Myeloid Dendritic Cells Are Enriched in Lymph Node Tissue of Early Rheumatoid Arthritis Patients but not in At Risk Individuals
Abstract
1. Introduction
2. Methods
2.1. Study Subjects and Lymph Node Biopsy Sampling
2.2. Isolation of Peripheral Blood Mononuclear Cells and Flow Cytometry Analysis
2.3. Immunofluorescence Microscopy
2.4. Statistics
3. Results
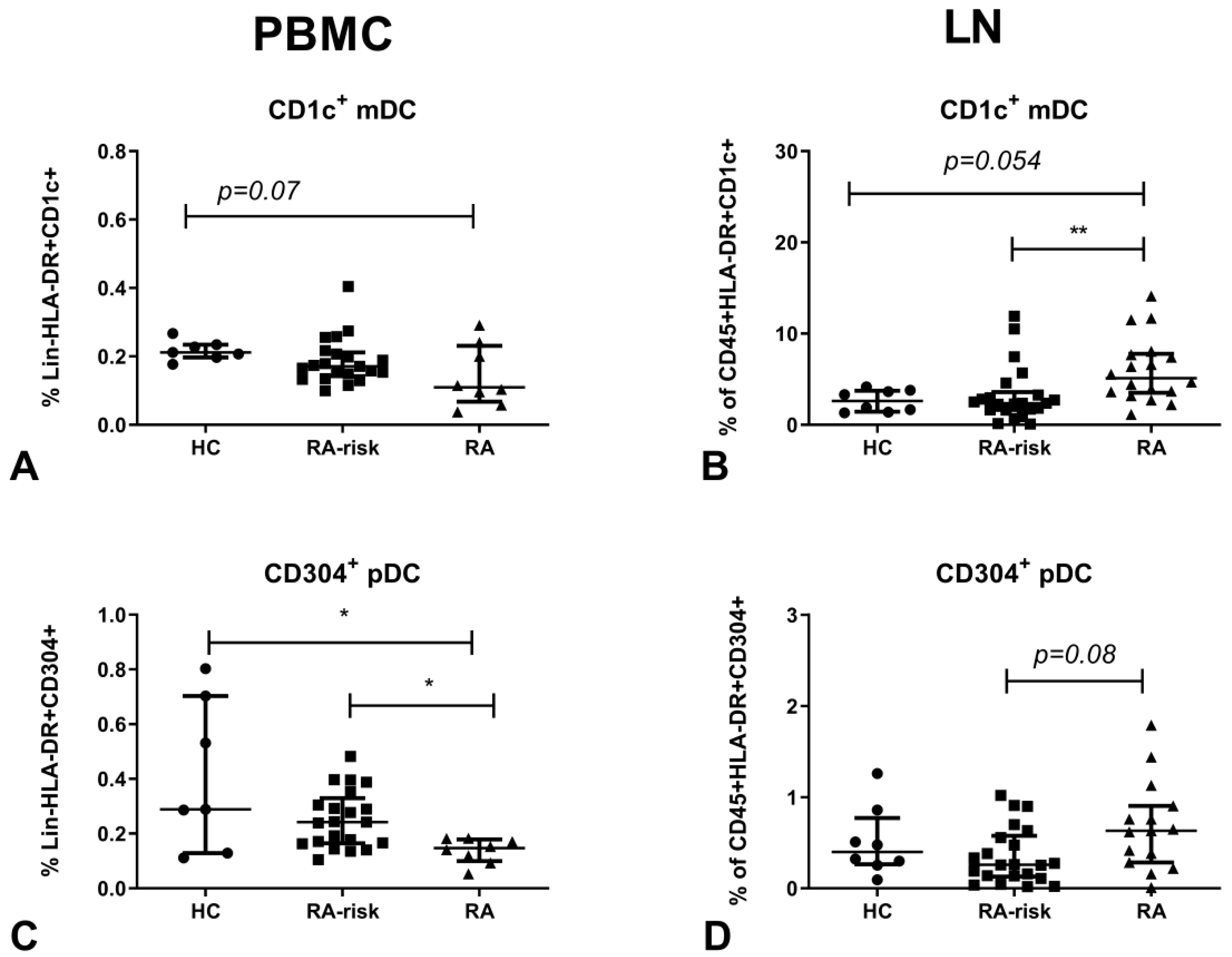
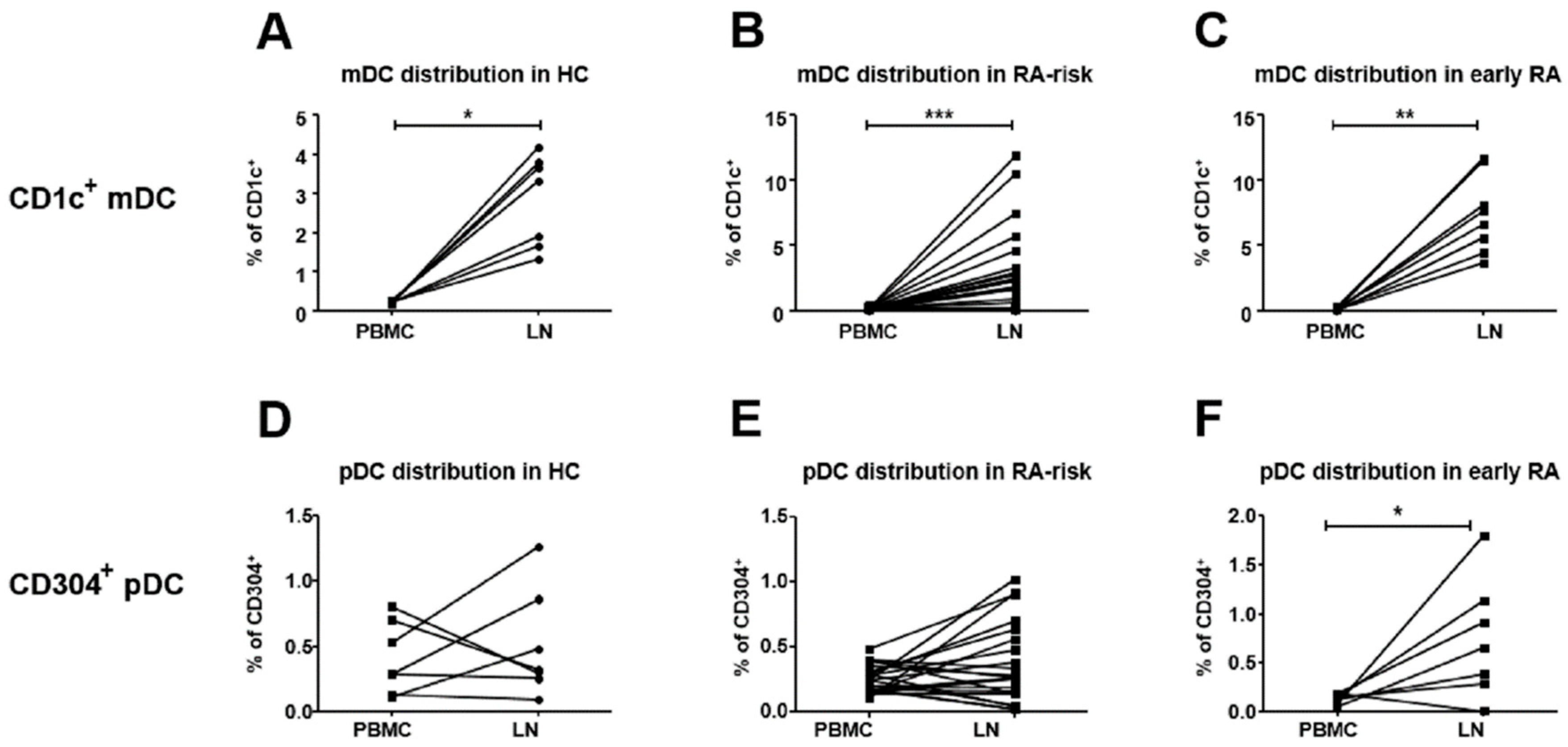
3.1. CD1c+ mDCs are Enriched in Human LN Tissue of Early-RA Patients
3.2. Compared to Blood, CD304+ DC Frequencies are Higher in Lymphoid Tissue of Early-RA Patients
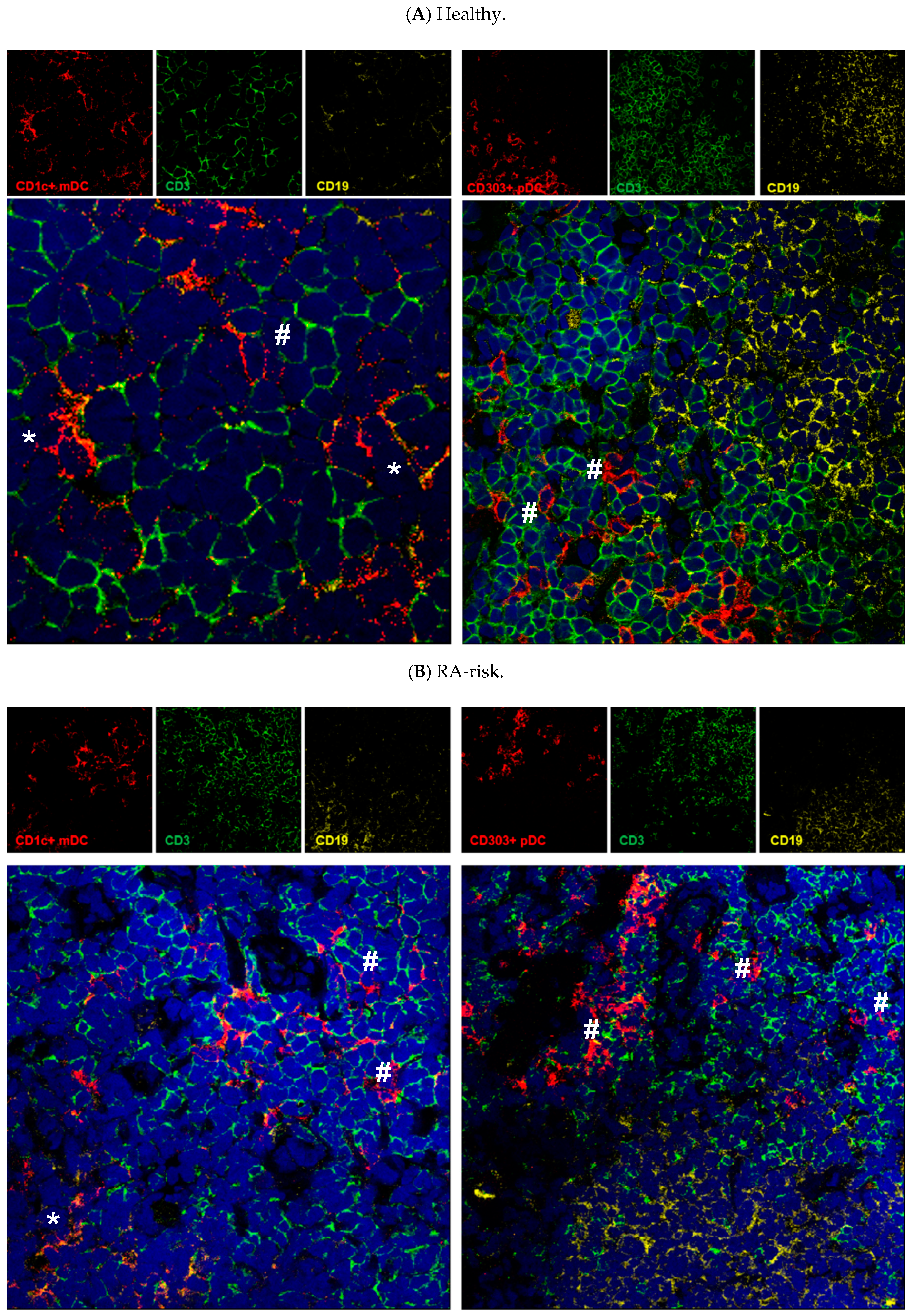
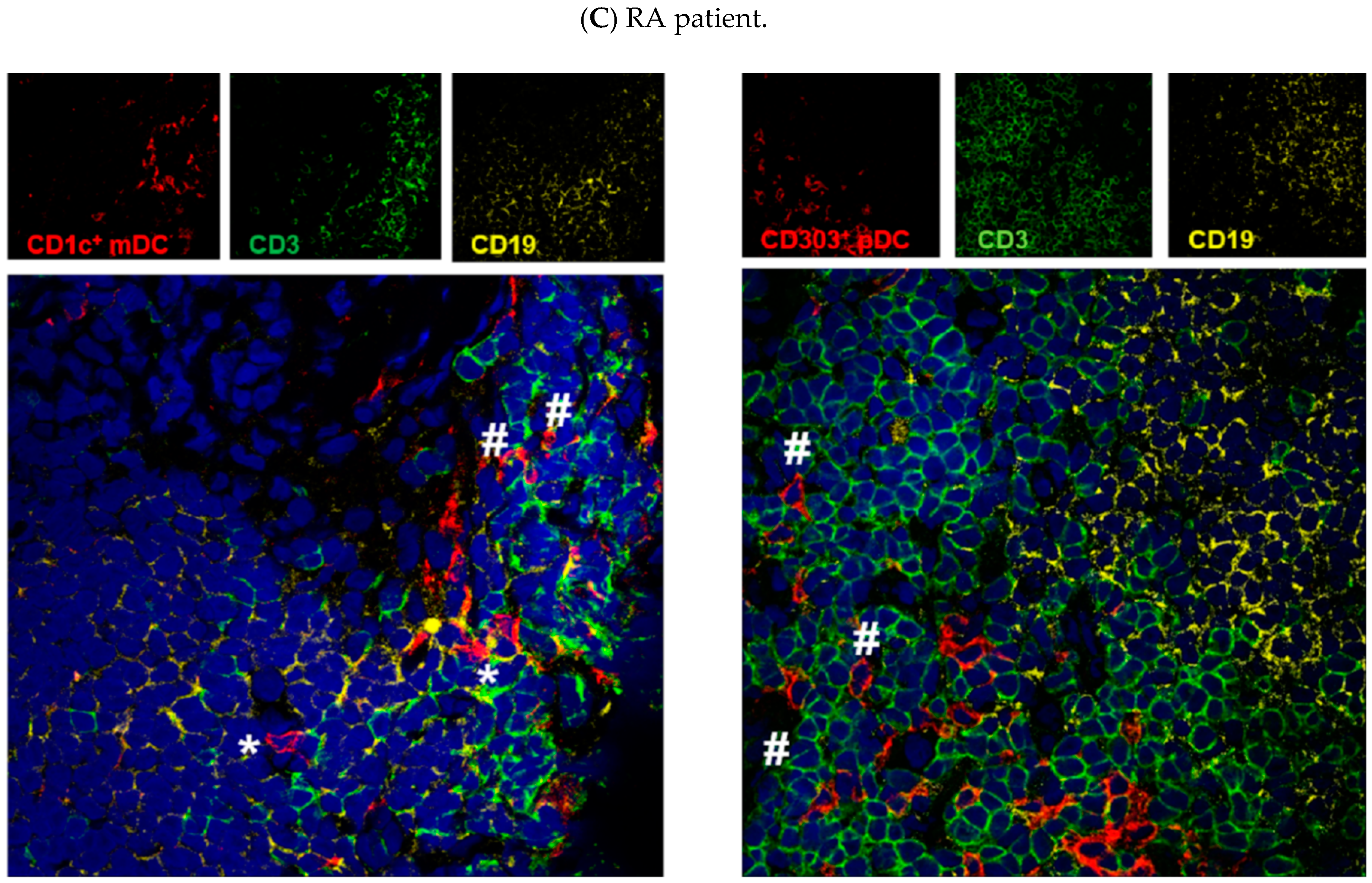
3.3. Both mDCs and pDCs Localize Mainly in T Cell Areas of Human Lymphoid Tissue
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Banchereau, J.; Schuler-Thurner, B.; Palucka, A.K.; Schuler, G. Dendritic cells as vectors for therapy. Cell 2001, 106, 271–274. [Google Scholar] [CrossRef]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cao, X. Regulatory dendritic cells in autoimmunity: A comprehensive review. J. Autoimmun. 2015, 63, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, D.; Haak, S.; Sisirak, V.; Reizis, B. The role of dendritic cells in autoimmunity. Nat. Rev. Immunol. 2013, 13, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Lipsky, P.E. Presentation of self peptides by dendritic cells: Possible implications for the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 1996, 39, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Merad, M.; Sathe, P.; Helft, J.; Miller, J.; Mortha, A. The dendritic cell lineage: Ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 2013, 31, 563–604. [Google Scholar] [CrossRef] [PubMed]
- Jongbloed, S.L.; Lebre, M.C.; Fraser, A.R.; Gracie, J.A.; Sturrock, R.D.; Tak, P.P.; McInnes, I.B. Enumeration and phenotypical analysis of distinct dendritic cell subsets in psoriatic arthritis and rheumatoid arthritis. Arthritis Res. Ther. 2006, 8, R15. [Google Scholar] [CrossRef]
- Lebre, M.C.; Jongbloed, S.L.; Tas, S.W.; Smeets, T.J.; McInnes, I.B.; Tak, P.P. Rheumatoid arthritis synovium contains two subsets of CD83-DC-LAMP- dendritic cells with distinct cytokine profiles. Am. J. Pathol. 2008, 172, 940–950. [Google Scholar] [CrossRef]
- Lebre, M.C.; Tak, P.P. Dendritic cell subsets: Their roles in rheumatoid arthritis. Acta Reumatol. Port. 2008, 33, 35–45. [Google Scholar]
- Amodio, G.; Gregori, S. Dendritic cells a double-edge sword in autoimmune responses. Front. Immunol. 2012, 3, 233. [Google Scholar] [CrossRef]
- Hawiger, D.; Inaba, K.; Dorsett, Y.; Guo, M.; Mahnke, K.; Rivera, M.; Ravetch, J.V.; Steinman, R.M.; Nussenzweig, M.C. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 2001, 194, 769–779. [Google Scholar] [CrossRef]
- Iberg, C.A.; Jones, A.; Hawiger, D. Dendritic Cells As Inducers of Peripheral Tolerance. Trends Immunol. 2017, 38, 793–804. [Google Scholar] [CrossRef]
- Drakesmith, H.; Chain, B.; Beverley, P. How can dendritic cells cause autoimmune disease? Immunol. Today 2000, 21, 214–217. [Google Scholar] [CrossRef]
- Benson, R.A.; Patakas, A.; Conigliaro, P.; Rush, C.M.; Garside, P.; McInnes, I.B.; Brewer, J.M. Identifying the cells breaching self-tolerance in autoimmunity. J. Immunol. 2010, 184, 6378–6385. [Google Scholar] [CrossRef]
- Lebre, M.C.; Tak, P.P. Dendritic cells in rheumatoid arthritis: Which subset should be used as a tool to induce tolerance? Hum. Immunol. 2009, 70, 321–324. [Google Scholar] [CrossRef]
- Jongbloed, S.L.; Benson, R.A.; Nickdel, M.B.; Garside, P.; McInnes, I.B.; Brewer, J.M. Plasmacytoid dendritic cells regulate breach of self-tolerance in autoimmune arthritis. J. Immunol. 2009, 182, 963–968. [Google Scholar] [CrossRef]
- Jaen, O.; Rulle, S.; Bessis, N.; Zago, A.; Boissier, M.C.; Falgarone, G. Dendritic cells modulated by innate immunity improve collagen-induced arthritis and induce regulatory T cells in vivo. Immunology 2009, 126, 35–44. [Google Scholar] [CrossRef]
- Leung, B.P.; Conacher, M.; Hunter, D.; McInnes, I.B.; Liew, F.Y.; Brewer, J.M. A novel dendritic cell-induced model of erosive inflammatory arthritis: Distinct roles for dendritic cells in T cell activation and induction of local inflammation. J. Immunol. 2002, 169, 7071–7077. [Google Scholar] [CrossRef]
- Moret, F.M.; Hack, C.E.; van der Wurff-Jacobs, K.M.; de Jager, W.; Radstake, T.R.; Lafeber, F.P.; van Roon, J.A. Intra-articular CD1c-expressing myeloid dendritic cells from rheumatoid arthritis patients express a unique set of T cell-attracting chemokines and spontaneously induce Th1, Th17 and Th2 cell activity. Arthritis Res. Ther. 2013, 15, R155. [Google Scholar] [CrossRef]
- Kavousanaki, M.; Makrigiannakis, A.; Boumpas, D.; Verginis, P. Novel role of plasmacytoid dendritic cells in humans: Induction of interleukin-10-producing Treg cells by plasmacytoid dendritic cells in patients with rheumatoid arthritis responding to therapy. Arthritis Rheum. 2010, 62, 53–63. [Google Scholar] [CrossRef]
- Lande, R.; Giacomini, E.; Serafini, B.; Rosicarelli, B.; Sebastiani, G.D.; Minisola, G.; Tarantino, U.; Riccieri, V.; Valesini, G.; Coccia, E.M. Characterization and recruitment of plasmacytoid dendritic cells in synovial fluid and tissue of patients with chronic inflammatory arthritis. J. Immunol. 2004, 173, 2815–2824. [Google Scholar] [CrossRef]
- van Beers-Tas, M.H.; Stuiver, M.M.; de Koning, M.; van de Stadt, L.A.; Geskus, R.B.; van Schaardenburg, D. Can an increase in autoantibody levels predict arthritis in arthralgia patients? Rheumatol. (Oxf.) 2018, 57, 932–934. [Google Scholar] [CrossRef]
- Gerlag, D.M.; Raza, K.; van Baarsen, L.G.; Brouwer, E.; Buckley, C.D.; Burmester, G.R.; Gabay, C.; Catrina, A.I.; Cope, A.P.; Cornelis, F.; et al. EULAR recommendations for terminology and research in individuals at risk of rheumatoid arthritis: Report from the Study Group for Risk Factors for Rheumatoid Arthritis. Ann. Rheum. Dis. 2012, 71, 638–641. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., III; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef]
- De Hair, M.J.; Zijlstra, I.A.; Boumans, M.J.; van de Sande, M.G.; Maas, M.; Gerlag, D.M.; Tak, P.P. Hunting for the pathogenesis of rheumatoid arthritis: Core-needle biopsy of inguinal lymph nodes as a new research tool. Ann. Rheum Dis. 2012, 71, 1911–1912. [Google Scholar] [CrossRef]
- Bertram, K.M.; Botting, R.A.; Baharlou, H.; Rhodes, J.W.; Rana, H.; Graham, J.D.; Patrick, E.; Fletcher, J.; Plasto, T.M.; Truong, N.R.; et al. Identification of HIV transmitting CD11c(+) human epidermal dendritic cells. Nat. Commun. 2019, 10, 2759. [Google Scholar] [CrossRef]
- Canavan, M.; Walsh, A.M.; Bhargava, V.; Wade, S.M.; McGarry, T.; Marzaioli, V.; Moran, B.; Biniecka, M.; Convery, H.; Wade, S.; et al. Enriched Cd141+ DCs in the joint are transcriptionally distinct, activated, and contribute to joint pathogenesis. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Angel, C.E.; Chen, C.J.; Horlacher, O.C.; Winkler, S.; John, T.; Browning, J.; MacGregor, D.; Cebon, J.; Dunbar, P.R. Distinctive localization of antigen-presenting cells in human lymph nodes. Blood 2009, 113, 1257–1267. [Google Scholar] [CrossRef]
- Cella, M.; Jarrossay, D.; Facchetti, F.; Alebardi, O.; Nakajima, H.; Lanzavecchia, A.; Colonna, M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 1999, 5, 919–923. [Google Scholar] [CrossRef]
- Cucak, H.; Yrlid, U.; Reizis, B.; Kalinke, U.; Johansson-Lindbom, B. Type I interferon signaling in dendritic cells stimulates the development of lymph-node-resident T follicular helper cells. Immunity 2009, 31, 491–501. [Google Scholar] [CrossRef]
- Jego, G.; Palucka, A.K.; Blanck, J.P.; Chalouni, C.; Pascual, V.; Banchereau, J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity 2003, 19, 225–234. [Google Scholar] [CrossRef]
- Poeck, H.; Wagner, M.; Battiany, J.; Rothenfusser, S.; Wellisch, D.; Hornung, V.; Jahrsdorfer, B.; Giese, T.; Endres, S.; Hartmann, G. Plasmacytoid dendritic cells, antigen, and CpG-C license human B cells for plasma cell differentiation and immunoglobulin production in the absence of T-cell help. Blood 2004, 103, 3058–3064. [Google Scholar] [CrossRef]
- Randolph, G.J.; Ochando, J.; Partida-Sanchez, S. Migration of dendritic cell subsets and their precursors. Annu. Rev. Immunol. 2008, 26, 293–316. [Google Scholar] [CrossRef]
- Nizzoli, G.; Krietsch, J.; Weick, A.; Steinfelder, S.; Facciotti, F.; Gruarin, P.; Bianco, A.; Steckel, B.; Moro, M.; Crosti, M.; et al. Human CD1c+ dendritic cells secrete high levels of IL-12 and potently prime cytotoxic T-cell responses. Blood 2013, 122, 932–942. [Google Scholar] [CrossRef]
- Schmitt, N.; Morita, R.; Bourdery, L.; Bentebibel, S.E.; Zurawski, S.M.; Banchereau, J.; Ueno, H. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity 2009, 31, 158–169. [Google Scholar] [CrossRef]
- Carvalheiro, H.; da Silva, J.A.; Souto-Carneiro, M.M. Potential roles for CD8(+) T cells in rheumatoid arthritis. Autoimmun. Rev. 2013, 12, 401–409. [Google Scholar] [CrossRef]
- Petrelli, A.; van Wijk, F. CD8(+) T cells in human autoimmune arthritis: The unusual suspects. Nat. Rev. Rheumatol 2016, 12, 421–428. [Google Scholar] [CrossRef]
- Wehr, P.; Purvis, H.; Law, S.C.; Thomas, R. Dendritic cells, T cells and their interaction in rheumatoid arthritis. Clin. Exp. Immunol. 2019, 196, 12–27. [Google Scholar] [CrossRef]
- Ueno, H.; Schmitt, N.; Palucka, A.K.; Banchereau, J. Dendritic cells and humoral immunity in humans. Immunol. Cell Biol. 2010, 88, 376–380. [Google Scholar] [CrossRef]
| Demographics | HCs | RA-Risk | Early Arthritis (RA) |
|---|---|---|---|
| n = 8 | n = 22 | n = 16 | |
| Sex, female (%) | 6 (75) | 18 (82) | 10 (63) |
| Age (years) (median (IQR)) | 34.0 (28.0–41.8) | 49.0 (43.5–57.5) | 49.0 (38.0–57.0) |
| IgM-RF positive (n (%)) | 0 (0) | 9 (41) | 15 (94) |
| IgM-RF level (kU/L) (median ((IQR)) | 1.0 (1.0–1.5) | 21.0 (3.0–117.5) | 182.0 (45.5–312.0) |
| ACPA positive (n (%)) | 0 (0) | 13 (59) | 14 (88) |
| ACPA level (kAU/L) (median (IQR)) | 2.5 (1.8–3.3) | 47.0 (4.5–202.0) | 119.0 (22.5–865.5) |
| IgM-RF and ACPA both pos. (n (%)) | 0 (0) | 0 (0) | 13 (81) |
| ESR (mm/h) median (IQR)) | nd | 8.0 (3.5–11.0) | 12.0 (6.5–22.0) |
| CRP (mg/L) (median (IQR)) | 0.7 (0.4–1.1) | 1.9 (0.9–4.3) | 4.6 (1.9–9.1) |
| 68 TJC (n) (median (IQR)) | 0 (0) | 2.0 (1.0–3.0) | 14.0 (5.0–23.5) |
| 66 SJC (n) (median (IQR)) | 0 (0) | 0 (0) | 7.0 (4.5–11.0) |
| DAS 28 (median (IQR)) | 4.6 (3.6–5.8) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramwadhdoebe, T.H.; Ramos, M.I.; Maijer, K.I.; van Lienden, K.P.; Maas, M.; Gerlag, D.M.; Tak, P.P.; Lebre, M.C.; van Baarsen, L.G.M. Myeloid Dendritic Cells Are Enriched in Lymph Node Tissue of Early Rheumatoid Arthritis Patients but not in At Risk Individuals. Cells 2019, 8, 756. https://doi.org/10.3390/cells8070756
Ramwadhdoebe TH, Ramos MI, Maijer KI, van Lienden KP, Maas M, Gerlag DM, Tak PP, Lebre MC, van Baarsen LGM. Myeloid Dendritic Cells Are Enriched in Lymph Node Tissue of Early Rheumatoid Arthritis Patients but not in At Risk Individuals. Cells. 2019; 8(7):756. https://doi.org/10.3390/cells8070756
Chicago/Turabian StyleRamwadhdoebe, T.H., M.I. Ramos, K.I. Maijer, K.P. van Lienden, M. Maas, D.M. Gerlag, P.P. Tak, M.C. Lebre, and L.G.M. van Baarsen. 2019. "Myeloid Dendritic Cells Are Enriched in Lymph Node Tissue of Early Rheumatoid Arthritis Patients but not in At Risk Individuals" Cells 8, no. 7: 756. https://doi.org/10.3390/cells8070756
APA StyleRamwadhdoebe, T. H., Ramos, M. I., Maijer, K. I., van Lienden, K. P., Maas, M., Gerlag, D. M., Tak, P. P., Lebre, M. C., & van Baarsen, L. G. M. (2019). Myeloid Dendritic Cells Are Enriched in Lymph Node Tissue of Early Rheumatoid Arthritis Patients but not in At Risk Individuals. Cells, 8(7), 756. https://doi.org/10.3390/cells8070756






