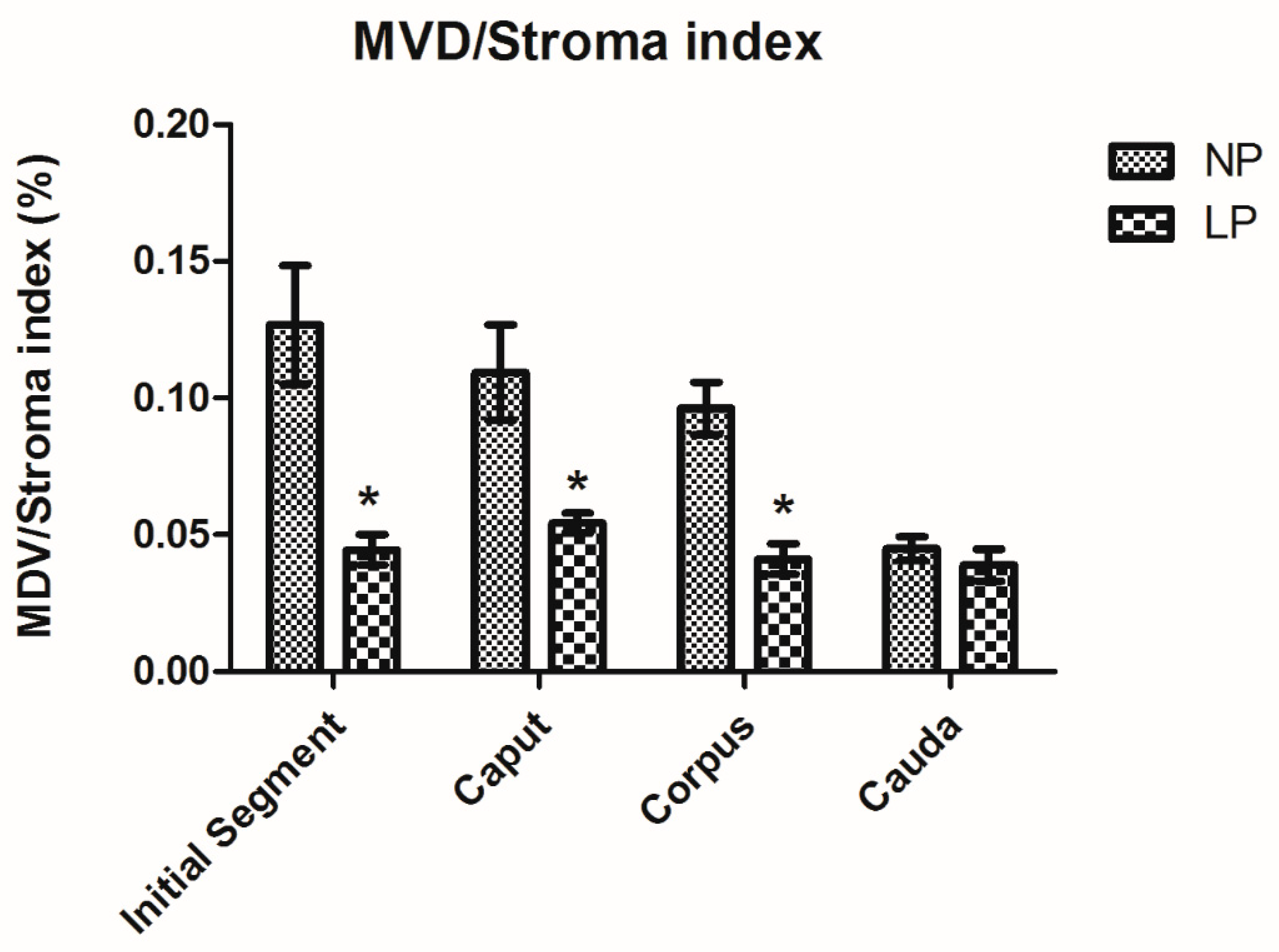
3.4. A Maternal Low-Protein Diet Decreases the Microvascular Density (MVD) of the Offspring at PND14
3.5. A Maternal Low-Protein Diet Changes the Epididymal Morphometry of the Offspring but Not the Morphology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumaran, K.; Osmond, C.; Fall, C.H.D. Early Origins of Cardiometabolic Disease. In Disease Control Priorities, Third Edition (Volume 5): Cardiovascular, Respiratory, and Related Disorders, 3rd ed.; Prabhakaran, D., Anand, S., Gaziano, T.A., Mbanya, J.-C., Wu, Y., Nugent, R., Eds.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2017. [Google Scholar]
- Lubinsky, M. Correction to: An epigenetic association of malformations, adverse reproductive outcomes, and fetal origins hypothesis related effects. J. Assist. Reprod. Genet. 2018, 35, 965. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.J. The developmental basis of disease: Update on environmental exposures and animal models. Basic Clin. Pharmacol. Toxicol. 2018, 125, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Bateson, P.; Barker, D.; Clutton-Brock, T.; Deb, D.; D’Udine, B.; Foley, R.A.; Gluckman, P.; Godfrey, K.; Kirkwood, T.; Lahr, M.M.; et al. Developmental plasticity and human health. Nature 2004, 430, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.P. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.K.; Manikkam, M.; Guerrero-Bosagna, C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol. Metab. 2010, 21, 214–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.-S. Impact of Maternal Diet on the Epigenome during In Utero Life and the Developmental Programming of Diseases in Childhood and Adulthood. Nutrients 2015, 7, 9492–9507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hales, C.; Barker, D. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Int. J. Epidemiol. 2013, 42, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.P.; Osmond, C.; Kajantie, E.; Eriksson, J.G. Growth and chronic disease: findings in the Helsinki Birth Cohort. Ann. Hum. Biol. 2009, 36, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.J.S.; Sellitti, D.F.; Lagranha, C.J. Protein undernutrition during development and oxidative impairment in the central nervous system (CNS): Potential factors in the occurrence of metabolic syndrome and CNS disease. J. Dev. Orig. Health Dis. 2016, 7, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Esteves, S.C.; Miyaoka, R.; Agarwal, A. An update on the clinical assessment of the infertile male. Clinics 2011, 66, 691–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faure, C.; Dupont, C.; Chavatte-Palmer, P.; Gautier, B.; Lévy, R. Are semen parameters related to birth weight? Fertil. Steril. 2015, 103, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Boeri, L.; Ventimiglia, E.; Capogrosso, P.; Ippolito, S.; Pecoraro, A.; Paciotti, A.; Scano, R.; Galdini, A.; Valsecchi, L.; Papaleo, E.; et al. Low Birth Weight Is Associated with a Decreased Overall Adult Health Status and Reproductive Capability—Results of a Cross-Sectional Study in Primary Infertile Patients. PLoS ONE 2016, 11, e0166728. [Google Scholar] [CrossRef] [PubMed]
- Langley-Evans, S.C.; McMullen, S. Developmental Origins of Adult Disease. Med. Princ. Pract. 2010, 19, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.P.; Watkins, A.J.; Sun, C.; Velazquez, M.A.; Smyth, N.R.; Eckert, J.J. Do little embryos make big decisions? How maternal dietary protein restriction can permanently change an embryo’s potential, affecting adult health. Reprod. Fertil. Dev. 2015, 27, 684. [Google Scholar] [CrossRef] [PubMed]
- Toledo, F.C.; Perobelli, J.E.; Pedrosa, F.P.; Anselmo-Franci, J.A.; Kempinas, W.D. In utero protein restriction causes growth delay and alters sperm parameters in adult male rats. Reprod. Biol. Endocrinol. 2011, 9, 94. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, P.J.; Xiong, F.; Li, Y.; Zhou, J.; Zhang, L. Fetal hypoxia increases vulnerability of hypoxic–ischemic brain injury in neonatal rats: Role of glucocorticoid receptors. Neurobiol. Dis. 2014, 65, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Jost, A. The age factor in the castration of male rabbit fetuses. Proc. Soc. Exp. Biol. Med. 1947, 66, 302–303. [Google Scholar] [CrossRef]
- Staack, A.; Donjacour, A.A.; Brody, J.; Cunha, G.R.; Carroll, P. Mouse urogenital development: A practical approach. Differentiation 2003, 71, 402–413. [Google Scholar] [CrossRef]
- Joseph, A.; Yao, H.; Hinton, B.T. Development and morphogenesis of the Wolffian/epididymal duct, more twists and turns. Dev. Biol. 2009, 325, 6–14. [Google Scholar] [CrossRef] [Green Version]
- Robaire, B.; Hinton, B.T. The epididymis. In The Physiology of Reproduction, 4th ed.; Knobil, E., Neill, J.D., Eds.; Elsevier: New York, NY, USA, 2015. [Google Scholar]
- Xu, B.; Yang, L.; Lye, R.J.; Hinton, B.T. p-MAPK1/3 and DUSP6 Regulate Epididymal Cell Proliferation and Survival in a Region-Specific Manner in Mice1. Biol. Reprod. 2010, 83, 807–817. [Google Scholar] [CrossRef]
- Yeung, C.H.; Sonnenberg-Riethmacher, E.; Cooper, T.G. Receptor tyrosine kinase c-ros knockout mice as a model for the study of epididymal regulation of sperm function. J. Reprod. Fertil. Suppl. 1998, 53, 137–147. [Google Scholar]
- Robaire, B.; Henderson, N. Actions of 5alpha-reductase inhibitors on the epididymis. Mol. Cell. Endocrinol. 2006, 250, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Gao, X.; Ma, X.; Yue, Y. Corrigendum #2 to “Effects of Chronic Exposure to Sodium Arsenite on Expressions of VEGF and VEGFR2 Proteins in the Epididymis of Rats”. BioMed. Res. Int. 2018, 2018, 1082961. [Google Scholar] [PubMed]
- Da Silva, N.; Pietrement, C.; Brown, D.; Breton, S. Segmental and cellular expression of aquaporins in the male excurrent duct. Biochim. Biophys. Acta (BBA)-Biomembr. 2006, 1758, 1025–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastor-Soler, N.; Bagnis, C.; Sabolic, I.; Tyszkowski, R.; McKee, M.; Van Hoek, A.; Breton, S.; Brown, D. Aquaporin 9 Expression along the Male Reproductive Tract1. Biol. Reprod. 2001, 65, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Badran, H.H.; Hermo, L.S. Expression and regulation of aquaporins 1, 8, and 9 in the testis, efferent ducts, and epididymis of adult rats and during postnatal development. J. Androl. 2002, 23, 358–373. [Google Scholar] [PubMed]
- Cavariani, M.M.; Santos, T.D.M.; Pereira, D.N.; Chuffa, L.G.D.A.; Pinheiro, P.F.F.; Scarano, W.R.; Domeniconi, R.F. Maternal Protein Restriction Differentially Alters the Expression of AQP1, AQP9 and VEGFr-2 in the Epididymis of Rat Offspring. Int. J. Mol. Sci. 2019, 20, 469. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantiWcation of microgram quantities of protein utilizing the principle of protein– dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Eriksson, J.; Forsén, T.; Barker, D.; Osmond, C. Fetal origins of adult disease: Strength of effects and biological basis. Int. J. Epidemiol. 2002, 31, 1235–1239. [Google Scholar]
- Gluckman, P.; Hanson, M. The Consequences of Being Born Small—An Adaptive Perspective. Horm. Res. Paediatr. 2006, 65, 5–14. [Google Scholar] [CrossRef]
- Indrio, F.; Martini, S.; Francavilla, R.; Corvaglia, L.; Cristofori, F.; Mastrolia, S.A.; Neu, J.; Rautava, S.; Russo Spena, G.; Raimondi, F.; et al. Epigenetic Matters: The Link between Early Nutrition, Microbiome, and Long-term Health Development. Front. Pediatr. 2017, 5, 178. [Google Scholar] [CrossRef] [PubMed]
- Reckmann, A.N.; Tomczyk, C.U.M.; Davidoff, M.S.; Michurina, T.V.; Arnhold, S.; Müller, D.; Mietens, A.; Middendorff, R. Nestin in the epididymis is expressed in vascular wall cells and is regulated during postnatal development and in case of testosterone deficiency. PLoS ONE 2018, 13, e0194585. [Google Scholar] [CrossRef] [PubMed]
- Karaman, S.; Leppänen, V.-M.; Alitalo, K. Vascular endothelial growth factor signaling in development and disease. Development 2018, 145, dev151019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ergün, S.; Luttmer, W.; Fiedler, W.; Holstein, A.F. Functional Expression and Localization of Vascular Endothelial Growth Factor and Its Receptors in the Human Epididymis1. Biol. Reprod. 1998, 58, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Colombelli, K.T.; Santos, S.A.; Camargo, A.C.; Constantino, F.B.; Barquilha, C.N.; Rinaldi, J.C.; Felisbino, S.L.; Justulin, L.A. Impairment of microvascular angiogenesis is associated with delay in prostatic development in rat offspring of maternal protein malnutrition. Gen. Comp. Endocrinol. 2017, 246, 258–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Setchell, B.; Breed, W. Anatomy, Vasculature, and Innervation of the Male Reproductive Tract. In Knobil and Neill’s Physiology of Reproduction; Elsevier: New York, NY, USA, 2006; pp. 771–825. [Google Scholar]
- Dacheux, J.-L.; Dacheux, F. New insights into epididymal function in relation to sperm maturation. Reproduction 2014, 147, R27–R42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agre, P. Aquaporin Water Channels (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 2004, 43, 4278–4290. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Mitra, A.K. Structure and function of aquaporin water channels. Am. J. Physiol. Renal Physiol. 2017, 278, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Pelagalli, A.; Squillacioti, C.; Mirabella, N.; Meli, R. Aquaporins in Health and Disease: An Overview Focusing on the Gut of Different Species. Int. J. Mol. Sci. 2016, 17, 1213. [Google Scholar] [CrossRef]
- Wei, X.; Dong, J. Aquaporin 1 promotes the proliferation and migration of lung cancer cell in vitro. Oncol. Rep. 2015, 34, 1440–1448. [Google Scholar] [CrossRef] [Green Version]
- Ricanek, P.; Lunde, L.K.; A Frye, S.; Stoen, M.; Nygård, S.; Morth, J.P.; Rydning, A.; Vatn, M.H.; Amiry-Moghaddam, M.; Tønjum, T. Reduced expression of aquaporins in human intestinal mucosa in early stage inflammatory bowel disease. Clin. Exp. Gastroenterol. 2015, 8, 49–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.H.; Yan, C.X.; Chen, X.J.; Zhu, Y.S. Expression of aquaglyceroporins in epithelial ovarian tumours and their clinical significance. J. Int. Med. Res. 2011, 39, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.G.; Li, C.F.; Liu, M.; Chen, X.F.; Shuai, K.; Kong, X.; Lv, L.; Mei, Z.C. Aquaporin 9 is down-regulated in hepatocellular carcinoma and its over-expression suppresses hepatoma cell invasion through inhibiting epithelial-to-mesenchymal transition. Cancer Lett. 2016, 378, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, M.; Waguri-Nagaya, Y.; Yamagami, T.; Aoyama, M.; Tada, T.; Inoue, K.; Asai, K.; Otsuka, T. TNF-alpha-induced aquaporin 9 in synoviocytes from patients with OA and RA. Rheumatology 2010, 49, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Park, J.H.; Yoon, J.-K.; Yoon, J.S.; Kim, J.S.; Lee, J.H.; Yun, B.H.; Park, J.H.; Seo, S.K.; Cho, S.; et al. Potential roles of aquaporin 9 in the pathogenesis of endometriosis. Mol. Hum. Reprod. 2019, 25, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M.; Morató, R.; Rodríguez-Gil, J.; Bonet, S.; Prieto-Martínez, N. Aquaporins in the male reproductive tract and sperm: Functional implications and cryobiology. Reprod. Domest. Anim. 2017, 52, 12–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.-F.; He, R.-H.; Sun, C.-C.; Zhang, Y.; Meng, Q.-X.; Ma, Y.-Y. Function of aquaporins in female and male reproductive systems. Hum. Reprod. Updat. 2006, 12, 785–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, D.; Verbavatz, J.M.; Valenti, G.; Lui, B.; Sabolić, I. Localization of the CHIP28 water channel in reabsorptive segments of the rat male reproductive tract. Eur. J. Cell Biol. 1993, 61, 264–273. [Google Scholar]
- Arrighi, S.; Aralla, M.; Genovese, P.; Picabea, N.; Bielli, A. Undernutrition during foetal to prepubertal life affects aquaporin 9 but not aquaporins 1 and 2 expression in the male genital tract of adult rats. Theriogenology 2010, 74, 1661–1669. [Google Scholar] [CrossRef]
- King, L.S.; Kozono, D.; Agre, P. From structure to disease: The evolving tale of aquaporin biology. Nat. Rev. Mol. Cell Biol. 2004, 5, 687–698. [Google Scholar] [CrossRef]
- Elkjær, M.-L.; Vajda, Z.; Nejsum, L.N.; Kwon, T.-H.; Jensen, U.B.; Amiry-Moghaddam, M.; Frøkiær, J.; Nielsen, S. Immunolocalization of AQP9 in Liver, Epididymis, Testis, Spleen, and Brain. Biochem. Biophys. Res. Commun. 2000, 276, 1118–1128. [Google Scholar] [CrossRef]
- Pastor-Soler, N.; Isnard-Bagnis, C.; Herak-Kramberger, C.; Sabolic, I.; Van Hoek, A.; Brown, D.; Breton, S. Expression of Aquaporin 9 in the Adult Rat Epididymal Epithelium Is Modulated by Androgens1. Biol. Reprod. 2002, 66, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.T.; Johnston, D.S.; Jelinsky, S.A.; Tomsig, J.L.; Finger, J.N. Segment boundaries of the adult rat epididymis limit interstitial signaling by potential paracrine factors and segments lose differential gene expression after efferent duct ligation. Asian J. Androl. 2007, 9, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, E.; Rodríguez-González, G.L.; Guzmán, C.; García-Becerra, R.; Boeck, L.; Díaz, L.; Menjivar, M.; Larrea, F.; Nathanielsz, P.W. A maternal low protein diet during pregnancy and lactation in the rat impairs male reproductive development. J. Physiol. 2005, 563, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; De Iuliis, G.N.; Dun, M.D.; Nixon, B. Characteristics of the Epididymal Luminal Environment Responsible for Sperm Maturation and Storage. Front. Endocrinol. 2018, 9, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agre, P.; Kozono, D. Aquaporin water channels: Molecular mechanisms for human diseases. FEBS Lett. 2003, 555, 72–78. [Google Scholar] [CrossRef]
- Rojek, A.M.; Skowronski, M.T.; Füchtbauer, E.-M.; Füchtbauer, A.C.; Fenton, R.A.; Agre, P.; Frøkiær, J.; Nielsen, S. Defective glycerol metabolism in aquaporin 9 (AQP9) knockout mice. Proc. Natl. Acad. Sci. USA 2007, 104, 3609–3614. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, S.; Moniaga, C.S.; Nielsen, S.; Hara-Chikuma, M. Aquaporin-9 facilitates membrane transport of hydrogen peroxide in mammalian cells. Biochem. Biophys. Res. Commun. 2016, 471, 191–197. [Google Scholar] [CrossRef]
- Laforenza, U.; Pellavio, G.; Marchetti, A.L.; Omes, C.; Todaro, F.; Gastaldi, G. Aquaporin-mediated water and hydrogen peroxide transport is involved in normal human spermatozoa functioning. Int. J. Mol. Sci. 2017, 18, 66. [Google Scholar] [CrossRef]
- Xu, B.; Turner, S.D.; Hinton, B.T. Alteration of transporter activities in the epididymides of infertile initial segment-specific Pten knockout mice†. Biol. Reprod. 2018, 99, 536–545. [Google Scholar] [CrossRef]
- Lei, Z.M.; Mishra, S.; Zou, W.; Xu, B.; Foltz, M.; Li, X.; Rao, C.V. Targeted Disruption of Luteinizing Hormone/Human Chorionic Gonadotropin Receptor Gene. Mol. Endocrinol. 2011, 15, 184–200. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-González, G.L.; Reyes-Castro, L.A.; Vega, C.C.; Boeck, L.; Ibáñez, C.; Nathanielsz, P.W.; Larrea, F.; Zambrano, E. Accelerated aging of reproductive capacity in male rat offspring of protein-restricted mothers is associated with increased testicular and sperm oxidative stress. Age 2014, 36, 9721. [Google Scholar] [CrossRef] [PubMed]



| Components * | Normoprotein (17% Protein) | Low-Protein (6% Protein) |
|---|---|---|
| Casein (84% of protein) ** | 202.00 | 71.50 |
| Corn flour | 397.00 | 480.00 |
| Dextrin | 130.50 | 159.00 |
| Sucrose | 100.00 | 121.00 |
| Soy oil | 70.00 | 70.00 |
| Fiber (microcellulose) | 50.00 | 50.00 |
| Mineral blend *** | 35.00 | 35.00 |
| Vitamin blend *** | 10.00 | 10.00 |
| L–cystine | 3.00 | 1.00 |
| Choline chloride | 2.50 | 2.50 |
| Parameters (µm) | PND7 | |||||||
|---|---|---|---|---|---|---|---|---|
| IS | Caput | Corpus | Cauda | |||||
| NP | LP | NP | LP | NP | LP | NP | LP | |
| Tubular diameter | 23.87 ± 0.37 | 21.86 ± 0.43 * | 25.90 ± 0.57 | 23.33 ± 0.27 * | 27.97 ± 0.25 | 25.58 ± 0.82 * | 35.85 ± 1.45 | 32.33 ± 2.53 |
| Epithelial height | 7.73 ± 0.22 | 7.22 ± 0.06 | 7.15 ± 0.29 | 6.78 ± 0.18 | 7.78 ± 0.01 | 7.268 ± 0.07 | 8.579 ± 0.83 | 7.14 ± 0.43 |
| Luminal diameter | 7.49 ± 0.15 | 6.70 ± 0.18 * | 10.97 ± 0.51 | 9.29 ± 0.68 | 12.10 ± 0.34 | 10.60 ± 1.27 | 19.23 ± 0.45 | 18.01 ± 3.11 |
| Parameters (µm) | PND14 | |||||||
|---|---|---|---|---|---|---|---|---|
| IS | IS | |||||||
| NP | LP | NP | LP | NP | LP | NP | LP | |
| Tubular diameter | 28.47 ± 0.80 | 25.80 ± 1.30 | 33.55 ± 2.24 | 29.41 ± 1.26 | 40.18 ± 1.05 | 32.46 ± 1.04 * | 39.82 ± 2.17 | 38.29 ± 1.97 |
| Epithelial height | 9.50 ± 0.28 | 8.17 ± 0.39 * | 8.90 ± 0.99 | 7.47 ± 0.60 | 11.85 ± 0.28 | 9.17 ± 0.67 * | 10.93 ± 1.72 | 8.07 ± 0.23 |
| Luminal diameter | 9.22 ± 0.62 | 8.56 ± 0.51 | 14.16 ± 1.51 | 14.18 ± 0.81 | 15.06 ± 0.62 | 14.52 ± 0.73 | 19.02 ± 1.86 | 21.99 ± 2.32 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Mello Santos, T.; Cavariani, M.M.; Pereira, D.N.; Schimming, B.C.; Chuffa, L.G.d.A.; Domeniconi, R.F. Maternal Protein Restriction Modulates Angiogenesis and AQP9 Expression Leading to a Delay in Postnatal Epididymal Development in Rat. Cells 2019, 8, 1094. https://doi.org/10.3390/cells8091094
de Mello Santos T, Cavariani MM, Pereira DN, Schimming BC, Chuffa LGdA, Domeniconi RF. Maternal Protein Restriction Modulates Angiogenesis and AQP9 Expression Leading to a Delay in Postnatal Epididymal Development in Rat. Cells. 2019; 8(9):1094. https://doi.org/10.3390/cells8091094
Chicago/Turabian Stylede Mello Santos, Talita, Marilia Martins Cavariani, Dhrielly Natália Pereira, Bruno César Schimming, Luiz Gustavo de Almeida Chuffa, and Raquel Fantin Domeniconi. 2019. "Maternal Protein Restriction Modulates Angiogenesis and AQP9 Expression Leading to a Delay in Postnatal Epididymal Development in Rat" Cells 8, no. 9: 1094. https://doi.org/10.3390/cells8091094