Gap Junction Intercellular Communication in the Carcinogenesis Hallmarks: Is This a Phenomenon or Epiphenomenon?
Abstract
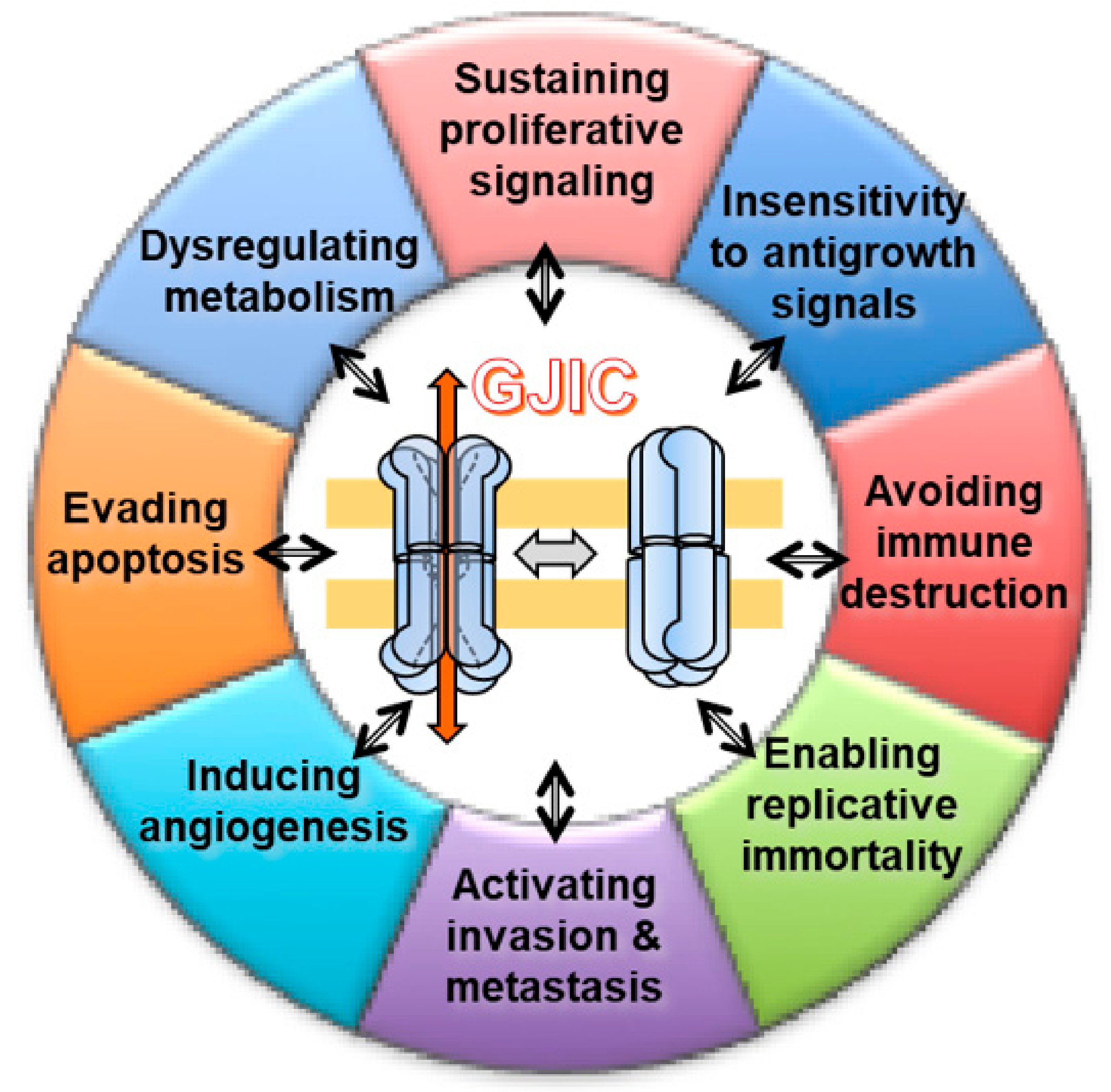
:1. Introduction
- Hallmarks of cancer are: “acquired functional capabilities that allow cancer cells to survive, proliferate, and disseminate; these functions are acquired in different tumor types via distinct mechanisms and at various times during the course of multistep tumorigenesis” [19];
- GJIC is the major mechanism used by biological systems enabling cells to work in an integrate way;
- Epiphenomenon is a phenomenon that occurs contemporary to another but is not related to it [20].
Gap Junctions, Hemichannels, and Connexins
2. GJ, GJIC, and Cancer
Carcinogenesis Models and Cancer Hallmarks
- (1)
- Self-sufficiency in growth signals (later renamed proliferative signaling)—i.e., cancer cells grow at a seemingly unlimited rate.
- (2)
- Insensitivity to antigrowth signals (evading growth suppressors)—i.e., cancer cells are not subject to antigrowth signals nor withdrawal of normal growth signals.
- (3)
- Evading apoptosis (resisting cell death)—i.e., cancer cells avoid the usual process, whereby abnormal or redundant cells trigger internal self-destroying (as opposed to cell death) mechanisms.
- (4)
- Limitless replicative potential (enabling replicative immortality)—i.e., cancer cells do not senesce (or age) and die after a limited number of cell divisions.
- (5)
- Sustained angiogenesis (inducing angiogenesis)—i.e., cancer cells elicit new blood vessels to sustain growth.
- (6)
- Tissue invasion and metastasis (activating invasion and metastasis)—i.e., in situ or non-invasive cancers grow into pre-existing spaces, but invasive tumors must create a space to expand into normal tissue.
3. GJIC and Hallmarks of Cancer
3.1. “Insensitivity to Antigrowth Signals” and “Sustaining Proliferative Signaling”
3.2. Dysregulating Metabolism
3.3. Evading Apoptosis
3.4. Enabling Replicative Immortality
3.5. Inducing Angiogenesis
3.6. Activating Invasion and Metastasis
3.7. Avoiding Immune Destruction
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackadar, C.B. Historical review of the causes of cancer. World J. Clin. Oncol. 2016, 7, 54–86. [Google Scholar] [CrossRef] [PubMed]
- Garber, J.E.; Offit, K. Hereditary Cancer Predisposition Syndromes. J. Clin. Oncol. 2005, 23, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Tomasetti, C.; Vogelstein, B. Cancer etiology: Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 2015, 347, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Powers, S.; Zhu, W.; Hannun, Y.A. Substantial contribution of extrinsic risk factors to cancer development. Nature 2016, 529, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Zefferino, R.; Piccoli, C.; Ricciardi, N.; Scrima, R.; Capitanio, N. Possible Mechanisms of Mercury Toxicity and Cancer Promotion: Involvement of Gap Junction Intercellular Communications and Inflammatory Cytokines. Oxid. Med. Cell. Longev. 2017, 2017, 7028583. [Google Scholar] [CrossRef] [PubMed]
- Trosko, E.J.; Ruch, R.J. Cell-cell communication in carcinogenesis. Front. Biosci. 1998, 3, d208–d236. [Google Scholar] [CrossRef]
- Mesnil, M.; Crespin, S.; Avanzo, J.L.; Zaidandagli, M.L. Defective gap junctional intercellular communication in the carcinogenic process. Biochim. Biophys. Acta Biomembr. 2005, 1719, 125–145. [Google Scholar] [CrossRef] [Green Version]
- Trosko, J.E.; Upham, B.L. A paradigm shift is required for the risk assessment of potential human health after exposure to low level chemical exposures: A response to the toxicity testing in the 21st century report. Int. J. Toxicol. 2010, 29, 344–357. [Google Scholar] [CrossRef]
- Trosko, J.E.; Chang, C.C. Mechanism of up-regulated gap junctional intercellular communication during chemoprevention and chemotherapy of cancer. Mutat. Res. 2001, 480, 219–229. [Google Scholar] [CrossRef]
- Bursch, W.; Oberhammer, F.; Schulte-Hermann, R. Cell death by apoptosis and its protective role against disease. Trends Pharmacol. Sci. 1992, 13, 245–251. [Google Scholar] [CrossRef]
- Wilson, M.; Close, T.W.; Trosko, J.E. Cell Population Dynamics (Apoptosis, Mitosis, and Cell–Cell Communication) during Disruption of Homeostasis. Exp. Cell Res. 2000, 254, 257–268. [Google Scholar] [CrossRef]
- Yotti, L.; Chang, C.; Trosko, J. Elimination of metabolic cooperation in Chinese hamster cells by a tumor promoter. Science 1979, 206, 1089–1091. [Google Scholar] [CrossRef] [PubMed]
- Trosko, J.E.; Chang, C.C.; Medcalf, A. Mechanisms of Tumor Promotion: Potential Role of Intercellular Communication. Cancer Investig. 1983, 1, 511–526. [Google Scholar] [CrossRef]
- Trosko, J.E. Commentary: Is the concept of “tumor promotion” a useful paradigm? Mol. Carcinog. 2001, 30, 131–137. [Google Scholar] [CrossRef]
- Trosko, J.E.; Goodman, J.I. Intercellular communication may facilitate apoptosis: Implications for tumor promotion. Mol. Carcinog. 1994, 11, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Trosko, J.E. Gap Junctional Intercellular Communication as a Biological “Rosetta Stone” in Understanding, in a Systems Biological Manner, Stem Cell Behavior, Mechanisms of Epigenetic Toxicology, Chemoprevention and Chemotherapy. J. Membr. Biol. 2007, 218, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Trosko, J.E.; Tai, M.-H. Adult Stem Cell Theory of the Multi-Stage, Multi-Mechanism Theory of Carcinogenesis: Role of Inflammation on the Promotion of Initiated Stem Cells. Contrib. Microbiol. 2006, 13, 45–65. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Simpson, J.A.; Weiner, E.S.C. Oxford English Dictionary; Murray, J., Ed.; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Sáez, J.C.; Retamal, M.A.; Basilio, D.; Bukauskas, F.F.; Bennett, M.V. Connexin-based gap junction hemichannels: Gating mechanisms. Biochim. Biophys. Acta BBA Biomembr. 2005, 1711, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Saez, J.C.; Berthoud, V.M.; Branes, M.C.; Martinez, A.D.; Beyer, E.C. Plasma membrane channels formed by connexins: Their regulation and functions. Physiol. Rev. 2003, 83, 1359–1400. [Google Scholar] [CrossRef] [PubMed]
- Valiunas, V.; Polosina, Y.Y.; Miller, H.; Potapova, I.A.; Valiuniene, L.; Doronin, S.; Mathias, R.T.; Robinson, R.B.; Rosen, M.R.; Cohen, I.S.; et al. Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. J. Physiol. 2005, 568, 459–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Söhl, G.; Willecke, K. An Update on Connexin Genes and their Nomenclature in Mouse and Man. Cell Commun. Adhes. 2003, 10, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Aasen, T.; Mesnil, M.; Naus, C.C.; Lampe, P.D.; Laird, D.W. Gap Junctions and Cancer: Communicating for 50 Years. Nat. Rev. Cancer 2016, 16, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Panchina, Y.; Kelmanson, I.; Matz, M.; Lukyanov, K.; Usman, N.; Lukyanov, S. A ubiquitous family of putative gap junction molecules. Curr. Biol. 2000, 10, R473–R474. [Google Scholar] [CrossRef] [Green Version]
- Dahl, G.; Locovei, S. Pannexin: To gap or not to gap, is that a question? IUBMB Life 2006, 58, 409–419. [Google Scholar] [CrossRef]
- Maeda, S.; Nakagawa, S.; Suga, M.; Yamashita, E.; Oshima, A.; Fujiyoshi, Y.; Tsukihara, T. Structure of the connexin 26 gap junction channel at 3.5 Å resolution. Nature 2009, 458, 597–602. [Google Scholar] [CrossRef]
- Myers, J.B.; Haddad, B.G.; O’Neill, S.E.; Chorev, D.S.; Yoshioka, C.C.; Robinson, C.V.; Zuckerman, D.M.; Reichow, S.L. Structure of native lens connexin-46/50 intercellular channels by CryoEM. Nature 2018, 564, 372–377. [Google Scholar] [CrossRef]
- Oshima, A. Structure and closure of connexin gap junction channels. FEBS Lett. 2014, 588, 1230–1237. [Google Scholar] [CrossRef] [Green Version]
- Revel, J.P.; Karnovsky, M. Hexagonal Array of Subunits In Intercellular Junctions of the Mouse Heart And Liver. J. Cell Biol. 1967, 33, C7–C12. [Google Scholar] [CrossRef]
- Fujimoto, K. Freeze-fracture replica electron microscopy combined with SDS digestion for cytochemical labeling of integral membrane proteins. Application to the immunogold labeling of intercellular junctional complexes. J. Cell Sci. 1995, 108, 3443–3449. [Google Scholar] [PubMed]
- Meier, C.; Beckmann, A. Freeze fracture: New avenues for the ultrastructural analysis of cells in vitro. Histochem. Cell Biol. 2018, 149, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, A.; Hainz, N.; Tschernig, T.; Meier, C. Facets of Communication: Gap Junction Ultrastructure and Function in Cancer Stem Cells and Tumor Cells. Cancers 2019, 11, 288. [Google Scholar] [CrossRef] [PubMed]
- Solan, J.L.; Lampe, P.D. Connexin phosphorylation as a regulatory event linked to gap junction channel assembly. Biochim. Biophys. Acta Biomembr. 2005, 1711, 154–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solan, J.L.; Lampe, P.D. Spatio-temporal regulation of connexin43 phosphorylation and gap junction dynamics. Biochim. Biophys. Acta Biomembr. 2018, 1860, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Sorgen, P.L.; Trease, A.J.; Spagnol, G.; Delmar, M.; Nielsen, M.S. Protein–Protein Interactions with Connexin 43: Regulation and Function. Int. J. Mol. Sci. 2018, 19, 1428. [Google Scholar] [CrossRef] [PubMed]
- Leithe, E.; Mesnil, M.; Aasen, T. The connexin 43 C-terminus: A tail of many tales. Biochim. Biophys. Acta Biomembr. 2018, 1860, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Meunier, C.; Wang, N.; Yi, C.; Dallerac, G.; Ezan, P.; Koulakoff, A.; Leybaert, L.; Giaume, C. Contribution of Astroglial Cx43 Hemichannels to the Modulation of Glutamatergic Currents by D-Serine in the Mouse Prefrontal Cortex. J. Neurosci. 2017, 37, 9064–9075. [Google Scholar] [CrossRef] [Green Version]
- Bol, M.; Wang, N.; De Bock, M.; Wacquier, B.; Decrock, E.; Gadicherla, A.; Decaluwe, K.; Vanheel, B.; van Rijen, H.V.; Krysko, D.V.; et al. At the cross-point of connexins, calcium, and ATP: Blocking hemichannels inhibits vasoconstriction of rat small mesenteric arteries. Cardiovasc. Res. 2017, 113, 195–206. [Google Scholar] [CrossRef]
- Orellana, J.A.; Saez, P.J.; Cortes-Campos, C.; Elizondo, R.J.; Shoji, K.F.; Contreras-Duarte, S.; Figueroa, V.; Velarde, V.; Jiang, J.X.; Nualart, F.; et al. Glucose increases intracellular free Ca2+ in tanycytes via ATP released through connexin 43 hemichannels. Glia 2012, 60, 53–68. [Google Scholar] [CrossRef]
- Saez, J.C.; Contreras-Duarte, S.; Gomez, G.I.; Labra, V.C.; Santibanez, C.A.; Gajardo-Gomez, R.; Avendano, B.C.; Diaz, E.F.; Montero, T.D.; Velarde, V.; et al. Connexin 43 Hemichannel Activity Promoted by Pro-Inflammatory Cytokines and High Glucose Alters Endothelial Cell Function. Front. Immunol. 2018, 9, 1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinyuk, M.; Mulkearns-Hubert, E.E.; Reizes, O.; Lathia, J. Cancer Connectors: Connexins, Gap Junctions, and Communication. Front. Oncol. 2018, 8, 646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinken, M.; Decrock, E.; Leybaert, L.; Bultynck, G.; Himpens, B.; Vanhaecke, T.; Rogiers, V. Non-channel functions of connexins in cell growth and cell death. Biochim. Biophys. Acta Biomembr. 2012, 1818, 2002–2008. [Google Scholar] [CrossRef] [PubMed]
- Loewenstein, W.R.; Kanno, Y. Intercellular Communication and the Control of Tissue Growth: Lack of Communication between Cancer Cells. Nature 1966, 209, 1248–1249. [Google Scholar] [CrossRef] [PubMed]
- Klaunig, J.E.; Ruch, R.J.; Weghorst, C.M. Comparative effects of phenobarbital, DDT, and lindane on mouse hepatocyte gap junctional intercellular communication. Toxicol. Appl. Pharmacol. 1990, 102, 553–563. [Google Scholar] [CrossRef]
- Klann, R.C.; Fitzgerald, D.J.; Piccoli, C.; Slaga, T.J.; Yamasaki, H. Gap-junctional intercellular communication in epidermal cell lines from selected stages of SENCAR mouse skin carcinogenesis. Cancer Res. 1989, 49, 699–705. [Google Scholar] [PubMed]
- Yamasaki, H.; Krutovskikh, V.; Mesnil, M.; Tanaka, T.; Zaidan-Dagli, M.L.; Omori, Y. Role of connexin (gap junction) genes in cell growth control and carcinogenesis. Comptes Rendus de l’Académie des Sciences Series III Sciences de la Vie 1999, 322, 151–159. [Google Scholar] [CrossRef]
- Babica, P.; Čtveráčková, L.; Lenčešová, Z.; Trosko, J.E.; Upham, B.L. Chemopreventive agents attenuate rapid inhibition of gap junctional intercellular communication induced by environmental toxicants. Nutr. Cancer 2016, 68, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Cronier, L.; Crespin, S.; Strale, P.O.; Defamie, N.; Mesnil, M. Gap Junctions and Cancer: New Functions for an Old Story. Antioxid. Redox Signal. 2009, 11, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Holder, J.W.; Elmore, E.; Barrett, J.C. Gap junction function and cancer. Cancer Res. 1993, 53, 3475–3485. [Google Scholar]
- Kar, R.; Batra, N.; Riquelme, M.A.; Jiang, J.X. Biological Role of Connexin Intercellular Channels and Hemichannels. Arch. Biochem. Biophys. 2012, 524, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Osswald, M.; Jung, E.; Sahm, F.; Solecki, G.; Venkataramani, V.; Blaes, J.; Weil, S.; Horstmann, H.; Wiestler, B.; Syed, M.; et al. Brain tumour cells interconnect to a functional and resistant network. Nature 2015, 528, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Boire, A.; Jin, X.; Valiente, M.; Er, E.E.; López-Soto, A.; Jacob, L.S.; Patwa, R.; Shah, H.; Xu, K.; et al. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 2016, 533, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Ezumi, K.; Yamamoto, H.; Murata, K.; Higashiyama, M.; Damdinsuren, B.; Nakamura, Y.; Kyo, N.; Okami, J.; Ngan, C.Y.; Takemasa, I.; et al. Aberrant Expression of Connexin 26 Is Associated with Lung Metastasis of Colorectal Cancer. Clin. Cancer Res. 2008, 14, 677–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teleki, I.; Szász, A.M.; Maros, M.E.; Győrffy, B.; Kulka, J.; Meggyeshazi, N.; Kiszner, G.; Balla, P.; Samu, A.; Krenacs, T. Correlations of Differentially Expressed Gap Junction Connexins Cx26, Cx30, Cx32, Cx43 and Cx46 with Breast Cancer Progression and Prognosis. PLoS ONE 2014, 9, e112541. [Google Scholar] [CrossRef] [PubMed]
- Sirnes, S.; Bruun, J.; Kolberg, M.; Kjenseth, A.; Lind, G.E.; Svindland, A.; Brech, A.; Nesbakken, A.; Lothe, R.A.; Leithe, E.; et al. Connexin43 acts as a colorectal cancer tumor suppressor and predicts disease outcome. Int. J. Cancer 2012, 131, 570–581. [Google Scholar] [CrossRef]
- Teleki, I.; Krenacs, T.A.; Szasz, M.; Kulka, J.; Wichmann, B.; Leo, C.; Papassotiropoulos, B.; Riemenschnitter, C.; Moch, H.; Varga, Z. The potential prognostic value of connexin 26 and 46 expression in neoadjuvant-treated breast cancer. BMC Cancer 2013, 13, 50. [Google Scholar] [CrossRef]
- Naoi, Y.; Miyoshi, Y.; Taguchi, T.; Kim, S.J.; Arai, T.; Tamaki, Y.; Noguchi, S. Connexin26 expression is associated with lymphatic vessel invasion and poor prognosis in human breast cancer. Breast Cancer Res. Treat. 2007, 106, 11–17. [Google Scholar] [CrossRef]
- Graham, S.V.; Jiang, J.X.; Mesnil, M. Connexins and Pannexins: Important Players in Tumorigenesis, Metastasis and Potential Therapeutics. Int. J. Mol. Sci. 2018, 19, 1645. [Google Scholar] [CrossRef]
- Elzarrad, M.K.; Haroon, A.; Willecke, K.; Dobrowolski, R.; Gillespie, M.N.; Al-Mehdi, A.B. Connexin-43 upregulation in micrometastases and tumor vasculature and its role in tumor cell attachment to pulmonary endothelium. BMC Med. 2008, 6, 20. [Google Scholar] [CrossRef]
- Sin, W.C.; Crespin, S.; Mesnil, M. Opposing roles of connexin43 in glioma progression. Biochim. Biophys. Acta Biomembr. 2012, 1818, 2058–2067. [Google Scholar] [CrossRef] [PubMed]
- Spray, D.C.; Hanstein, R.; Lopez-Quintero, S.V.; Stout, R.F., Jr.; Suadicani, S.O.; Thi, M.M. Gap junctions and Bystander Effects: Good Samaritans and executioners. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2013, 2, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Vineis, P.; Schatzkin, A.; Potter, J.D. Models of carcinogenesis: An overview. Carcinogenesis 2010, 31, 1703–1709. [Google Scholar] [CrossRef]
- Goodson, W.H., 3rd; Lowe, L.; Carpenter, D.O.; Gilbertson, M.; Manaf Ali, A.; Lopez de Cerain Salsamendi, A.; Lasfar, A.; Carnero, A.; Azqueta, A.; Amedei, A.; et al. Assessing the carcinogenic potential of low-dose exposures to chemical mixtures in the environment: The challenge ahead. Carcinogenesis 2015, 36, S254–S296. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef]
- Loch-Caruso, R.; Galvez, M.; Brant, K.; Chung, D. Cell and toxicant specific phosphorylation of conexin43: Effects of lindane and TPA on rat myometrial and WB-F344 liver cell gap junctions. Cell Biol. Toxicol. 2004, 20, 147–169. [Google Scholar] [CrossRef]
- Nahta, R.; Al-Mulla, F.; Al-Temaimi, R.; Amedei, A.; Andrade-Vieira, R.; Bay, S.N.; Brown, D.G.; Calaf, G.M.; Castellino, R.C.; Cohen-Solal, K.A.; et al. Mechanisms of environmental chemicals that enable the cancer hallmark of evasion of growth suppression. Carcinogenesis 2015, 36, S2–S18. [Google Scholar] [CrossRef] [Green Version]
- Dairkee, S.H.; Luciani-Torres, M.G.; Moore, D.H.; Goodson, W.H., 3rd. Bisphenol-A-induced inactivation of the p53 axis underlying deregulation of proliferation kinetics, and cell death in non-malignant human breast epithelial cells. Carcinogenesis 2013, 34, 703–712. [Google Scholar] [CrossRef]
- Andersson, H.; Brittebo, E. Proangiogenic effects of environmentally relevant levels of bisphenol A in human primary endothelial cells. Arch. Toxicol. 2012, 86, 465–474. [Google Scholar] [CrossRef]
- Li, M.W.M.; Mruk, D.D.; Lee, W.M.; Cheng, C.Y. Connexin 43 is critical to maintain the homeostasis of the blood–testis barrier via its effects on tight junction reassembly. Proc. Natl. Acad. Sci. USA 2010, 107, 17998–18003. [Google Scholar] [CrossRef] [PubMed]
- Little, J.B.; Nagasawa, H.; De Toledo, S.M.; Azzam, E. Transmission of damage signals from irradiated to nonirradiated cells. Int. Congr. Ser. 2002, 1236, 229–235. [Google Scholar] [CrossRef]
- Warburg, O. The Metabolism of Tumours: Investigations from the Kaiser Wilhelm Institute for Biology, Berlin-Dahlem; Warburg, O., Ed.; Constable & Co. Ltd.: London, UK, 1930. [Google Scholar]
- Aisenberg, A.C. The Glycolysis and Respiration of Tumors; Academic Press: New York, NY, USA, 1961. [Google Scholar]
- Phan, L.M.; Yeung, S.C.J.; Lee, M.H. Cancer metabolic reprogramming: Importance, main features, and potentials for precise targeted anti-cancer therapies. Cancer Biol. Med. 2014, 11, 1–19. [Google Scholar] [PubMed]
- Ippolito, L.; Morandi, A.; Giannoni, E.; Chiarugi, P. Lactate: A Metabolic Driver in the Tumour Landscape. Trends Biochem. Sci. 2019, 44, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Jiang, Y.C.; Sun, C.K.; Chen, Q.M. Role of the tumor microenvironment in tumor progression and the clinical applications (Review). Oncol. Rep. 2016, 35, 2499–2515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, M.; Luo, Y.; Mao, N.; Huang, G.; Teng, C.; Wang, H.; Wu, J.; Liao, X.; Yang, J. Cancer-Associated Fibroblasts Accelerate Malignant Progression of Non-Small Cell Lung Cancer via Connexin 43-Formed Unidirectional Gap Junctional Intercellular Communication. Cell. Physiol. Biochem. 2018, 51, 315–336. [Google Scholar] [CrossRef] [PubMed]
- Kameritsch, P.; Khandoga, N.; Pohl, U.; Pogoda, K. Gap junctional communication promotes apoptosis in a connexin-type-dependent manner. Cell Death Dis. 2013, 4, e584. [Google Scholar] [CrossRef] [PubMed]
- Kanczuga-Koda, L.; Sulkowski, S.; Koda, M.; Skrzydlewska, E.; Sulkowska, M. Connexin 26 correlates with Bcl-xL and Bax proteins expression in colorectal cancer. World J. Gastroenterol. 2005, 11, 1544–1548. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Galluzzi, L.; Kroemer, G. Cell biology. Metabolic control of cell death. Science 2014, 345, 1250256. [Google Scholar] [CrossRef]
- Badrinath, N.; Yoo, S.Y. Mitochondria in cancer: In the aspects of tumorigenesis and targeted therapy. Carcinogenesis 2018, 39, 1419–1430. [Google Scholar] [CrossRef]
- Boengler, K.; Dodoni, G.; Rodriguez-Sinovas, A.; Cabestrero, A.; Ruiz-Meana, M.; Gres, P.; Konietzka, I.; Lopez-Iglesias, C.; Garcia-Dorado, D.; Di Lisa, F.; et al. Connexin 43 in cardiomyocyte mitochondria and its increase by ischemic preconditioning. Cardiovasc. Res. 2005, 67, 234–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boengler, K.; Schulz, R. Connexin 43 and Mitochondria in Cardiovascular Health and Disease. Adv. Exp. Med. Biol. 2017, 982, 227–246. [Google Scholar] [PubMed]
- Ku, W.C.; Cheng, A.J.; Wang, T.C.V. Inhibition of Telomerase Activity by PKC Inhibitors in Human Nasopharyngeal Cancer Cells in Culture. Biochem. Biophys. Res. Commun. 1997, 241, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Joshi, C.N.; Martin, D.N.; Shaver, P.; Madamanchi, C.; Muller-Borer, B.J.; Tulis, D.A. Control of Vascular Smooth Muscle Cell Growth by Connexin 43. Front. Physiol. 2012, 3, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.C.; Wang, S.W.; Hung, H.Y.; Chang, C.C.; Wu, I.C.; Huang, Y.L.; Lin, T.M.; Tsai, J.L.; Chen, A.; Kuo, F.C.; et al. Isolation and characterization of human gastric cell lines with stem cell phenotypes. J. Gastroenterol. Hepatol. 2007, 22, 1460–1468. [Google Scholar] [CrossRef] [PubMed]
- Trosko, J.E.; Chang, C.; Upham, B.L.; Tai, M. Ignored Hallmarks of Carcinogenesis: Stem Cells and Cell-Cell Communication. Ann. N. Y. Acad. Sci. 2004, 1028, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Trosko, J.E. The Role of Stem Cells and Gap Junctional Intercellular Communication in Carcinogenesis. BMB Rep. 2003, 36, 43–48. [Google Scholar] [CrossRef]
- Chang, C.C.; Trosko, E.J.; El-Fouly, M.H.; E Gibson-D’Ambrosio, R.; D’Ambrosio, S.M. Contact insensitivity of a subpopulation of normal human fetal kidney epithelial cells and of human carcinoma cell lines. Cancer Res. 1987, 47, 1634–1645. [Google Scholar]
- Kao, C.Y.; Nomata, K.; Oakley, C.S.; Welsch, C.W.; Chang, C.C. Two types of normal human breast epithelial cells derived from reduction mammoplasty: Phenotypic characterization and response to SV40 transfection. Carcinogenesis 1995, 16, 531–538. [Google Scholar] [CrossRef]
- Matic, M.; Evans, W.H.; Brink, P.R.; Simon, M. Epidermal Stem Cells do not Communicate through Gap Junctions. J. Investig. Dermatol. 2002, 118, 110–116. [Google Scholar] [CrossRef]
- Matic, M.; Petrov, I.N.; Chen, S.; Wang, C.; Wolosin, J.M.; Dimitrijevich, S.D. Stem cells of the corneal epithelium lack connexins and metabolite transfer capacity. Differentiation 1997, 61, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Kang, K.S.; Morita, I.; Trosko, J.E.; Chang, C.C. High susceptibility of a human breast epithelial cell type with stem cell characteristics to telomerase activation and immortalization. Cancer Res. 1999, 59, 6118–6123. [Google Scholar] [PubMed]
- Dowling-Warriner, C.V.; Trosko, J.E. Induction of gap junctional intercellular communication, connexin43 expression, and subsequent differentiation in human fetal neuronal cells by stimulation of the cyclic AMP pathway. Neuroscience 2000, 95, 859–868. [Google Scholar] [CrossRef]
- Batlle, E.; Clevers, H. Cancer stem cells revisited. Nat. Med. 2017, 23, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Colak, S.; Medema, J.P. Cancer stem cells—Important players in tumor therapy resistance. FEBS J. 2014, 281, 4779–4791. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Hawkins, C.; Clarke, I.D.; Squire, J.A.; Bayani, J.; Hide, T.; Henkelman, R.M.; Cusimano, M.D.; Dirks, P.B. Identification of human brain tumour initiating cells. Nature 2004, 432, 396–401. [Google Scholar] [CrossRef]
- Dawood, S.; Austin, L.; Cristofanilli, M. Cancer stem cells: Implications for cancer therapy. Oncology (Williston Park. N.Y.) 2014, 28, 1101–1107. [Google Scholar]
- Evans, W.H.; Martin, P.E.M. Gap junctions: Structure and function (Review). Mol. Membr. Biol. 2002, 19, 121–136. [Google Scholar] [CrossRef]
- Foss, B.; Hervig, T.; Bruserud, Ø. Connexins Are Active Participants of Hematopoietic Stem Cell Regulation. Stem Cells Dev. 2009, 18, 807–812. [Google Scholar] [CrossRef]
- Jäderstad, J.; Jäderstad, L.M.; Li, J.; Chintawar, S.; Salto, C.; Pandolfo, M.; Ourednik, V.; Teng, Y.D.; Sidman, R.L.; Arenas, E.; et al. Communication via gap junctions underlies early functional and beneficial interactions between grafted neural stem cells and the host. Proc. Natl. Acad. Sci. USA 2010, 107, 5184–5189. [Google Scholar] [CrossRef] [Green Version]
- Badri, L.; Walker, N.M.; Ohtsuka, T.; Wang, Z.; Delmar, M.; Flint, A.; Peters-Golden, M.; Toews, G.B.; Pinsky, D.J.; Krebsbach, P.H.; et al. Epithelial Interactions and Local Engraftment of Lung-Resident Mesenchymal Stem Cells. Am. J. Respir. Cell Mol. Biol. 2011, 45, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Tazuke, S.I.; Schulz, C.; Gilboa, L.; Fogarty, M.; Mahowald, A.P.; Guichet, A.; Ephrussi, A.; Wood, C.G.; Lehmann, R.; Fuller, M.T. A germline-specific gap junction protein required for survival of differentiating early germ cells. Development 2002, 129, 2529–2539. [Google Scholar] [PubMed]
- Todorova, M.G.; Soria, B.; Quesada, I. Gap junctional intercellular communication is required to maintain embryonic stem cells in a non-differentiated and proliferative state. J. Cell. Physiol. 2008, 214, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.C.; Pebay, A. Study of gap junctions in human embryonic stem cells. Methods Mol. Biol. 2010, 584, 211–228. [Google Scholar] [PubMed]
- Patel, J.S.; Hu, M.; Sinha, G.; Walker, N.D.; Sherman, L.S.; Gallagher, A.; Rameshwar, P. Non-coding RNA as mediators in microenvironment–breast cancer cell communication. Cancer Lett. 2016, 380, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Omori, Y.; Li, Q.; Nishikawa, Y.; Yoshioka, T.; Yoshida, M.; Ishikawa, K.; Enomoto, K. Cytoplasmic accumulation of connexin32 expands cancer stem cell population in human HuH7 hepatoma cells by enhancing its self-renewal. Int. J. Cancer 2011, 128, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Hitomi, M.; Deleyrolle, L.P.; Mulkearns-Hubert, E.E.; Jarrar, A.; Li, M.; Sinyuk, M.; Otvos, B.; Brunet, S.; Flavahan, W.A.; Hubert, C.G.; et al. Differential Connexin Function Enhances Self-Renewal in Glioblastoma. Cell Rep. 2015, 11, 1031–1042. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Li, Y.; Ma, X.; Wan, Q.; Jiang, Z.; Liu, Y.; Zhang, D.; Liu, X.; Wu, W. Connexin 43 SUMOylation improves gap junction functions between liver cancer stem cells and enhances their sensitivity to HSVtk/GCV. Int. J. Oncol. 2018, 52, 872–880. [Google Scholar] [CrossRef]
- Yu, S.C.; Xiao, H.L.; Jiang, X.-F.; Wang, Q.L.; Li, Y.; Yang, X.J.; Ping, Y.F.; Duan, J.J.; Jiang, J.Y.; Ye, X.Z.; et al. Connexin 43 Reverses Malignant Phenotypes of Glioma Stem Cells by Modulating E-Cadherin. Stem Cells 2012, 30, 108–120. [Google Scholar] [CrossRef]
- Thiagarajan, P.S.; Sinyuk, M.; Turaga, S.M.; Mulkearns-Hubert, E.E.; Hale, J.S.; Rao, V.; Demelash, A.; Saygin, C.; China, A.; Alban, T.J.; et al. Cx26 drives self-renewal in triple-negative breast cancer via interaction with NANOG and focal adhesion kinase. Nat. Commun. 2018, 9, 578. [Google Scholar] [CrossRef]
- Hanahan, D.; Folkman, J. Patterns and Emerging Mechanisms of the Angiogenic Switch during Tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, T.; Usuda, H.; Tanaka, T.; Wada, K.; Shimaoka, M. The Functional Implications of Endothelial Gap Junctions and Cellular Mechanics in Vascular Angiogenesis. Cancers 2019, 11, 237. [Google Scholar] [CrossRef] [PubMed]
- Alonso, F.; Domingos-Pereira, S.; Le Gal, L.; Derré, L.; Meda, P.; Jichlinski, P.; Nardelli-Haefliger, D.; Haefliger, J.A. Targeting endothelial connexin40 inhibits tumor growth by reducing angiogenesis and improving vessel perfusion. Oncotarget 2016, 7, 14015–14028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLachlan, E.; Shao, Q.; Laird, D.W. Connexins and Gap Junctions in Mammary Gland Development and Breast Cancer Progression. J. Membr. Biol. 2007, 218, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Wang, H.; McLachlan, E.; Veitch, G.I.; Laird, D.W. Down-regulation of Cx43 by Retroviral Delivery of Small Interfering RNA Promotes an Aggressive Breast Cancer Cell Phenotype. Cancer Res. 2005, 65, 2705–2711. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, R.C.; Yamada, K.A.; Kléber, A.G.; Saffitz, J.E.; Kléber, A.G. Autocrine Regulation of Myocyte Cx43 Expression by VEGF. Circ. Res. 2002, 90, 671–677. [Google Scholar] [CrossRef]
- Suarez, S.; Ballmer-Hofer, K. VEGF transiently disrupts gap junctional communication in endothelial cells. J. Cell Sci. 2001, 114, 1229–1235. [Google Scholar]
- Wang, W.K.; Chen, M.C.; Leong, H.F.; Kuo, Y.L.; Kuo, C.Y.; Lee, C.H. Connexin 43 suppresses tumor angiogenesis by down-regulation of vascular endothelial growth factor via hypoxic-induced factor-1alpha. Int. J. Mol. Sci. 2014, 16, 439–451. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Kaneda, M.; Morita, I.; Lane, W.S.; Houng, A.K.; Polgár, J.; Chung, S.H.; Reed, G.L. The Gap Junction-independent Tumor-suppressing Effect of Connexin 43. J. Biol. Chem. 2003, 278, 44852–44856. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.X.; Gu, S. Gap junction- and hemichannel-independent actions of connexins. Biochim. Biophys. Acta 2005, 1711, 208–214. [Google Scholar] [CrossRef] [Green Version]
- Gleisner, M.A.; Navarrete, M.; Hofmann, F.; Salazar-Onfray, F.; Tittarelli, A. Mind the Gaps in Tumor Immunity: Impact of Connexin-Mediated Intercellular Connections. Front. Immunol. 2017, 8, 1067. [Google Scholar] [CrossRef] [PubMed]
- Thuringer, D.; Berthenet, K.; Cronier, L.; Solary, E.; Garrido, C. Primary tumor- and metastasis-derived colon cancer cells differently modulate connexin expression and function in human capillary endothelial cells. Oncotarget 2015, 6, 28800–28815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thuringer, D.; Boucher, J.; Jego, G.; Pernet, N.; Cronier, L.; Hammann, A.; Solary, E.; Garrido, C. Transfer of functional microRNAs between glioblastoma and microvascular endothelial cells through gap junctions. Oncotarget 2016, 7, 73925–73934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chantrain, C.F.; Feron, O.; Marbaix, E.; Declerck, Y.A. Bone Marrow Microenvironment and Tumor Progression. Cancer Microenviron. 2008, 1, 23–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, A.; Katoh, F.; Kataoka, T.R.; Okada, M.; Tsubota, N.; Asada, H.; Yoshikawa, K.; Maeda, S.; Kitamura, Y.; Yamasaki, H.; et al. A role for heterologous gap junctions between melanoma and endothelial cells in metastasis. J. Clin. Investig. 2000, 105, 1189–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villares, G.J.; Dobroff, A.S.; Wang, H.; Zigler, M.; Melnikova, V.O.; Huang, L.; Bar-Eli, M. Overexpression of Protease Activated Receptor-1 Contributes to Melanoma Metastasis via Regulation of Connexin 43. Cancer Res. 2009, 69, 6730–6737. [Google Scholar] [CrossRef] [PubMed]
- Stoletov, K.; Strnadel, J.; Zardouzian, E.; Momiyama, M.; Park, F.D.; Kelber, J.A.; Pizzo, D.P.; Hoffman, R.; Vandenberg, S.R.; Klemke, R.L. Role of connexins in metastatic breast cancer and melanoma brain colonization. J. Cell Sci. 2013, 126, 904–913. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Couldwell, W.T.; Simard, M.F.; Song, H.; Lin, J.H.; Nedergaard, M. Direct gap junction communication between malignant glioma cells and astrocytes. Cancer Res. 1999, 59, 1994–2003. [Google Scholar]
- Lin, J.H.C.; Takano, T.; Cotrina, M.L.; Arcuino, G.; Kang, J.; Liu, S.; Gao, Q.; Jiang, L.; Li, F.; Lichtenberg-Fraté, H.; et al. Connexin 43 Enhances the Adhesivity and Mediates the Invasion of Malignant Glioma Cells. J. Neurosci. 2002, 22, 4302–4311. [Google Scholar] [CrossRef] [Green Version]
- Sin, W.C.; Aftab, Q.; Bechberger, J.F.; Leung, J.H.; Chen, H.; Naus, C.C. Astrocytes promote glioma invasion via the gap junction protein connexin43. Oncogene 2016, 35, 1504–1516. [Google Scholar] [CrossRef]
- Oliveira, R.; Christov, C.; Guillamo, J.S.; de Bouard, S.; Palfi, S.; Venance, L.; Tardy, M.; Peschanski, M. Contribution of gap junctional communication between tumor cells and astroglia to the invasion of the brain parenchyma by human glioblastomas. BMC Cell Biol. 2005, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Czyż, J.; Piwowarczyk, K.; Paw, M.; Luty, M.; Wróbel, T.; Catapano, J.; Madeja, Z.; Ryszawy, D. Connexin-dependent intercellular stress signaling in tissue homeostasis and tumor development. Acta Biochim. Pol. 2017, 64, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Eugenín, E.A.; Brañes, M.C.; Berman, J.W.; Sáez, J.C. TNF-alpha plus IFN-gamma induce connexin43 expression and formation of gap junctions between human monocytes/macrophages that enhance physiological responses. J. Immunol. 2003, 170, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Kwak, B.R.; Mulhaupt, F.; Veillard, N.; Gros, D.B.; Mach, F. Altered Pattern of Vascular Connexin Expression in Atherosclerotic Plaques. Arter. Thromb. Vasc. Biol. 2002, 22, 225–230. [Google Scholar] [CrossRef] [Green Version]
- Alexander, D.B.; Ichikawa, H.; Bechberger, J.F.; Valiunas, V.; Ohki, M.; Naus, C.C.G.; Kunimoto, T.; Tsuda, H.; Miller, W.T.; Goldberg, G.S. Normal Cells Control the Growth of Neighboring Transformed Cells Independent of Gap Junctional Communication and Src Activity. Cancer Res. 2004, 64, 1347–1358. [Google Scholar] [CrossRef]
- Kamibayashi, Y.; Oyamada, Y.; Mori, M.; Oyamada, M. Aberrant expression of gap junction proteins (connexins) is associated with tumor progression during multistage mouse skin carcinogenesis in vivo. Carcinogenesis 1995, 16, 1287–1297. [Google Scholar] [CrossRef]
- Miękus, K.; Czernik, M.; Sroka, J.; Czyż, J.; Madeja, Z. Contact stimulation of prostate cancer cell migration: The role of gap junctional coupling and migration stimulated by heterotypic cell-to-cell contacts in determination of the metastatic phenotype of Dunning rat prostate cancer cells. Biol. Cell 2005, 97, 893–903. [Google Scholar] [CrossRef]
- Tate, A.W.; Lung, T.; Radhakrishnan, A.; Lim, S.D.; Lin, X.; Edlund, M. Changes in gap junctional connexin isoforms during prostate cancer progression. Prostate 2006, 66, 19–31. [Google Scholar] [CrossRef]
- Kanczuga-Koda, L.; Sulkowski, S.; Lenczewski, A.; Koda, M.; Wincewicz, A.; Baltaziak, M.; Sulkowska, M. Increased expression of connexins 26 and 43 in lymph node metastases of breast cancer. J. Clin. Pathol. 2006, 59, 429–433. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Nwagwu, C.; Le, D.M.; Yong, V.W.; Song, H.; Couldwell, W.T. Increased invasive capacity of connexin43-overexpressing malignant glioma cells. J. Neurosurg. 2003, 99, 1039–1046. [Google Scholar] [CrossRef]
- Graeber, S.H.; Hülser, D.F. Connexin Transfection Induces Invasive Properties in HeLa Cells. Exp. Cell Res. 1998, 243, 142–149. [Google Scholar] [CrossRef] [PubMed]
- El-Sabban, M.E.; Pauli, B.U. Cytoplasmic dye transfer between metastatic tumor cells and vascular endothelium. J. Cell Biol. 1991, 115, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Tommelein, J.; Verset, L.; Boterberg, T.; Demetter, P.; Bracke, M.; De Wever, O. Cancer-Associated Fibroblasts Connect Metastasis-Promoting Communication in Colorectal Cancer. Front. Oncol. 2015, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, G.S.; Poutahidis, T.; Erdman, S.E.; Kirsch, R.; Riddell, R.H.; Diamandis, E.P. Cancer-Associated Fibroblasts Drive the Progression of Metastasis through both Paracrine and Mechanical Pressure on Cancer Tissue. Mol. Cancer Res. 2012, 10, 1403–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Zhang, W.; Sun, X.; Lin, Y.; Chen, W. Cancer-associated fibroblasts induce epithelial-mesenchymal transition through secreted cytokines in endometrial cancer cells. Oncol. Lett. 2018, 15, 5694–5702. [Google Scholar] [CrossRef] [PubMed]
- Husoy, T.; Knutsen, H.K.; Cruciani, V.; Olstorn, H.B.; Mikalsen, S.O.; Loberg, E.M.; Alexander, J. Connexin43 is overexpressed in ApcMin/+-mice adenomas and colocalises with COX-2 in myofibroblasts. Int. J. 2005, 116, 351–358. [Google Scholar]
- Pollmann, M.A.; Shao, Q.; Laird, D.W.; Sandig, M. Connexin 43 mediated gap junctional communication enhances breast tumor cell diapedesis in culture. Breast Cancer Res. 2005, 7, R522–R534. [Google Scholar] [CrossRef] [PubMed]
- Bazarbachi, A.; Merhi, R.A.; Gessain, A.; Talhouk, R.; El-Khoury, H.; Nasr, R.; Gout, O.; Sulahian, R.; Homaidan, F.; Hermine, O.; et al. Human T-Cell Lymphotropic Virus Type I-Infected Cells Extravasate through the Endothelial Barrier by a Local Angiogenesis-Like Mechanism. Cancer Res. 2004, 64, 2039–2046. [Google Scholar] [CrossRef] [Green Version]
- Haddad, L.; El Hajj, H.; Abou-Merhi, R.; Kfoury, Y.; Mahieux, R.; El-Sabban, M.; Bazarbachi, A. KSHV-transformed primary effusion lymphoma cells induce a VEGF-dependent angiogenesis and establish functional gap junctions with endothelial cells. Leukemia 2008, 22, 826–834. [Google Scholar] [CrossRef]
- Mitsiades, C.S.; McMillin, D.W.; Klippel, S.; Hideshima, T.; Chauhan, D.; Richardson, P.G.; Munshi, N.C.; Anderson, K.C. The role of the bone marrow microenvironment in the pathophysiology of myeloma and its significance in the development of more effective therapies. Hematol. Oncol. Clin. N. Am. 2007, 21, 1007–1034. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Rafii, S.; Lyden, D.; Benezra, R.; Hattori, K.; Heissig, B. Vascular and haematopoietic stem cells: Novel targets for anti-angiogenesis therapy? Nat. Rev. Cancer 2002, 2, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, H.; Xu, C.; Deng, M.; Song, M.; Yu, X.; Xu, S.; Zhao, X. VEGF promotes endothelial progenitor cell differentiation and vascular repair through connexin 43. Stem Cell Res. Ther. 2017, 8, 237. [Google Scholar] [CrossRef] [PubMed]
- Aasen, T.; Leithe, E.; Graham, S.V.; Kameritsch, P.; Mayán, M.D.; Mesnil, M.; Pogoda, K.; Tabernero, A. Connexins in cancer: Bridging the gap to the clinic. Oncogene 2019, 38, 4426–4451. [Google Scholar] [CrossRef] [PubMed]
- Tittarelli, A.; Mendoza-Naranjo, A.; Farias, M.; Guerrero, I.; Ihara, F.; Wennerberg, E.; Riquelme, S.; Gleisner, A.; Kalergis, A.; Lundqvist, A.; et al. Gap junction intercellular communications regulate NK cell activation and modulate NK cytotoxic capacity. J. Immunol. 2014, 192, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Neijssen, J.; Pang, B.; Neefjes, J. Gap junction-mediated intercellular communication in the immune system. Prog. Biophys. Mol. Biol. 2007, 94, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, N.C.; Lozier, A.; Flament, C.; Ricciardi-Castagnoli, P.; Bellet, D.; Suter, M.; Perricaudet, M.; Tursz, T.; Maraskovsky, E.; Zitvogel, L. Dendritic cells directly trigger NK cell functions: Cross-talk relevant in innate anti-tumor immune responses in vivo. Nat. Med. 1999, 5, 405–411. [Google Scholar] [CrossRef]
- Jacobs, B.; Ullrich, E. The interaction of NK cells and dendritic cells in the tumor environment: How to enforce NK cell & DC action under immunosuppressive conditions? Curr. Med. Chem. 2012, 19, 1771–1779. [Google Scholar]
- Da Silva, R.B.; Münz, C. Natural killer cell activation by dendritic cells: Balancing inhibitory and activating signals. Cell. Mol. Life Sci. 2011, 68, 3505–3518. [Google Scholar] [CrossRef]
- Orange, J.S. Formation and function of the lytic NK-cell immunological synapse. Nat. Rev. Immunol. 2008, 8, 713–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiery, J.; Keefe, D.; Boulant, S.; Boucrot, E.; Walch, M.; Martinvalet, D.; Goping, I.S.; Bleackley, R.C.; Kirchhausen, T.; Lieberman, J. Perforin pores in the endosomal membrane trigger the release of endocytosed granzyme B into the cytosol of target cells. Nat. Immunol. 2011, 12, 770–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thuringer, D.; Jego, G.; Berthenet, K.; Hammann, A.; Solary, E.; Garrido, C. Gap junction-mediated transfer of miR-145-5p from microvascular endothelial cells to colon cancer cells inhibits angiogenesis. Oncotarget 2016, 7, 28160–28168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Liu, Y.; Si, Y.J.; Chen, X.H.; Li, Z.J.; Gao, L.; Zhang, C. Effect of Cx43 gene-modified leukemic bone marrow stromal cells on the regulation of Jurkat cell line in vitro. Leuk. Res. 2012, 36, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Z.; Riquelme, M.A.; Gu, S.; Kar, R.; Gao, X.; Sun, L.; Jiang, J.X. Osteocytic Connexin Hemichannels Suppress Breast Cancer Growth and Bone Metastasis. Oncogene 2016, 35, 5597–5607. [Google Scholar] [CrossRef] [PubMed]
- Dilber, M.S.; Abedi, M.R.; Christensson, B.; Björkstrand, B.; Kidder, G.M.; Naus, C.C.; Gahrton, G.; Smith, C.I. Gap junctions promote the bystander effect of herpes simplex virus thymidine kinase in vivo. Cancer Res. 1997, 57, 1523–1528. [Google Scholar] [PubMed]
- Tittarelli, A.; Janji, B.; Van Moer, K.; Noman, M.Z.; Chouaib, S. The Selective Degradation of Synaptic Connexin 43 Protein by Hypoxia-induced Autophagy Impairs Natural Killer Cell-mediated Tumor Cell Killing. J. Biol. Chem. 2015, 290, 23670–23679. [Google Scholar] [CrossRef] [Green Version]
- Salameh, A.; Dhein, S. Pharmacology of Gap junctions. New pharmacological targets for treatment of arrhythmia, seizure and cancer? Biochim. Biophys. Acta Biomembr. 2005, 1719, 36–58. [Google Scholar] [CrossRef] [Green Version]
- Evans, W.H.; Leybaert, L. Mimetic Peptides as Blockers of Connexin Channel—Facilitated Intercellular Communication. Cell Commun. Adhes. 2007, 14, 265–273. [Google Scholar] [CrossRef]
- Trosko, J.E. Cancer Prevention and Therapy of Two Types of Gap Junctional Intercellular Communication–Deficient “Cancer Stem Cell”. Cancers 2019, 11, 87. [Google Scholar] [CrossRef]
- Ogawa, T.; Hayashi, T.; Tokunou, M.; Yorioka, N.; Nakachi, K.; Trosko, J.E.; Chang, C.C. Suberoylanilide Hydroxamic Acid Enhances Gap Junctional Intercellular Communication via Acetylation of Histone Containing Connexin 43 Gene Locus. Cancer Res. 2005, 65, 9771–9778. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zefferino, R.; Piccoli, C.; Di Gioia, S.; Capitanio, N.; Conese, M. Gap Junction Intercellular Communication in the Carcinogenesis Hallmarks: Is This a Phenomenon or Epiphenomenon? Cells 2019, 8, 896. https://doi.org/10.3390/cells8080896
Zefferino R, Piccoli C, Di Gioia S, Capitanio N, Conese M. Gap Junction Intercellular Communication in the Carcinogenesis Hallmarks: Is This a Phenomenon or Epiphenomenon? Cells. 2019; 8(8):896. https://doi.org/10.3390/cells8080896
Chicago/Turabian StyleZefferino, Roberto, Claudia Piccoli, Sante Di Gioia, Nazzareno Capitanio, and Massimo Conese. 2019. "Gap Junction Intercellular Communication in the Carcinogenesis Hallmarks: Is This a Phenomenon or Epiphenomenon?" Cells 8, no. 8: 896. https://doi.org/10.3390/cells8080896




