MicroRNA-99a is a Potential Target for Regulating Hypothalamic Synaptic Plasticity in the Peri/Postmenopausal Depression Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Model Established as Described Previously
2.2. Metabolic Chambers
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Electrophysiological Analysis
Hippocampal Slice Preparations
2.5. Field Potential Recording
2.6. Transmission Electron Microscope
2.7. Immunofluorescence
2.8. Nuclear Protein Extraction
2.9. Western Blotting
2.10. Chip-Based miRNA Expression Analysis
2.11. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
2.12. Statistical Analysis
3. Results
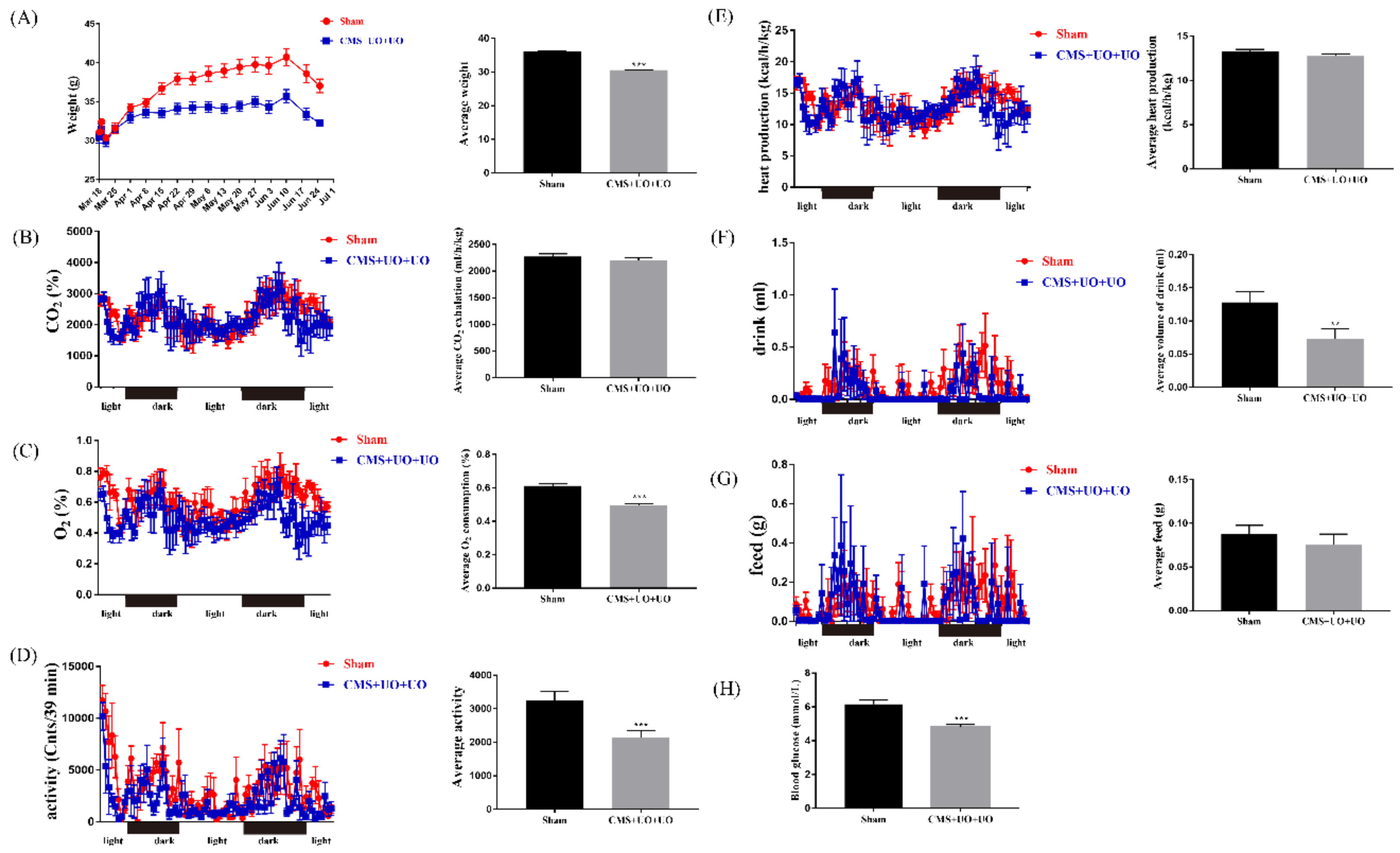
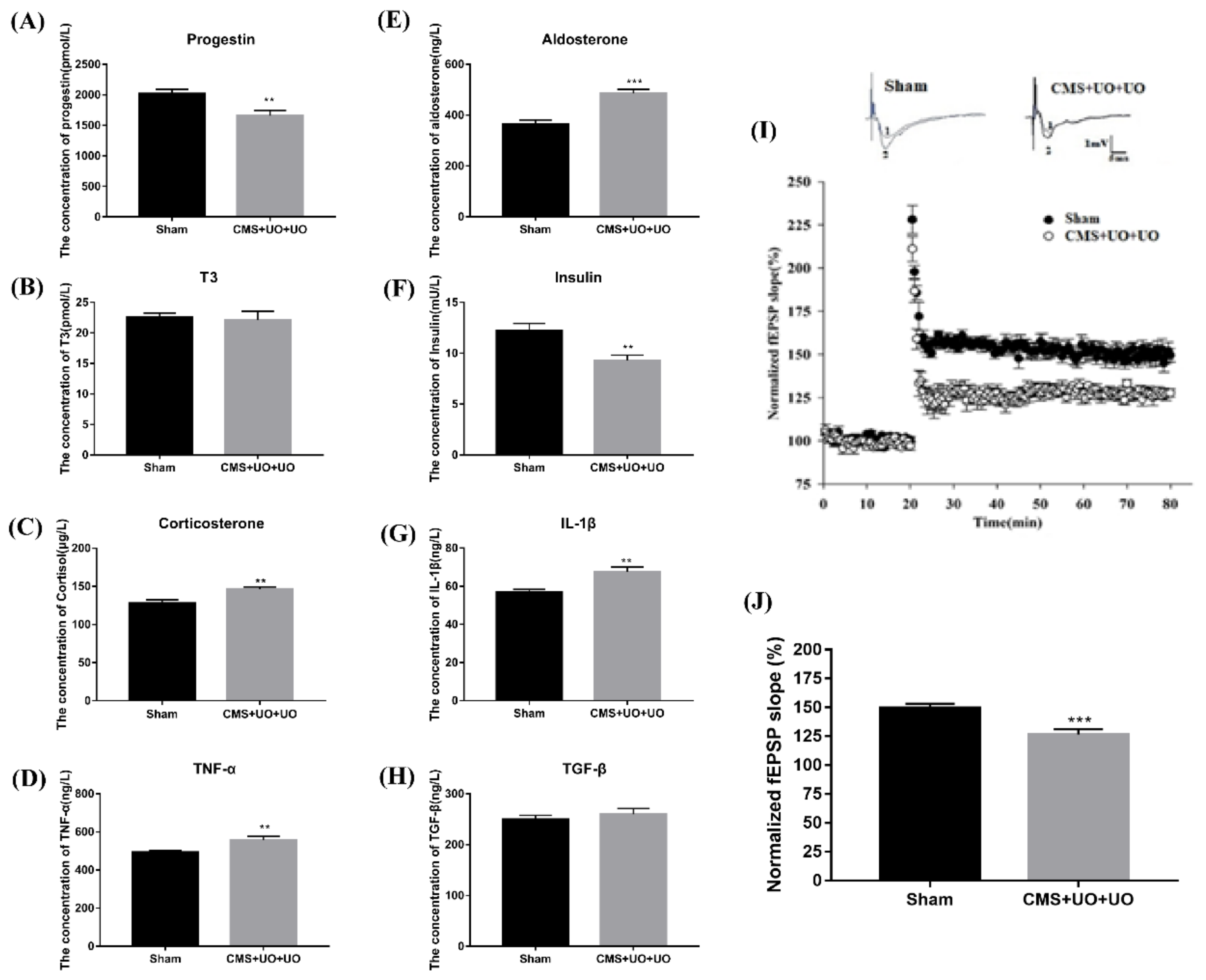
3.1. Peri/Postmenopausal Depressive Mice Had Endocrine and Metabolic Disorders and Synaptic Transmission Compromise
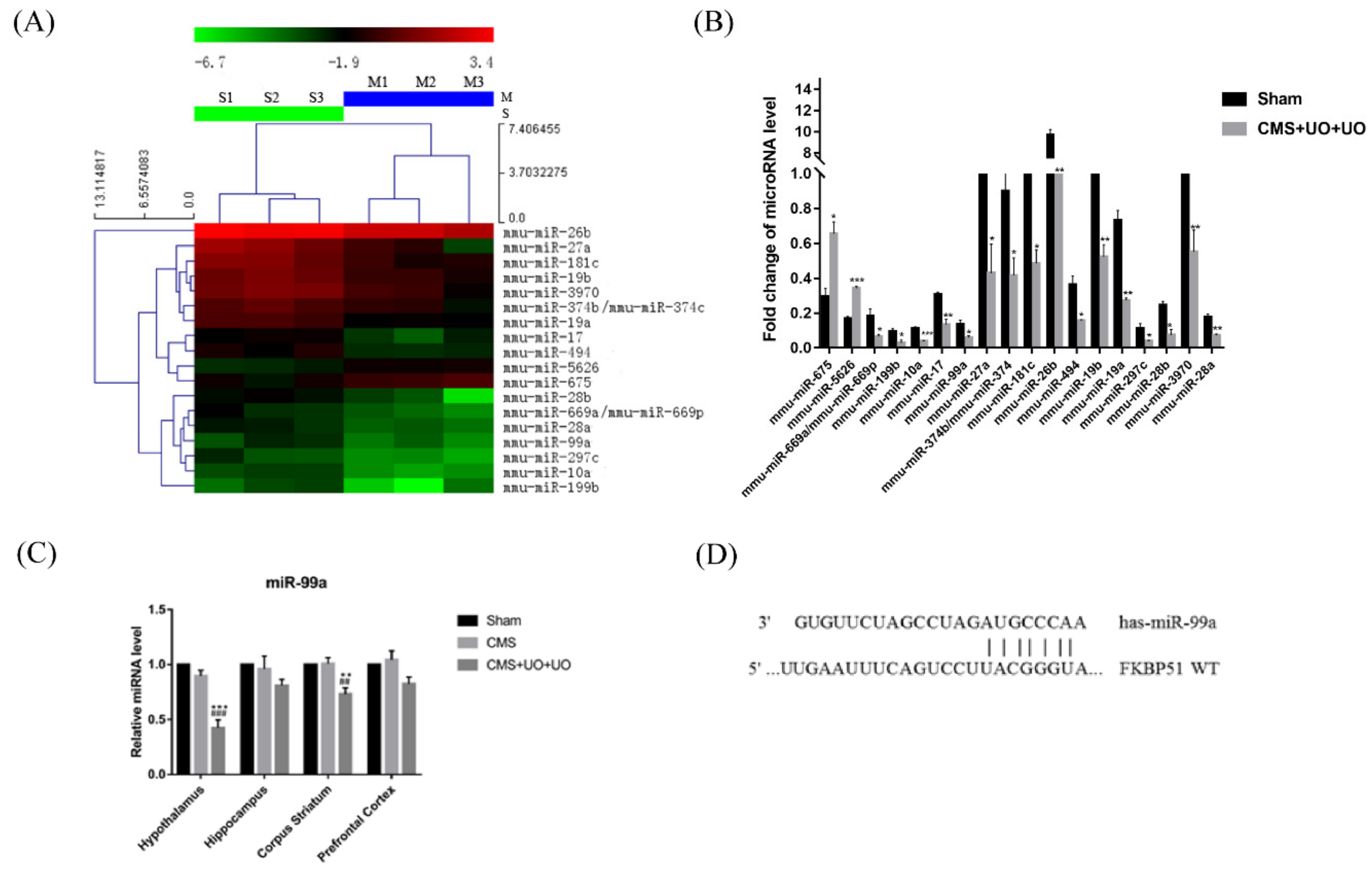
3.2. Downregulation of miR-99a in the Hypothalamus of Peri/Postmenopausal Depressive Mice
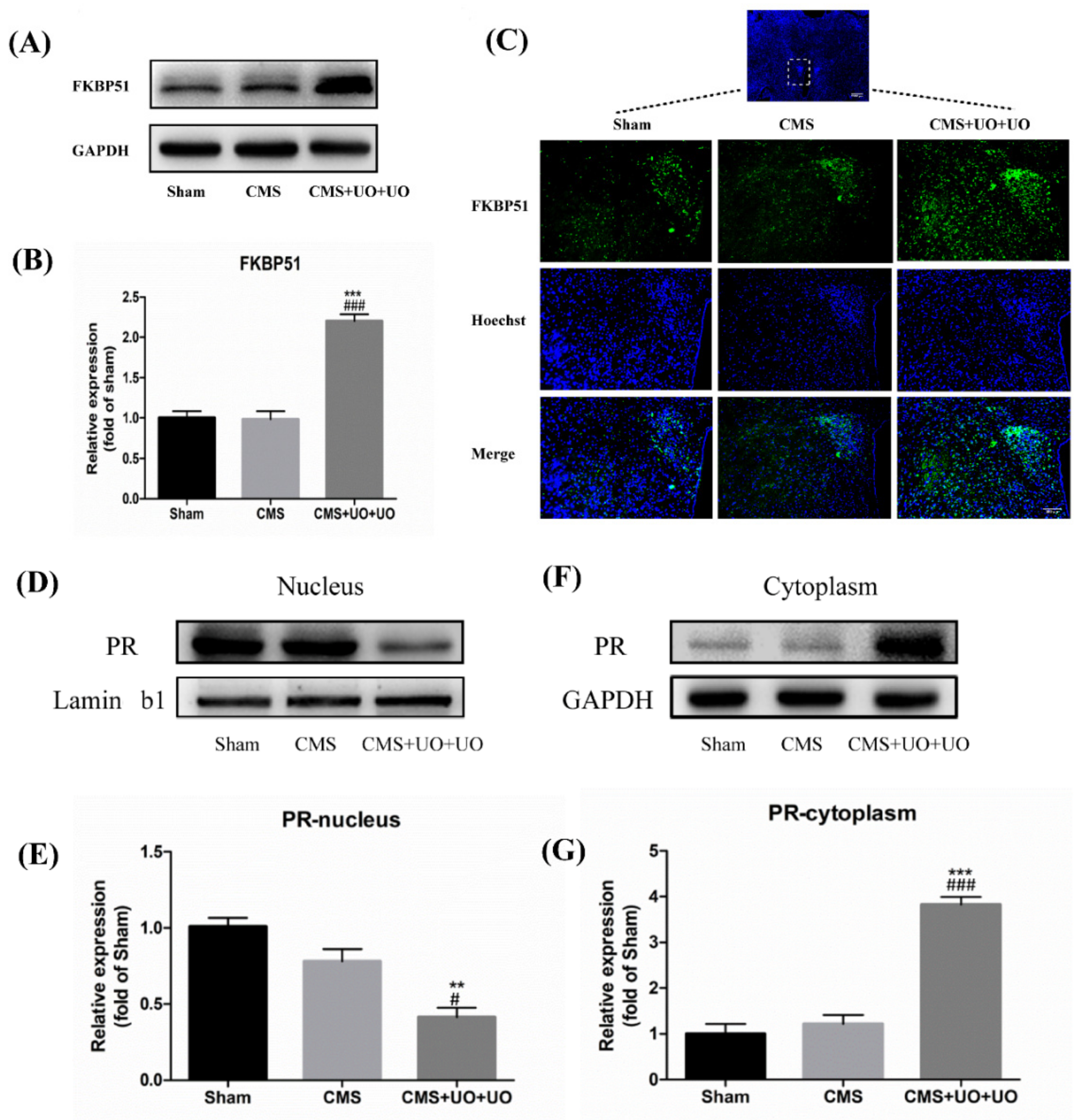
3.3. Hypothalamic FKBP51 Was Upregulated and Progesterone (PR) Nuclear Translocation Decreased in the Peri/Postmenopausal Depression Mice
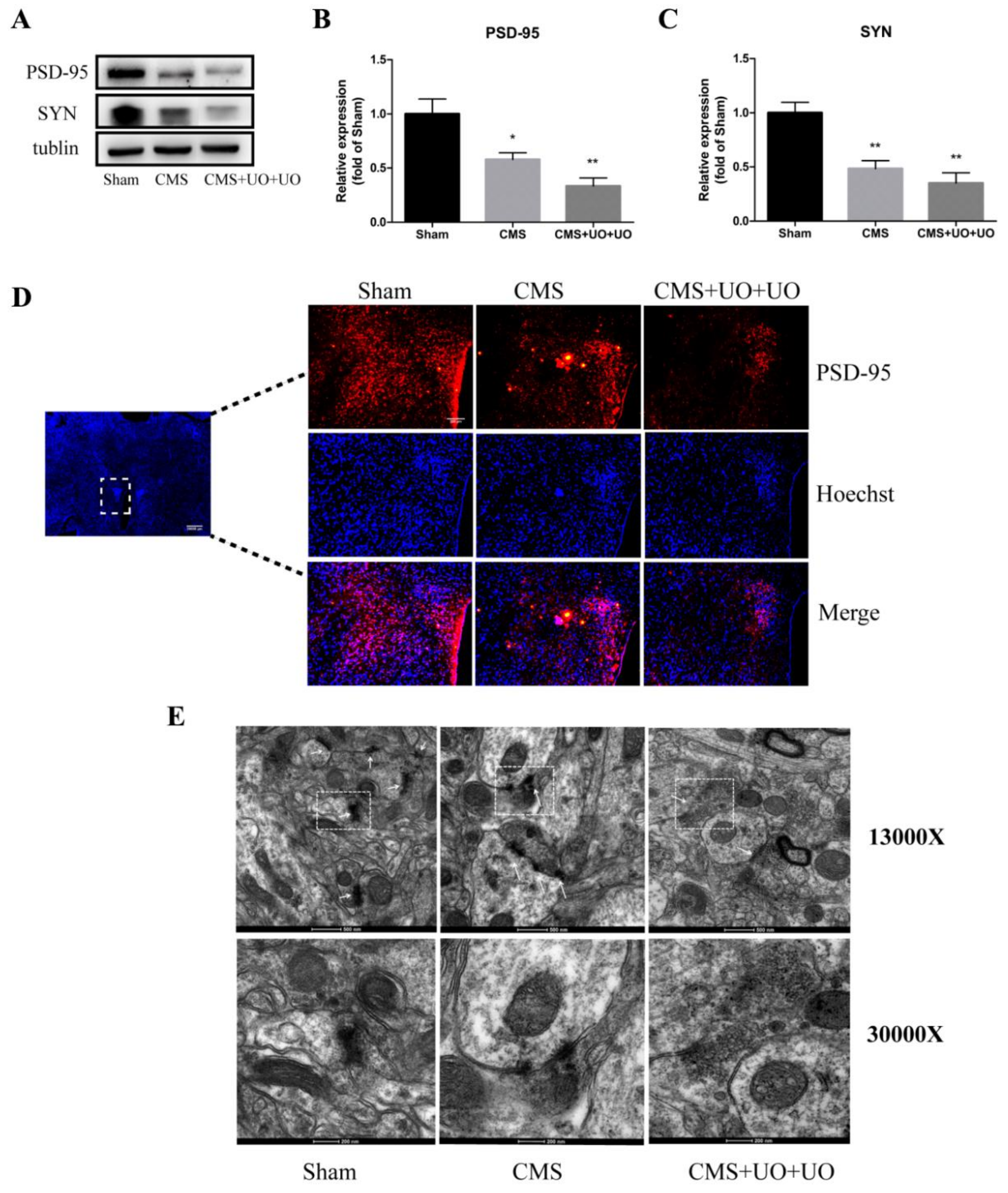
3.4. Hypothalamic Synaptic Plasticity Injury Was More Severe in the Peri/Postmenopausal Depressive Mice
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saad, M.A.; El-Sahar, A.E.; Sayed, R.H.; Elbaz, E.M.; Helmy, H.S.; Senousy, M.A. Venlafaxine Mitigates Depressive-Like Behavior in Ovariectomized Rats by Activating the EPO/EPOR/JAK2 Signaling Pathway and Increasing the Serum Estradiol Level. Neurotherapeutics 2018, 16, 404–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, K.E.; Dennerstein, L.; Finch, S.; Szoeke, C.E. Impact of menopausal status on negative mood and depressive symptoms in a longitudinal sample spanning 20 years. Menopause 2017, 24, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.N. Depression in Peri- and Postmenopausal Women: Prevalence, Pathophysiology and Pharmacological Management. Drugs Aging 2013, 30, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Fattah, A. Effect of Phytoestrogen on Depression and Anxiety in Menopausal Women: A Systematic Review. J. Menopausal Med. 2017, 23, 160–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, S.; Jing, L.; Li, Y.; Huang, J.H.; Wang, F. Stress Induced Hormone and Neuromodulator Changes in Menopausal Depressive Rats. Front. Psychol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.T.; Maki, P.M.; McDermott, M.P. Cognition and mood in perimenopause: A systematic review and meta-analysis. J. Steroid Biochem. Mol. Biol 2014, 142, 90–98. [Google Scholar] [CrossRef]
- Rubinow, D.R.; Johnson, S.L.; Schmidt, P.J.; Girdler, S.; Gaynes, B. Efficacy of estradiol in perimenopausal depression: So much promise and so few answers. Depress. Anxiety 2015, 32, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Borrow, A.P.; Cameron, N.M. Estrogenic mediation of serotonergic and neurotrophic systems: Implications for female mood disorders. Prog. Neuropsychopharmacol. Boil. Psychiatry 2014, 54, 13–25. [Google Scholar] [CrossRef]
- Hou, X.; Adeosun, S.O.; Zhao, X.; Hill, R.; Zheng, B.; Reddy, R.; Su, X.; Meyer, J.; Mosley, T.; Wang, J.M. ERbeta agonist alters RNA splicing factor expression and has a longer window of antidepressant effectiveness than estradiol after long-term ovariectomy. J. Psychiatry Neurosci. JPN 2019, 44, 19–31. [Google Scholar] [CrossRef]
- Maki, P.M.; Kornstein, S.G.; Joffe, H.; Bromberger, J.T.; Freeman, E.W.; Athappilly, G.; Bobo, W.V.; Rubin, L.H.; Koleva, H.K.; Cohen, L.S.; et al. Guidelines for the Evaluation and Treatment of Perimenopausal Depression: Summary and Recommendations. J. Women’s Heal. 2018, 10, 1069–1085. [Google Scholar] [CrossRef]
- Guo, M.; Li, C.; Lei, Y.; Xu, S.; Zhao, D.; Lu, X.Y. Role of the adipose PPARgamma-adiponectin axis in susceptibility to stress and depression/anxiety-related behaviors. Mol. Psychiatry 2017, 22, 1056–1068. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Aghajanian, G.K.; Sanacora, G.; Krystal, J.H. Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat. Med. 2016, 22, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Wohleb, E.S.; Franklin, T.; Iwata, M.; Duman, R.S. Integrating neuroimmune systems in the neurobiology of depression. Nat. Rev. Neurosci. 2016, 17, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Voleti, B.; Hajszan, T.; Rajkowska, G.; Stockmeier, C.; Licznerski, P.; Lepack, A.; Majik, M.S.; Jeong, L.S.; Banasr, M.; et al. Decreased Expression of Synapse-Related Genes and Loss of Synapses in Major Depressive Disorder. Nat. Med. 2012, 18, 1413–1417. [Google Scholar] [CrossRef] [PubMed]
- Marsden, W.N. Synaptic plasticity in depression: Molecular, cellular and functional correlates. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 43, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Tomasetti, C.; Iasevoli, F.; Buonaguro, E.; De Berardis, D.; Fornaro, M.; Fiengo, A.; Martinotti, G.; Orsolini, L.; Valchera, A.; Di Giannantonio, M.; et al. Treating the Synapse in Major Psychiatric Disorders: The Role of Postsynaptic Density Network in Dopamine-Glutamate Interplay and Psychopharmacologic Drugs Molecular Actions. Int. J. Mol. Sci. 2017, 18, 135. [Google Scholar] [CrossRef] [PubMed]
- Ogundele, O.M.; Ebenezer, P.J.; Lee, C.C.; Francis, J. Stress-altered synaptic plasticity and DAMP signaling in the hippocampus-PFC axis; elucidating the significance of IGF-1/IGF-1R/CaMKIIalpha expression in neural changes associated with a prolonged exposure therapy. Neuroscience 2017, 353, 147–165. [Google Scholar] [CrossRef]
- Caruana, D.A.; Alexander, G.M.; Dudek, S.M. New insights into the regulation of synaptic plasticity from an unexpected place: Hippocampal area CA2. Learn. Mem. 2012, 19, 391–400. [Google Scholar] [CrossRef] [Green Version]
- Bains, J.S.; Cusulin, J.I.W.; Inoue, W. Stress-related synaptic plasticity in the hypothalamus. Nat. Rev. Neurosci. 2015, 16, 377–388. [Google Scholar] [CrossRef]
- Baudry, A.; Mouillet-Richard, S.; Schneider, B.; Launay, J.-M.; Kellermann, O. MiR-16 Targets the Serotonin Transporter: A New Facet for Adaptive Responses to Antidepressants. Science 2010, 329, 1537–1541. [Google Scholar] [CrossRef] [Green Version]
- Deng, Z.F.; Zheng, H.L.; Chen, J.G.; Luo, Y.; Xu, J.F.; Zhao, G.; Lu, J.J.; Li, H.H.; Gao, S.Q.; Zhang, D.Z.; et al. miR-214-3p Targets beta-Catenin to Regulate Depressive-like Behaviors Induced by Chronic Social Defeat Stress in Mice. Cereb. Cortex 2018, 4, 1509–1519. [Google Scholar]
- Mendell, J.T.; Olson, E.N. MicroRNAs in stress signaling and human disease. Cell 2012, 148, 1172–1187. [Google Scholar] [CrossRef]
- Fiore, R.; Rajman, M.; Schwale, C.; Bicker, S.; Antoniou, A.; Bruehl, C.; Draguhn, A.; Schratt, G. MiR-134-dependent regulation of Pumilio-2 is necessary for homeostatic synaptic depression. EMBO J. 2014, 33, 2231–2246. [Google Scholar] [CrossRef]
- Rajman, M.; Metge, F.; Fiore, R.; Khudayberdiev, S.; Aksoy-Aksel, A.; Bicker, S.; Reschke, C.R.; Raoof, R.; Brennan, G.P.; Delanty, N.; et al. A microRNA-129-5p/Rbfox crosstalk coordinates homeostatic downscaling of excitatory synapses. EMBO J. 2017, 36, 1770–1787. [Google Scholar] [CrossRef]
- Mannironi, C.; Biundo, A.; Rajendran, S.; De Vito, F.; Saba, L.; Caioli, S.; Zona, C.; Ciotti, T.; Caristi, S.; Perlas, E.; et al. miR-135a Regulates Synaptic Transmission and Anxiety-Like Behavior in Amygdala. Mol. Neurobiol. 2018, 55, 3301–3315. [Google Scholar] [CrossRef]
- Edbauer, D.; Neilson, J.R.; Foster, K.A.; Wang, C.-F.; Seeburg, D.P.; Batterton, M.N.; Tada, T.; Dolan, B.M.; Sharp, P.A.; Sheng, M. Regulation of Synaptic Structure and Function by FMRP-Associated MicroRNAs miR-125b and miR-132. Neuron 2010, 65, 373–384. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J.E.; Lee, P.R.; Chen, S.; Li, W.; Fields, R.D. MicroRNA regulation of homeostatic synaptic plasticity. Proc. Nat. Acad. Sci. USA 2011, 108, 11650–11655. [Google Scholar] [CrossRef] [Green Version]
- Magill, S.T.; Cambronne, X.A.; Luikart, B.W.; Lioy, D.T.; Leighton, B.H.; Westbrook, G.L.; Mandel, G.; Goodman, R.H. microRNA-132 regulates dendritic growth and arborization of newborn neurons in the adult hippocampus. Proc. Nat. Acad. Sci. USA 2010, 107, 20382–20387. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Cao, L.-L.; Yang, D.-D.; Ding, J.-H.; Guo, X.-D.; Xue, T.-F.; Zhao, X.-J.; Sun, X.-L. Establishment and evaluation of a novel mouse model of peri/postmenopausal depression. Heliyon 2019, 5. [Google Scholar] [CrossRef]
- Kleinridders, A.; Cai, W.; Cappellucci, L.; Ghazarian, A.; Collins, W.R.; Vienberg, S.G.; Pothos, E.N.; Kahn, C.R. Insulin resistance in brain alters dopamine turnover and causes behavioral disorders. Proc. Natl. Acad. Sci. USA 2015, 112, 3463–3468. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Yang, Q.; Yang, L.; Li, Y.-J.; Wang, X.-S.; Li, Y.-J.; Dang, R.-L.; Guan, S.-Y.; Guo, Y.-Y.; Sun, T.; et al. CB1 agonism prolongs therapeutic window for hormone replacement in ovariectomized mice. J. Clin. Investig. 2019, 129, 2333–2350. [Google Scholar] [CrossRef]
- Xie, J.; Cao, Y. Expression of TGF-beta1 and miR-99a in serum of patients with early spontaneous abortion and correlation with hormone levels during pregnancy. Exp. Ther. Med. 2019, 17, 4593–4597. [Google Scholar]
- Li, X.-J.; Luo, X.-Q.; Han, B.-W.; Duan, F.-T.; Wei, P.-P.; Chen, Y.-Q. MicroRNA-100/99a, deregulated in acute lymphoblastic leukaemia, suppress proliferation and promote apoptosis by regulating the FKBP51 and IGF1R/mTOR signalling pathways. Br. J. Cancer 2013, 109, 2189–2198. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, A.; Periyasamy, S.; Wolf, I.M.; Hinds, T.D.; Yong, W.; Shou, W.; Sanchez, E.R. Control of Glucocorticoid and Progesterone Receptor Subcellular Localization by the Ligand-binding Domain is Mediated by Distinct Interactions with Tetratricopeptide Repeat Proteins. Biochem. 2008, 47, 10471–10480. [Google Scholar] [CrossRef]
- Acharya, K.D.; Nettles, S.A.; Sellers, K.J.; Im, D.D.; Harling, M.; Pattanayak, C.; Vardar-Ulu, D.; Lichti, C.F.; Huang, S.; Edwards, D.P.; et al. The Progestin Receptor Interactome in the Female Mouse Hypothalamus: Interactions with Synaptic Proteins Are Isoform Specific and Ligand Dependent. eNeuro 2017, 4. [Google Scholar] [CrossRef]
- De Kruif, M.; Spijker, A.T.; Molendijk, M.L. Depression during the perimenopause: A meta-analysis. J. Affect. Disord. 2016, 206, 174–180. [Google Scholar] [CrossRef]
- Hoyt, L.T.; Falconi, A.M. Puberty and perimenopause: Reproductive transitions and their implications for women’s health. Soc. Sci. Med. 2015, 132, 103–112. [Google Scholar] [CrossRef]
- Chhibber, A.; Woody, S.K.; Rumi, M.K.; Soares, M.J.; Zhao, L. Estrogen receptor beta deficiency impairs BDNF-5-HT2A signaling in the hippocampus of female brain: A possible mechanism for menopausal depression. Psychoneuroendocrinology 2017, 82, 107–116. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Zhang, L.; Cao, L.-L.; Qi, J.; Li, P.; Wang, X.-P.; Sun, X.-L. MicroRNA-99a is a Potential Target for Regulating Hypothalamic Synaptic Plasticity in the Peri/Postmenopausal Depression Model. Cells 2019, 8, 1081. https://doi.org/10.3390/cells8091081
Yang J, Zhang L, Cao L-L, Qi J, Li P, Wang X-P, Sun X-L. MicroRNA-99a is a Potential Target for Regulating Hypothalamic Synaptic Plasticity in the Peri/Postmenopausal Depression Model. Cells. 2019; 8(9):1081. https://doi.org/10.3390/cells8091081
Chicago/Turabian StyleYang, Jin, Ling Zhang, Lu-Lu Cao, Jun Qi, Ping Li, Xi-Peng Wang, and Xiu-Lan Sun. 2019. "MicroRNA-99a is a Potential Target for Regulating Hypothalamic Synaptic Plasticity in the Peri/Postmenopausal Depression Model" Cells 8, no. 9: 1081. https://doi.org/10.3390/cells8091081
APA StyleYang, J., Zhang, L., Cao, L.-L., Qi, J., Li, P., Wang, X.-P., & Sun, X.-L. (2019). MicroRNA-99a is a Potential Target for Regulating Hypothalamic Synaptic Plasticity in the Peri/Postmenopausal Depression Model. Cells, 8(9), 1081. https://doi.org/10.3390/cells8091081




