Immune-Deficient Pfp/Rag2−/− Mice Featured Higher Adipose Tissue Mass and Liver Lipid Accumulation with Growing Age than Wildtype C57BL/6N Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Cell Culture Experiments
2.3. Serum Analysis
2.4. Histology
2.5. Immunofluorescence
2.6. Gene Expression Analyses
2.7. Adipokine Profiling
2.8. Statistics
3. Results
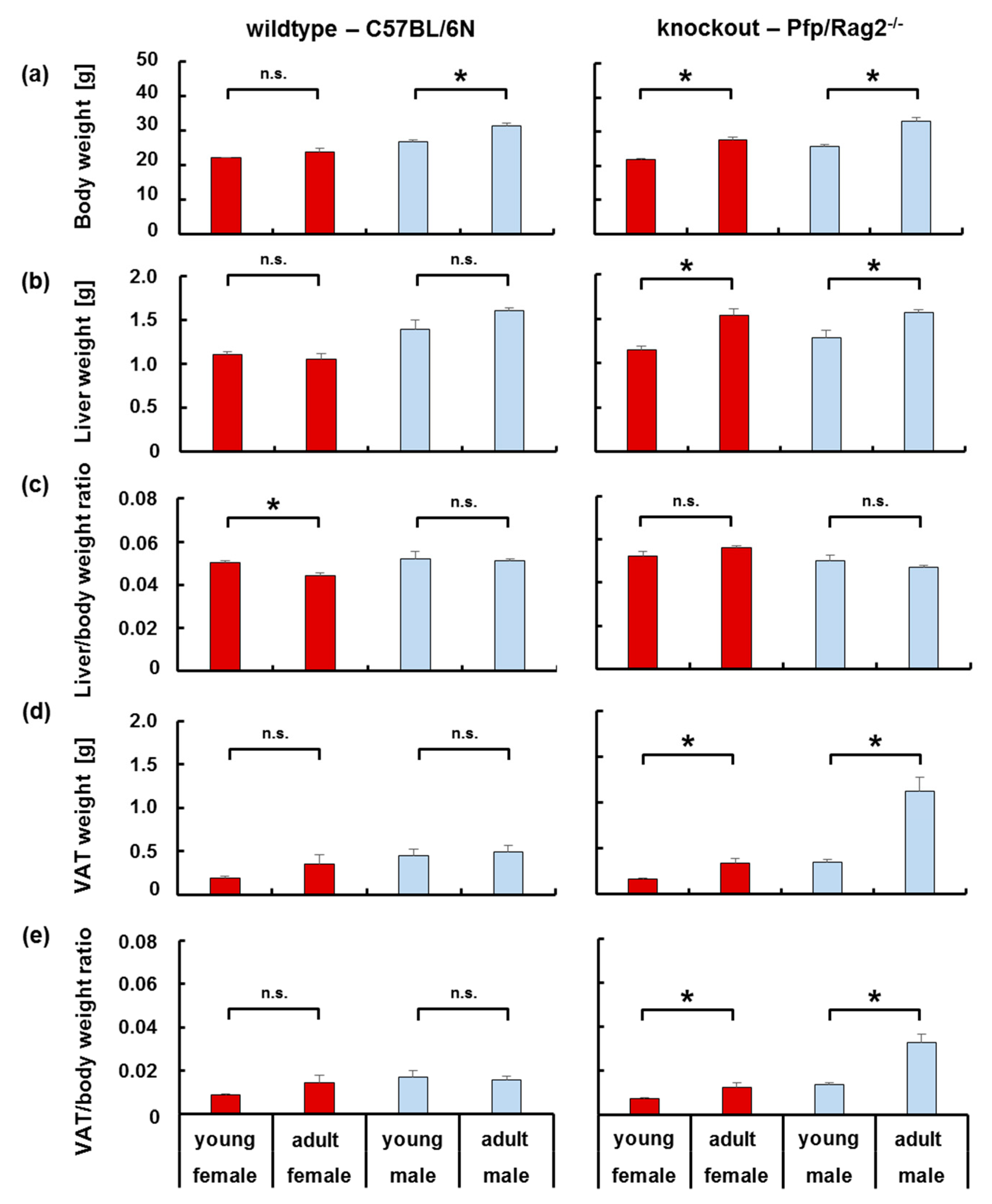
3.1. The Age-Related Increase in Body Weight Coincided with a Disproportionate Increase in Adipose Tissue in Immune-Deficient, but Not Wildtype Mice
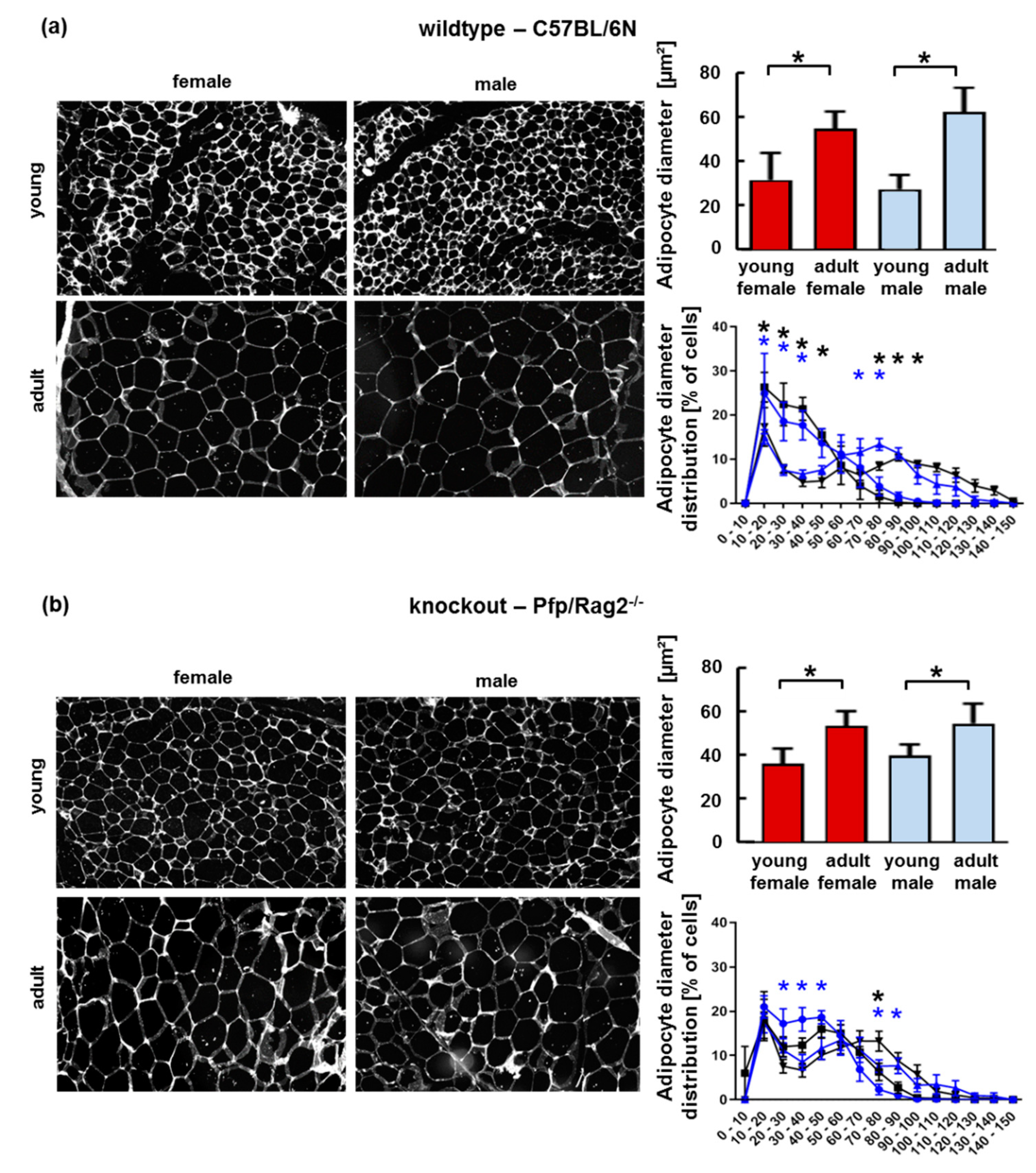
3.2. The Age-Related Increase of Visceral Adipose Tissue Mass in Immune-Deficient Mice Was Likely Attributable to Hyperplasia
3.3. Adipose Tissue Hypertrophy and Hyperplasia, Respectively, Coincided with Enhanced Fat Storage in Livers of WTFA and KOFA as Well as of KOMA Mice
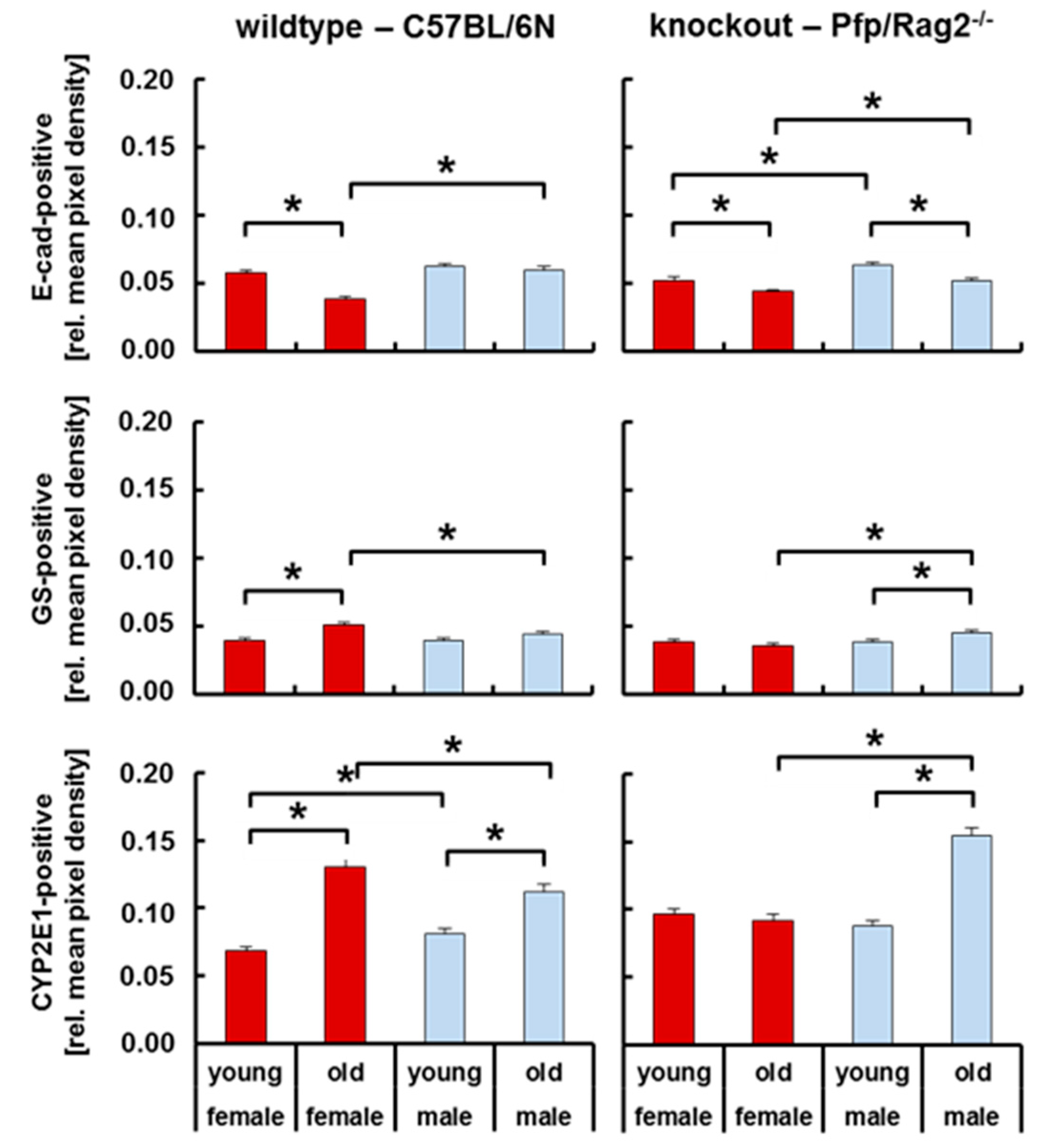
3.4. Livers of WTFA and KOMA Mice Presented Damage-Associated Signs of Tissue Remodeling
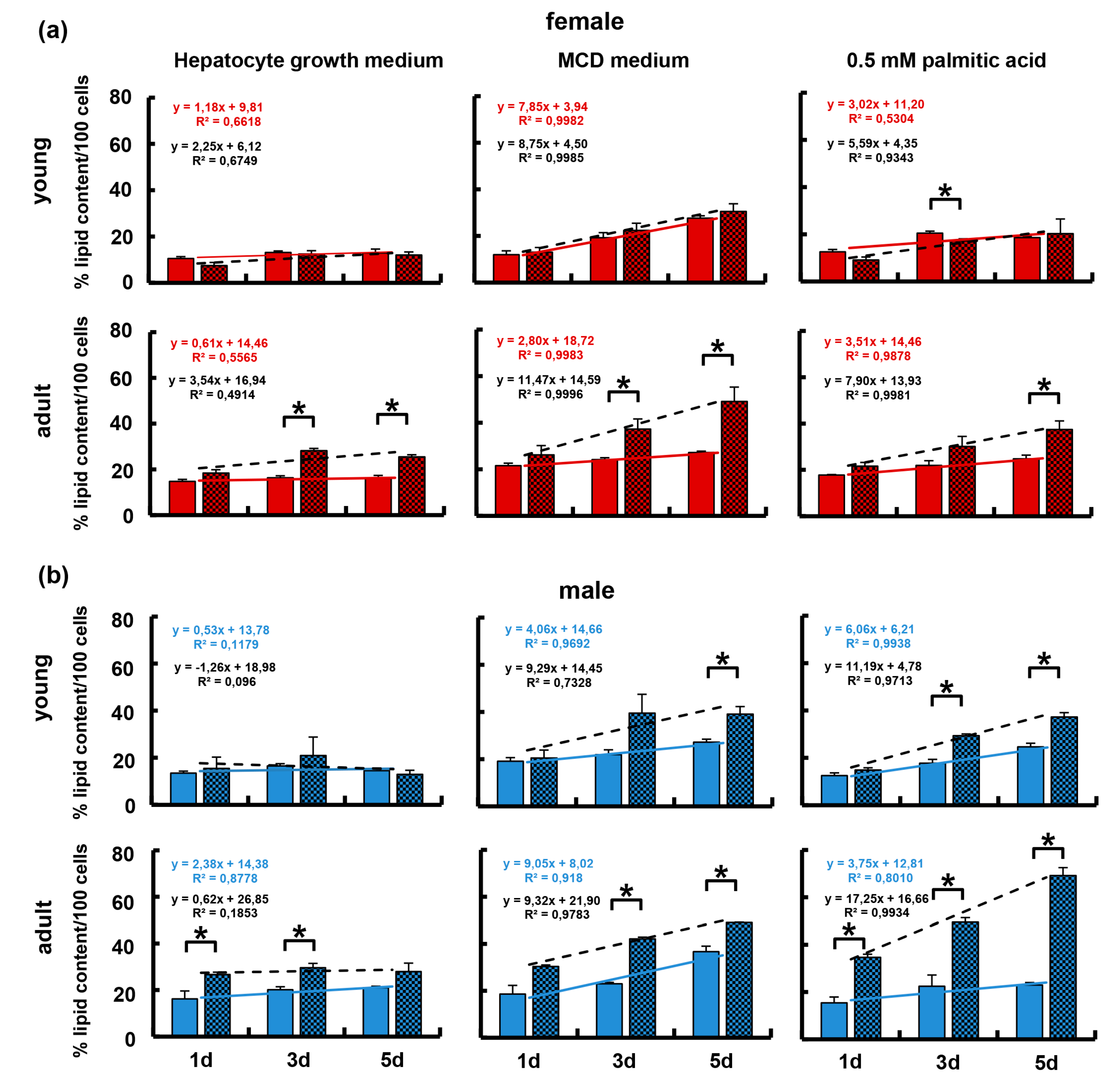
3.5. The Immune-Deficient Phenotype Favored Lipid Storage in Isolated Hepatocytes
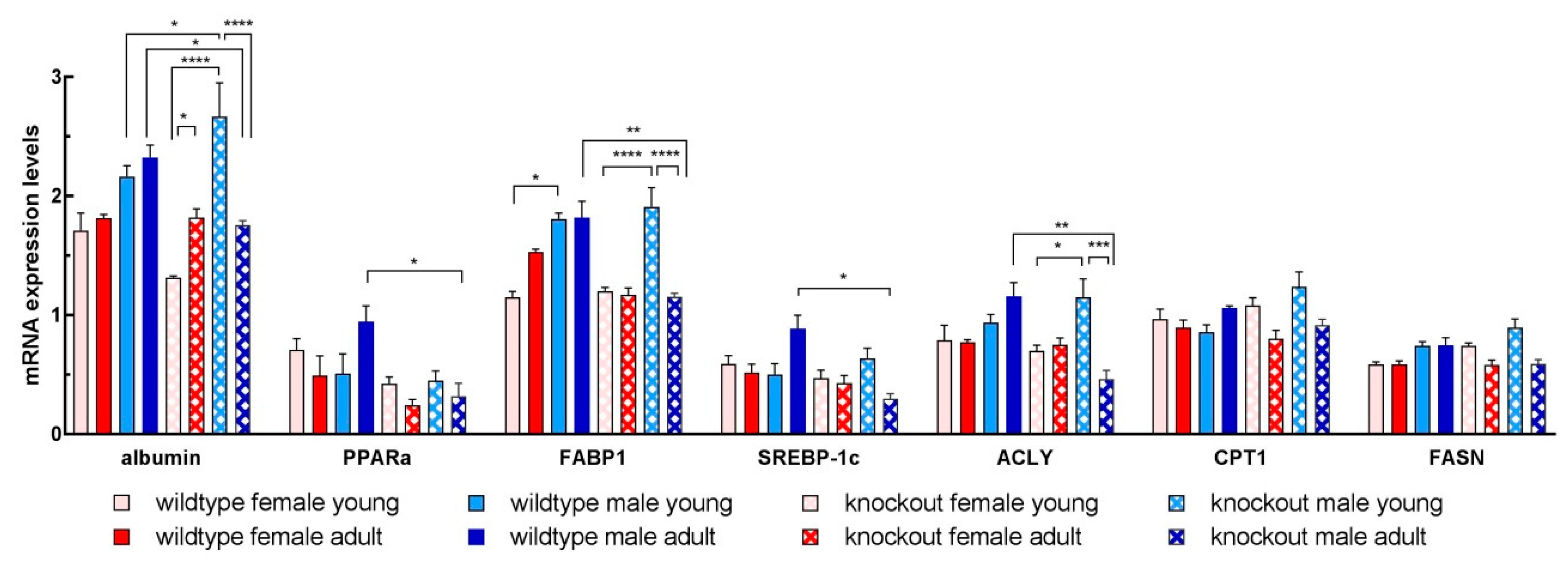
3.6. Isolated Hepatocytes from KOMA Mice Featured Low Lipogenic and Low Fatty Acid Utilization Capacity
3.7. Adipose Tissue-Derived Adipokines and Cytokines Potentially Mediate Interactions with the Liver
4. Discussion
4.1. Age-Related Mechanisms to Promote Adipose Tissue Pathologies in Adult Mice
4.2. Hepatic Mechanisms Involved in Increased Lipid Storage in Old Mice
5. Conclusions/Hypothesis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tilg, H.; Moschen, A.R. Mechanisms behind the link between obesity and gastrointestinal cancers. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. Adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Buch, S.C.; Kondragunta, V.; Branch, R.A.; Carr, B.I. Gender-based outcomes differences in unresectable hepatocellular carcinoma. Hepatol. Int. 2008, 2, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.T.; Chang, C.W.; Wei, R.J.; Wang, S.N. Progesterone and related compounds in hepatocellular carcinoma: Basic and clinical aspects. BioMed Res. Int. 2013, 2013, 290575. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA A Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samanic, C.; Chow, W.H.; Gridley, G.; Jarvholm, B.; Fraumeni, J.F., Jr. Relation of body mass index to cancer risk in 362,552 swedish men. Cancer Causes Control 2006, 17, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. J. Int. Assoc. Study Obes. 2010, 11, 11–18. [Google Scholar] [CrossRef]
- Graffy, P.M.; Pickhardt, P.J. Quantification of hepatic and visceral fat by ct and mr imaging: Relevance to the obesity epidemic, metabolic syndrome and nafld. Br. J. Radiol. 2016, 89, 20151024. [Google Scholar] [CrossRef]
- Yaskolka Meir, A.; Tene, L.; Cohen, N.; Shelef, I.; Schwarzfuchs, D.; Gepner, Y.; Zelicha, H.; Rein, M.; Bril, N.; Serfaty, D.; et al. Intrahepatic fat, abdominal adipose tissues, and metabolic state: Magnetic resonance imaging study. Diabetes/Metab. Res. Rev. 2017, 33, e2888. [Google Scholar] [CrossRef]
- Navarro, E.; Funtikova, A.N.; Fito, M.; Schroder, H. Can metabolically healthy obesity be explained by diet, genetics, and inflammation? Mol. Nutr. Food Res. 2015, 59, 75–93. [Google Scholar] [CrossRef]
- Boonchaya-anant, P.; Apovian, C.M. Metabolically healthy obesity-does it exist? Curr. Atheroscler. Rep. 2014, 16, 441. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.E.; Kim, J.M.; Joung, K.H.; Lee, J.H.; You, B.R.; Choi, M.J.; Ryu, M.J.; Ko, Y.B.; Lee, M.A.; Lee, J.; et al. The roles of adipokines, proinflammatory cytokines, and adipose tissue macrophages in obesity-associated insulin resistance in modest obesity and early metabolic dysfunction. PLoS ONE 2016, 11, e0154003. [Google Scholar] [CrossRef] [PubMed]
- Cautivo, K.M.; Molofsky, A.B. Regulation of metabolic health and adipose tissue function by group 2 innate lymphoid cells. Eur. J. Immunol. 2016, 46, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Heeren, J.; Scheja, L. Regulation of immunometabolism in adipose tissue. Semin. Immunopathol. 2018, 40, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Park, Y.J.; Ham, M.; Kim, J.B. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Mol. Cells 2014, 37, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Bjorntorp, P. Do stress reactions cause abdominal obesity and comorbidities? Obes. Rev. J. Int. Assoc. Study Obes. 2001, 2, 73–86. [Google Scholar] [CrossRef]
- Freedland, E.S. Role of a critical visceral adipose tissue threshold (cvatt) in metabolic syndrome: Implications for controlling dietary carbohydrates: A review. Nutr. Metab. 2004, 1, 12. [Google Scholar] [CrossRef]
- Ande, S.R.; Nguyen, K.H.; Gregoire Nyomba, B.L.; Mishra, S. Prohibitin-induced, obesity-associated insulin resistance and accompanying low-grade inflammation causes nash and hcc. Sci. Rep. 2016, 6, 23608. [Google Scholar] [CrossRef]
- Lopez-Lluch, G. Mitochondrial activity and dynamics changes regarding metabolism in ageing and obesity. Mech. Ageing Dev. 2017, 162, 108–121. [Google Scholar] [CrossRef] [Green Version]
- Kapetanovic, R.; Bokil, N.J.; Sweet, M.J. Innate immune perturbations, accumulating damps and inflammasome dysregulation: A ticking time bomb in ageing. Ageing Res. Rev. 2015, 24, 40–53. [Google Scholar] [CrossRef]
- Shaw, A.C.; Goldstein, D.R.; Montgomery, R.R. Age-dependent dysregulation of innate immunity. Nat. Rev. Immunol. 2013, 13, 875–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelz, S.; Stock, P.; Bruckner, S.; Christ, B. A methionine-choline-deficient diet elicits nash in the immunodeficient mouse featuring a model for hepatic cell transplantation. Exp. Cell Res. 2012, 318, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Winkler, S.; Hempel, M.; Bruckner, S.; Mallek, F.; Weise, A.; Liehr, T.; Tautenhahn, H.M.; Bartels, M.; Christ, B. Mouse white adipose tissue-derived mesenchymal stem cells gain pericentral and periportal hepatocyte features after differentiation in vitro, which are preserved in vivo after hepatic transplantation. Acta Physiol. 2015, 215, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Koenig, S.; Aurich, H.; Schneider, C.; Krause, P.; Haftendorn, R.; Becker, H.; Christ, B. Zonal expression of hepatocytic marker enzymes during liver repopulation. Histochem. Cell Biol. 2007, 128, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, R.; Sundermann, S.; Ludwig, D.; Ceglarek, U.; Holdt, L.M.; Thiery, J.; Teupser, D. Cosegregation of aortic root atherosclerosis and intermediate lipid phenotypes on chromosomes 2 and 8 in an intercross of c57bl/6 and balbc/byj low-density lipoprotein receptor/mice. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Braune, J.; Weyer, U.; Matz-Soja, M.; Hobusch, C.; Kern, M.; Kunath, A.; Kloting, N.; Kralisch, S.; Bluher, M.; Gebhardt, R.; et al. Hedgehog signalling in myeloid cells impacts on body weight, adipose tissue inflammation and glucose metabolism. Diabetologia 2017, 60, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Maurizi, G.; Della Guardia, L.; Maurizi, A.; Poloni, A. Adipocytes properties and crosstalk with immune system in obesity-related inflammation. J. Cell. Physiol. 2018, 233, 88–97. [Google Scholar] [CrossRef]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose tissue remodeling: Its role in energy metabolism and metabolic disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef]
- Adolph, T.E.; Grander, C.; Grabherr, F.; Tilg, H. Adipokines and non-alcoholic fatty liver disease: Multiple interactions. Int. J. Mol. Sci. 2017, 18, 1649. [Google Scholar] [CrossRef]
- Parker, R.; Kim, S.J.; Gao, B. Alcohol, adipose tissue and liver disease: Mechanistic links and clinical considerations. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 50–59. [Google Scholar] [CrossRef]
- Hempel, M.; Schmitz, A.; Winkler, S.; Kucukoglu, O.; Bruckner, S.; Niessen, C.; Christ, B. Pathological implications of cadherin zonation in mouse liver. Cell. Mol. Life Sci. 2015, 72, 2599–2612. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.; Castro, J. Zonation of fatty acid metabolism in rat liver. Biochem. J. 1989, 264, 107–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hijmans, B.S.; Grefhorst, A.; Oosterveer, M.H.; Groen, A.K. Zonation of glucose and fatty acid metabolism in the liver: Mechanism and metabolic consequences. Biochimie 2014, 96, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Swiderska-Syn, M.; Jewell, M.L.; Premont, R.T.; Diehl, A.M. Liver regeneration requires yap1-tgfbeta-dependent epithelial-mesenchymal transition in hepatocytes. J. Hepatol. 2018, 69, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Van Zutphen, T.; Ciapaite, J.; Bloks, V.W.; Ackereley, C.; Gerding, A.; Jurdzinski, A.; de Moraes, R.A.; Zhang, L.; Wolters, J.C.; Bischoff, R.; et al. Malnutrition-associated liver steatosis and atp depletion is caused by peroxisomal and mitochondrial dysfunction. J. Hepatol. 2016, 65, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, J.; van Pelt, J.; Korf, H.; Mathieu, C.; van der Schueren, B.; Lannoo, M.; Oyen, T.; Topal, B.; Fetter, G.; Nayler, S.; et al. Association of adipose tissue inflammation with histologic severity of nonalcoholic fatty liver disease. Gastroenterology 2015, 149, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Vegiopoulos, A.; Rohm, M.; Herzig, S. Adipose tissue: Between the extremes. EMBO J. 2017, 36, 1999–2017. [Google Scholar] [CrossRef] [PubMed]
- Kloting, N.; Bluher, M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev. Endocr. Metab. Disord. 2014, 15, 277–287. [Google Scholar] [CrossRef]
- McQuaid, S.E.; Hodson, L.; Neville, M.J.; Dennis, A.L.; Cheeseman, J.; Humphreys, S.M.; Ruge, T.; Gilbert, M.; Fielding, B.A.; Frayn, K.N.; et al. Downregulation of adipose tissue fatty acid trafficking in obesity: A driver for ectopic fat deposition? Diabetes 2011, 60, 47–55. [Google Scholar] [CrossRef]
- Trujillo, M.E.; Scherer, P.E. Adipose tissue-derived factors: Impact on health and disease. Endocr. Rev. 2006, 27, 762–778. [Google Scholar] [CrossRef]
- Wensveen, F.M.; Valentic, S.; Sestan, M.; Turk Wensveen, T.; Polic, B. The “big bang” in obese fat: Events initiating obesity-induced adipose tissue inflammation. Eur. J. Immunol. 2015, 45, 2446–2456. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.R.; Milner, J.J.; Makowski, L. The inflammation highway: Metabolism accelerates inflammatory traffic in obesity. Immunol. Rev. 2012, 249, 218–238. [Google Scholar] [CrossRef] [PubMed]
- Perez, L.M.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Emanuele, E.; Lucia, A.; Galvez, B.G. “Adipaging”: Ageing and obesity share biological hallmarks related to a dysfunctional adipose tissue. J. Physiol. 2016, 594, 3187–3207. [Google Scholar] [CrossRef] [PubMed]
- Stout, M.B.; Justice, J.N.; Nicklas, B.J.; Kirkland, J.L. Physiological aging: Links among adipose tissue dysfunction, diabetes, and frailty. Physiology 2017, 32, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.H.; Doria, A.; et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Gesta, S.; Tseng, Y.H.; Kahn, C.R. Developmental origin of fat: Tracking obesity to its source. Cell 2007, 131, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Fried, S.K.; Lee, M.J.; Karastergiou, K. Shaping fat distribution: New insights into the molecular determinants of depot- and sex-dependent adipose biology. Obesity 2015, 23, 1345–1352. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.N.; Jung, Y.S.; Kwon, H.J.; Seong, J.K.; Granneman, J.G.; Lee, Y.H. Sex differences in sympathetic innervation and browning of white adipose tissue of mice. Biol. Sex Differ. 2016, 7, 67. [Google Scholar] [CrossRef] [Green Version]
- Ramseyer, V.D.; Granneman, J.G. Adrenergic regulation of cellular plasticity in brown, beige/brite and white adipose tissues. Adipocyte 2016, 5, 119–129. [Google Scholar] [CrossRef] [Green Version]
- Ogrodnik, M.; Miwa, S.; Tchkonia, T.; Tiniakos, D.; Wilson, C.L.; Lahat, A.; Day, C.P.; Burt, A.; Palmer, A.; Anstee, Q.M.; et al. Cellular senescence drives age-dependent hepatic steatosis. Nat. Commun. 2017, 8, 15691. [Google Scholar] [CrossRef]
- Guillory, B.; Jawanmardi, N.; Iakova, P.; Anderson, B.; Zang, P.; Timchenko, N.A.; Garcia, J.M. Ghrelin deletion protects against age-associated hepatic steatosis by downregulating the c/ebpalpha-p300/dgat1 pathway. Aging Cell 2018, 17, e12688. [Google Scholar] [CrossRef]
- Munukka, E.; Pekkala, S.; Wiklund, P.; Rasool, O.; Borra, R.; Kong, L.; Ojanen, X.; Cheng, S.M.; Roos, C.; Tuomela, S.; et al. Gut-adipose tissue axis in hepatic fat accumulation in humans. J. Hepatol. 2014, 61, 132–138. [Google Scholar] [CrossRef]
- Van der Poorten, D.; Milner, K.L.; Hui, J.; Hodge, A.; Trenell, M.I.; Kench, J.G.; London, R.; Peduto, T.; Chisholm, D.J.; George, J. Visceral fat: A key mediator of steatohepatitis in metabolic liver disease. Hepatology 2008, 48, 449–457. [Google Scholar] [CrossRef]
- Tikka, A.; Jauhiainen, M. The role of angptl3 in controlling lipoprotein metabolism. Endocrine 2016, 52, 187–193. [Google Scholar] [CrossRef]
- Chi, X.; Britt, E.C.; Shows, H.W.; Hjelmaas, A.J.; Shetty, S.K.; Cushing, E.M.; Li, W.; Dou, A.; Zhang, R.; Davies, B.S.J. Angptl8 promotes the ability of angptl3 to bind and inhibit lipoprotein lipase. Mol. Metab. 2017, 6, 1137–1149. [Google Scholar] [CrossRef]
- Hesse, D.; Trost, J.; Schafer, N.; Schwerbel, K.; Hoeflich, A.; Schurmann, A.; Brockmann, G.A. Effect of adipocyte-derived igf-i on adipose tissue mass and glucose metabolism in the berlin fat mouse. Growth Factors 2018, 36, 78–88. [Google Scholar] [CrossRef]
- Stengel, A.; Goebel-Stengel, M.; Teuffel, P.; Hofmann, T.; Busse, P.; Kobelt, P.; Rose, M.; Klapp, B.F. Obese patients have higher circulating protein levels of dipeptidyl peptidase iv. Peptides 2014, 61, 75–82. [Google Scholar] [CrossRef]
- Lazar, M.A. Resistin- and obesity-associated metabolic diseases. Horm. Metab. Res. 2007, 39, 710–716. [Google Scholar] [CrossRef]
- McTernan, C.L.; McTernan, P.G.; Harte, A.L.; Levick, P.L.; Barnett, A.H.; Kumar, S. Resistin, central obesity, and type 2 diabetes. Lancet 2002, 359, 46–47. [Google Scholar] [CrossRef]
- Sakamoto, K.; Sakamoto, T.; Ogawa, H. Effects of metabolic risk factors on production of plasminogen activator inhibitor-1 and adiponectin by adipocytes. Circ. J. Jpn. Circ. Soc. 2008, 72, 844–846. [Google Scholar] [CrossRef]
- You, T.; Yang, R.; Lyles, M.F.; Gong, D.; Nicklas, B.J. Abdominal adipose tissue cytokine gene expression: Relationship to obesity and metabolic risk factors. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E741–E747. [Google Scholar] [CrossRef]
- Meex, R.C.R.; Watt, M.J. Hepatokines: Linking nonalcoholic fatty liver disease and insulin resistance. Nat. Rev. Endocrinol. 2017, 13, 509–520. [Google Scholar] [CrossRef]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The hormone resistin links obesity to diabetes. Nature 2001, 409, 307–312. [Google Scholar] [CrossRef]
- Daigo, K.; Inforzato, A.; Barajon, I.; Garlanda, C.; Bottazzi, B.; Meri, S.; Mantovani, A. Pentraxins in the activation and regulation of innate immunity. Immunol. Rev. 2016, 274, 202–217. [Google Scholar] [CrossRef] [Green Version]
- Lago, F.; Dieguez, C.; Gomez-Reino, J.; Gualillo, O. The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine Growth Factor Rev. 2007, 18, 313–325. [Google Scholar] [CrossRef]
- Kietzmann, T. Metabolic zonation of the liver: The oxygen gradient revisited. Redox Biol. 2017, 11, 622–630. [Google Scholar] [CrossRef]
- Gebhardt, R.; Matz-Soja, M. Liver zonation: Novel aspects of its regulation and its impact on homeostasis. World J. Gastroenterol. 2014, 20, 8491–8504. [Google Scholar] [CrossRef]
- Schleicher, J.; Tokarski, C.; Marbach, E.; Matz-Soja, M.; Zellmer, S.; Gebhardt, R.; Schuster, S. Zonation of hepatic fatty acid metabolism - the diversity of its regulation and the benefit of modeling. Biochim. Biophys. Acta 2015, 1851, 641–656. [Google Scholar] [CrossRef]
- Raucy, J.L.; Lasker, J.M.; Kraner, J.C.; Salazar, D.E.; Lieber, C.S.; Corcoran, G.B. Induction of cytochrome p450iie1 in the obese overfed rat. Mol. Pharmacol 1991, 39, 275–280. [Google Scholar]
- Porubsky, P.R.; Meneely, K.M.; Scott, E.E. Structures of human cytochrome p-450 2e1. Insights into the binding of inhibitors and both small molecular weight and fatty acid substrates. J. Biol. Chem. 2008, 283, 33698–33707. [Google Scholar] [CrossRef]
- Gorsky, L.D.; Koop, D.R.; Coon, M.J. On the stoichiometry of the oxidase and monooxygenase reactions catalyzed by liver microsomal cytochrome p-450. Products of oxygen reduction. J. Biol. Chem. 1984, 259, 6812–6817. [Google Scholar]
- Ingelman-Sundberg, M.; Johansson, I. Mechanisms of hydroxyl radical formation and ethanol oxidation by ethanol-inducible and other forms of rabbit liver microsomal cytochromes p-450. J. Biol. Chem. 1984, 259, 6447–6458. [Google Scholar]
- Nanji, A.A.; Zhao, S.; Sadrzadeh, S.M.; Dannenberg, A.J.; Tahan, S.R.; Waxman, D.J. Markedly enhanced cytochrome p450 2e1 induction and lipid peroxidation is associated with severe liver injury in fish oil-ethanol-fed rats. Alcohol. Clin. Exp. Res. 1994, 18, 1280–1285. [Google Scholar] [CrossRef]
- Leclercq, I.A.; Farrell, G.C.; Field, J.; Bell, D.R.; Gonzalez, F.J.; Robertson, G.R. Cyp2e1 and cyp4a as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J. Clin. Investig. 2000, 105, 1067–1075. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, S.H.; Kim, S.N.; Kwon, H.J.; Kim, J.D.; Oh, J.Y.; Jung, Y.S. Sex-specific metabolic interactions between liver and adipose tissue in mcd diet-induced non-alcoholic fatty liver disease. Oncotarget 2016, 7, 46959–46971. [Google Scholar] [CrossRef]
- Cvitanovic Tomas, T.; Urlep, Z.; Moskon, M.; Mraz, M.; Rozman, D. Liversex computational model: Sexual aspects in hepatic metabolism and abnormalities. Front. Physiol. 2018, 9, 360. [Google Scholar] [CrossRef]
- Dyar, K.A.; Lutter, D.; Artati, A.; Ceglia, N.J.; Liu, Y.; Armenta, D.; Jastroch, M.; Schneider, S.; de Mateo, S.; Cervantes, M.; et al. Atlas of circadian metabolism reveals system-wide coordination and communication between clocks. Cell 2018, 174, 1571–1585. [Google Scholar] [CrossRef]
- Tal, Y.; Chapnik, N.; Froy, O. Non-obesogenic doses of fatty acids modulate the functionality of the circadian clock in the liver. Cell. Mol. Life Sci. 2019, 76, 1795–1806. [Google Scholar] [CrossRef]
- Sato, S.; Solanas, G.; Peixoto, F.O.; Bee, L.; Symeonidi, A.; Schmidt, M.S.; Brenner, C.; Masri, S.; Benitah, S.A.; Sassone-Corsi, P. Circadian reprogramming in the liver identifies metabolic pathways of aging. Cell 2017, 170, 664–677. [Google Scholar] [CrossRef]
- Rodriguez, A.; Ezquerro, S.; Mendez-Gimenez, L.; Becerril, S.; Fruhbeck, G. Revisiting the adipocyte: A model for integration of cytokine signaling in the regulation of energy metabolism. Am. J. Physiol. Endocrinol. Metab. 2015, 309, 691–714. [Google Scholar] [CrossRef]
- Trim, W.; Turner, J.E.; Thompson, D. Parallels in immunometabolic adipose tissue dysfunction with ageing and obesity. Front. Immunol. 2018, 9, 169. [Google Scholar] [CrossRef]
- Brenner, C.; Galluzzi, L.; Kepp, O.; Kroemer, G. Decoding cell death signals in liver inflammation. J. Hepatol. 2013, 59, 583–594. [Google Scholar] [CrossRef] [Green Version]
- Tacke, F. Targeting hepatic macrophages to treat liver diseases. J. Hepatol. 2017, 66, 1300–1312. [Google Scholar] [CrossRef]
- Peverill, W.; Powell, L.W.; Skoien, R. Evolving concepts in the pathogenesis of nash: Beyond steatosis and inflammation. Int. J. Mol. Sci. 2014, 15, 8591–8638. [Google Scholar] [CrossRef]


| Wildtype | Knockout | |||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| Young | 13.7 ± 0.47 weeks | 14.3 ± 0.48 weeks | 13.8 ± 0.62 weeks | 13.6 ± 0.52 weeks |
| Mean weight [g] | 22.11 ± 0.18 | 26.77 ± 0.55 | 21.94 ± 0.27 | 25.64 ± 0.47 |
| Group abbreviation | WTFY | WTMY | KOFY | KOMY |
| Adult | 29.3 ± 0.49 weeks | 29.0 ± 1.26 weeks | 29.8 ± 1.47 weeks | 30.3 ± 0.77 weeks |
| Mean weight [g] | 23.81 ± 1.13 | 31.44 ± 0.69 | 27.44 ± 0.98 | 32.93 ± 1.16 |
| Group abbreviation | WTFA | WTMA | KOFA | KOMA |
| Gene | Accession No. | Forward Primer 5′ → 3′ | Reverse Primer 5′ → 3′ |
|---|---|---|---|
| ACLY | NM_001199296.1 | CCCAAGTCCAAGATCCCTGC | CTGCTTGTGATCCCCAGTGA |
| Albumin | NM_009654.4 | CCAATCCTCCCGCATGCTAC | GCGAACTAGAATGGCATTTTGGAA |
| B2M | NM_009735.3 | TCTACTGGGATCGAGACATGTGA | ATTGCTATTTCTTTCTGCGTGCAT |
| CPT1A | NM_013495.2 | CCATGATGGACCCCACAACA | ATGGCTCAGACAGTACCTCCT |
| FABP1 | NM_017399.4 | GGAAAAAGTCAAGGCAGTCGTC | CTCTTGTAGACAATGTCGCCC |
| FASN | NM_007988.3 | TGCACCTCACAGGCATCAAT | GTCCCACTTGATGTGAGGGG |
| HPRT1 | NM_013556.2 | ACAGGCCAGACTTTGTTGGAT | TGCAGATTCAACTTGCGCTC |
| PPARα | NM_011144.6 | GTTCACGCATGTGAAGGCTG | AGCGAATTGCATTGTGTGACAT |
| SREBP1c | NM_011480.4 | GATTTGGCCCGGGGAGATTT | TGGCGGATGAGGTTCCAAAG |
| Wildtype C57BL/6N | Knockout Pfp/Rag2−/− | Significance | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Young (WTMY) | Adult (WTMA) | Young (KOMY) | Adult (KOMA) | ||||||
| Relative Mean Pixel Density/mg Serum Protein | |||||||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | P | |
| Adiponectin | 50.10 | 0.38 | 51.20 | 0.26 | 51.67 | 1.07 | 51.05 | 0.14 | |
| AgRP | 1.82 | 0.89 | 0.76 | 0.28 | 2.53 | 0.75 | 0.47 | 0.24 | KOMY/KOMA 0.004 |
| ANGPL3 | 11.90 | 1.62 | 16.10 | 0.50 | 21.51 | 6.80 | 23.82 | 1.30 | WTMY/KOMY 0.022 WTMA/KOMA 0.001 |
| CRP | 38.79 | 3.76 | 44.95 | 1.61 | 44.08 | 4.82 | 48.81 | 0.83 | |
| DPPIV | 11.86 | 2.16 | 11.08 | 0.61 | 16.59 | 5.47 | 21.62 | 1.58 | WTMY/KOMY 0,043 WTMA/KOMA 0.001 |
| Endocan | 2.34 | 0.27 | 2.55 | 0.15 | 3.35 | 1.01 | 2.83 | 0.42 | WTMY/KOMY 0.033 |
| FetuinA | 22.87 | 2.52 | 18.28 | 1.17 | 31.95 | 7.29 | 25.19 | 1.25 | WTMY/KOMY 0,034 WTMA/KOMA 0.007 |
| FGFacidic | 3.28 | 0.58 | 1.22 | 0.12 | 4.82 | 2.55 | 0.93 | 0.22 | WTMY/WTMA 0.013 |
| ICAM1 | 14.53 | 1.86 | 10.26 | 1.04 | 19.46 | 6.80 | 18.31 | 0.37 | WTMA/KOMA 0.000 |
| IGFI | 37.22 | 5.83 | 21.46 | 5.40 | 40.62 | 9.08 | 49.75 | 0.23 | WTMA/KOMA 0.013 |
| IGFII | 4.02 | 0.92 | 3.98 | 0.15 | 5.41 | 1.08 | 4.96 | 0.59 | |
| IGFBP1 | 8.31 | 2.25 | 32.31 | 6.15 | 12.05 | 4.98 | 9.02 | 1.83 | WTMY/WTMA 0.011 WTMA/KOMA 0.011 |
| IGFBP2 | 29.03 | 3.56 | 35.86 | 0.82 | 36.38 | 10.66 | 48.53 | 0.73 | WTMY/KOMY 0.015 WTMA/KOMA 0.000 |
| IGFBP3 | 43.01 | 2.98 | 47.01 | 0.85 | 42.05 | 6.59 | 50.82 | 0.23 | WTMA/KOMA 0.005 |
| IGFBP5 | 4.47 | 0.57 | 5.13 | 0.22 | 8.83 | 3.02 | 6.34 | 0.65 | WTMY/KOMY 0.014 KOMY/KOMA 0.044 |
| IGFBP6 | 18.08 | 3.13 | 20.75 | 2.53 | 23.59 | 9.55 | 37.90 | 4.73 | WTMA/KOMA 0.019 |
| IL11 | 1.14 | 0.10 | 1.59 | 0.27 | 2.95 | 1.21 | 0.68 | 0.17 | WTMA/KOMA 0.030 |
| Leptin | 1.73 | 0.21 | 1.17 | 0.26 | 2.83 | 1.21 | 2.72 | 0.53 | WTMA/KOMA 0.039 |
| Liopcalin2 | 15.96 | 1.33 | 18.60 | 2.11 | 29.45 | 9.48 | 19.83 | 1.58 | |
| MCSF | 3.35 | 0.89 | 5.60 | 0.87 | 5.69 | 1.85 | 6.16 | 0.59 | WTMY/KOMY 0.037 |
| OncostatinM | 0.82 | 0.16 | 1.64 | 0.22 | 3.02 | 1.01 | 1.14 | 0.08 | WTMY/WTMA 0.023 |
| Pentraxin2 | 5.29 | 1.94 | 9.56 | 1.82 | 16.90 | 7.17 | 5.40 | 1.02 | |
| Pentraxin3 | 2.13 | 0.42 | 2.37 | 0.34 | 4.32 | 1.63 | 2.11 | 0.24 | WTMY/KOMY 0.038 KOMY/KOMA 0.031 |
| RBP4 | 12.34 | 1.14 | 10.30 | 0.98 | 16.72 | 4.68 | 12.37 | 1.01 | WTMY/KOMY 0.047 |
| Resistin | 25.96 | 4.31 | 24.51 | 3.03 | 28.58 | 11.08 | 48.34 | 1.17 | WTMA/KOMA 0.000 |
| SerpinE1 | 1.75 | 0.31 | 2.06 | 0.39 | 3.11 | 1.15 | 0.85 | 0.15 | WTMA/KOMA 0.028 KOMY/KOMA 0.017 |
| TIMP1 | 0.52 | 0.10 | 0.93 | 0.20 | 2.71 | 0.99 | 0.50 | 0.11 | |
| Knockout Pfp/Rag2−/− | Significance | ||||
|---|---|---|---|---|---|
| Young (KOMY) | Adult (KOMA) | ||||
| Relative Mean Pixel Density/mg Protein | |||||
| Mean | SEM | Mean | SEM | p | |
| Adiponectin | 249.76 | 1.05 | 247.81 | 3.50 | |
| AgRP | 8.44 | 1.35 | 12.52 | 0.88 | |
| ANGPL3 | 64.21 | 3.45 | 72.86 | 11.18 | |
| CRP | 249.80 | 0.27 | 249.49 | 1.34 | |
| DPPIV | 133.98 | 9.61 | 139.65 | 10.08 | |
| Endocan | 38.38 | 8.63 | 63.27 | 8.99 | |
| FetuinA | 183.56 | 11.24 | 196.04 | 6.99 | |
| FGFacidic | 250.70 | 0.85 | 249.87 | 0.33 | |
| FGF21 | 14.39 | 2.75 | 24.10 | 1.69 | 0.024 |
| HGF | 15.26 | 4.69 | 26.14 | 3.40 | |
| ICAM1 | 225.11 | 8.89 | 238.95 | 4.28 | |
| IGFI | 23.40 | 3.83 | 33.52 | 6.00 | |
| IGFII | 14.10 | 0.89 | 17.69 | 2.16 | |
| IGFBP1 | 63.02 | 18.20 | 31.54 | 5.64 | |
| IGFBP2 | 135.32 | 5.14 | 161.04 | 23.43 | |
| IGFBP3 | 211.48 | 6.13 | 230.40 | 7.45 | |
| IGFBP5 | 60.57 | 6.19 | 82.33 | 1.99 | 0.015 |
| IGFBP6 | 241.15 | 5.05 | 249.88 | 0.67 | |
| IL6 | 9.57 | 1.81 | 13.54 | 1.48 | |
| IL10 | 14.67 | 1.88 | 23.60 | 2.49 | 0.029 |
| IL11 | 15.93 | 2.44 | 22.92 | 1.46 | 0.049 |
| Leptin | 141.31 | 27.71 | 225.14 | 7.41 | 0.027 |
| LIF | 11.18 | 1.58 | 20.27 | 3.36 | 0.050 |
| Liopcalin2 | 249.91 | 1.90 | 242.53 | 4.43 | |
| MCP1 | 23.25 | 4.65 | 43.67 | 8.51 | |
| MCSF | 47.88 | 6.20 | 63.50 | 2.23 | 0.056 |
| OncostatinM | 14.31 | 1.64 | 16.99 | 2.05 | |
| Pentraxin2 | 23.80 | 2.94 | 19.12 | 2.71 | |
| Pentraxin3 | 32.22 | 3.04 | 43.15 | 4.21 | |
| Pref1 | 14.04 | 2.54 | 24.49 | 2.46 | 0.025 |
| RAGE | 19.37 | 3.23 | 21.24 | 2.43 | |
| RANTES | 17.97 | 2.22 | 32.60 | 5.21 | 0.041 |
| RBP4 | 94.19 | 9.55 | 92.23 | 4.47 | |
| Resistin | 248.49 | 1.82 | 245.53 | 1.03 | |
| SerpinE1 | 60.81 | 14.27 | 88.45 | 26.72 | |
| TIMP1 | 28.02 | 3.93 | 47.08 | 11.84 | |
| TNFa | 8.52 | 1.11 | 12.33 | 1.59 | |
| VEGF | 55.13 | 14.65 | 55.56 | 2.77 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winkler, S.; Hempel, M.; Hsu, M.-J.; Gericke, M.; Kühne, H.; Brückner, S.; Erler, S.; Burkhardt, R.; Christ, B. Immune-Deficient Pfp/Rag2−/− Mice Featured Higher Adipose Tissue Mass and Liver Lipid Accumulation with Growing Age than Wildtype C57BL/6N Mice. Cells 2019, 8, 775. https://doi.org/10.3390/cells8080775
Winkler S, Hempel M, Hsu M-J, Gericke M, Kühne H, Brückner S, Erler S, Burkhardt R, Christ B. Immune-Deficient Pfp/Rag2−/− Mice Featured Higher Adipose Tissue Mass and Liver Lipid Accumulation with Growing Age than Wildtype C57BL/6N Mice. Cells. 2019; 8(8):775. https://doi.org/10.3390/cells8080775
Chicago/Turabian StyleWinkler, Sandra, Madlen Hempel, Mei-Ju Hsu, Martin Gericke, Hagen Kühne, Sandra Brückner, Silvio Erler, Ralph Burkhardt, and Bruno Christ. 2019. "Immune-Deficient Pfp/Rag2−/− Mice Featured Higher Adipose Tissue Mass and Liver Lipid Accumulation with Growing Age than Wildtype C57BL/6N Mice" Cells 8, no. 8: 775. https://doi.org/10.3390/cells8080775





