The Role of Phosphatases in Nuclear Envelope Disassembly and Reassembly and Their Relevance to Pathologies
Abstract
:1. Introduction
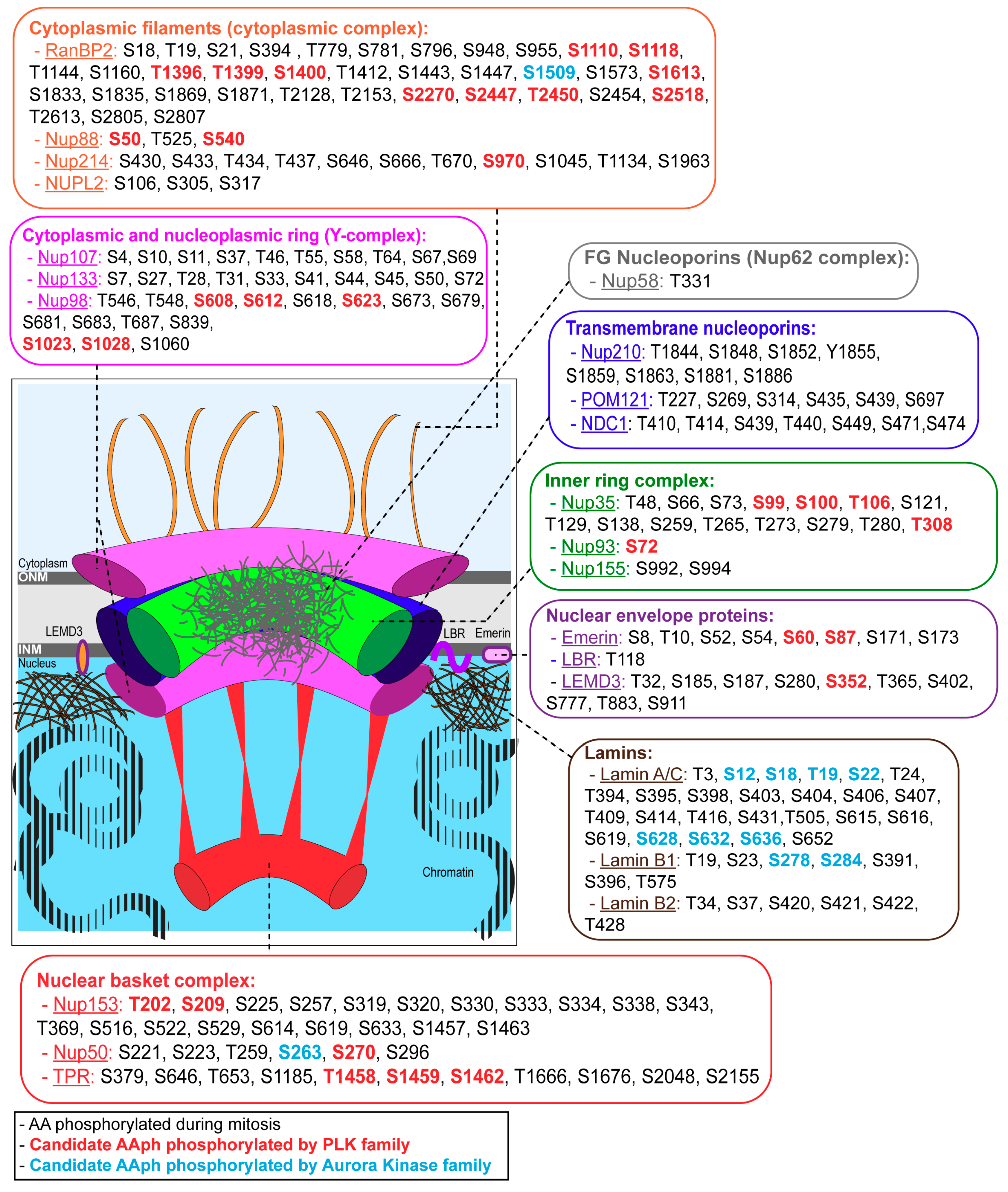
2. Nuclear Envelope Structure and Composition
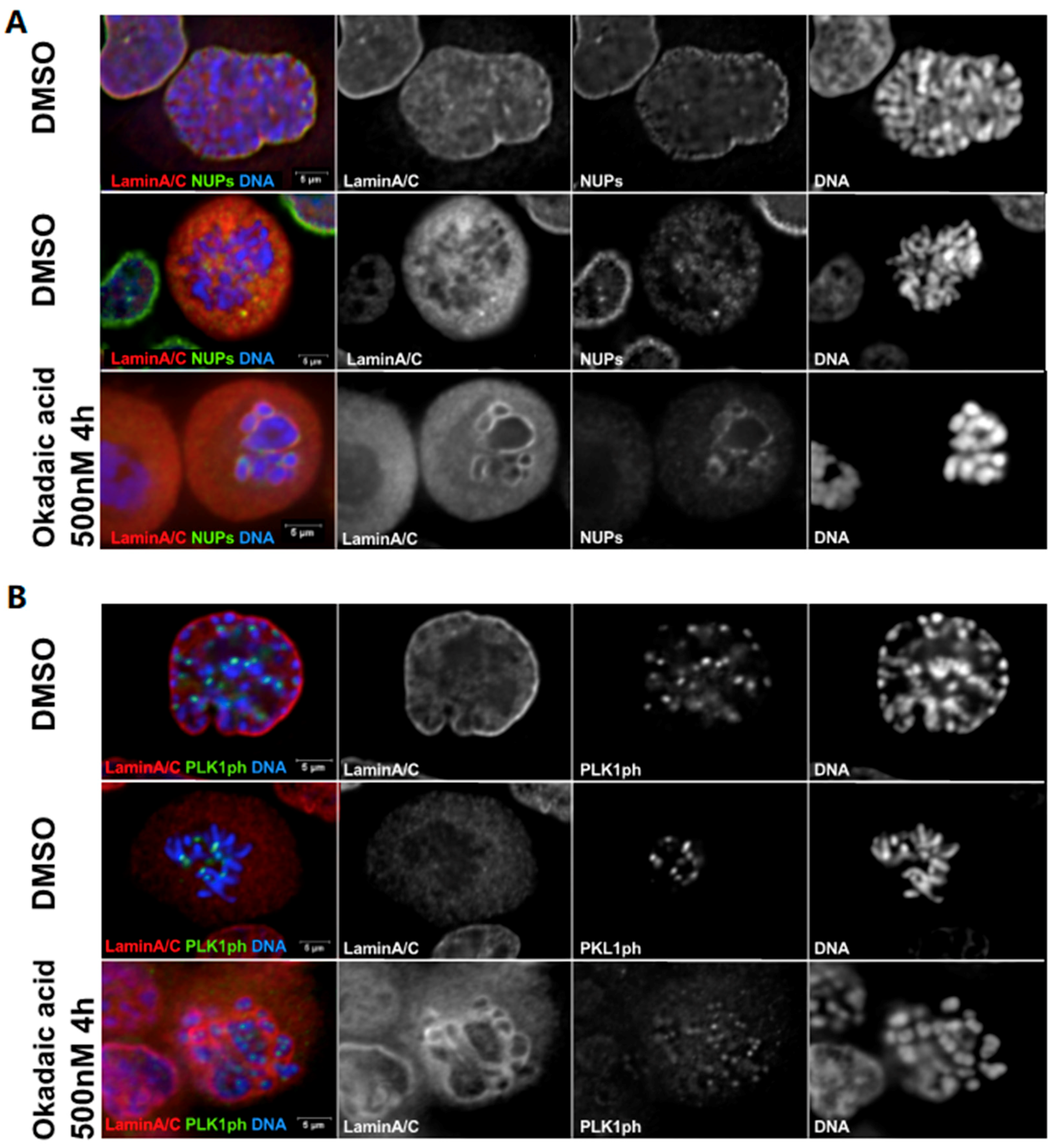
3. Nuclear Envelope Disassembly
4. Nuclear Envelope Reassembly
4.1. Lamina Re-Assembly
4.2. NPC Re-Assembly
4.3. Membrane Re-Assembly
5. Potential for Disease
6. Conclusion and Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Egli, D.; Birkhoff, G.; Eggan, K. Mediators of reprogramming: Transcription factors and transitions through mitosis. Nat. Rev. Mol. Cell Biol. 2008, 9, 505. [Google Scholar] [CrossRef] [PubMed]
- Vagnarelli, P.; Ribeiro, S.; Sennels, L.; Sanchez-Pulido, L.; de, L.A.; Verheyen, T.; Kelly, D.A.; Ponting, C.P.; Rappsilber, J.; Earnshaw, W.C. Repo-Man coordinates chromosomal reorganization with nuclear envelope reassembly during mitotic exit. Dev. Cell 2011, 21, 328–342. [Google Scholar] [CrossRef] [PubMed]
- Gerace, L.; Burke, B. Functional Organization of the Nuclear Envelope. Annu. Rev. Cell Biol. 1988, 4, 335–374. [Google Scholar] [CrossRef] [PubMed]
- Reichelt, R.; Holzenburg, A.; Buhle, E.L.; Jarnik, M.; Engel, A.; Aebi, U. Correlation between structure and mass distribution of the nuclear pore complex and of distinct pore complex components. J. Cell Biol. 1990, 110, 883. [Google Scholar] [CrossRef] [PubMed]
- Strambio-De-Castillia, C.; Niepel, M.; Rout, M.P. The nuclear pore complex: Bridging nuclear transport and gene regulation. Nat. Rev. Mol. Cell Biol. 2010, 11, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; Osmani, S.A. Poring over chromosomes: Mitotic nuclear pore complex segregation. Curr. Opin. Cell Biol. 2019, 58, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Cronshaw, J.M.; Krutchinsky, A.N.; Zhang, W.; Chait, B.T.; Matunis, M.J. Proteomic analysis of the mammalian nuclear pore complex. J. Cell Biol. 2002, 158, 915–927. [Google Scholar] [CrossRef] [Green Version]
- Beck, M.; Hurt, E. The nuclear pore complex: Understanding its function through structural insight. Nat. Reviews. Mol. Cell Biol. 2017, 18, 73–89. [Google Scholar] [CrossRef]
- Dechat, T.; Pfleghaar, K.; Sengupta, K.; Shimi, T.; Shumaker, D.K.; Solimando, L.; Goldman, R.D. Nuclear lamins: Major factors in the structural organization and function of the nucleus and chromatin. Genes Dev. 2008, 22, 832–853. [Google Scholar] [CrossRef]
- De Leeuw, R.; Gruenbaum, Y.; Medalia, O. Nuclear Lamins: Thin Filaments with Major Functions. Trends Cell Biol. 2018, 28, 34–45. [Google Scholar] [CrossRef]
- Wurzenberger, C.; Gerlich, D.W. Phosphatases: Providing safe passage through mitotic exit. Nat. Rev. Mol. Cell Biol. 2011, 12, 469. [Google Scholar] [CrossRef] [PubMed]
- Castilho, P.V.; Williams, B.C.; Mochida, S.; Zhao, Y.; Goldberg, M.L. The M Phase Kinase Greatwall (Gwl) Promotes Inactivation of PP2A/B55δ, a Phosphatase Directed Against CDK Phosphosites. Mol. Biol. Cell 2009, 20, 4777–4789. [Google Scholar] [CrossRef] [PubMed]
- Burgess, A.; Vigneron, S.; Brioudes, E.; Labbé, J.; Lorca, T.; Castro, A. Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc. Natl. Acad. Sci. USA 2010, 107, 12564. [Google Scholar] [CrossRef] [PubMed]
- Lorca, T.; Castro, A. The Greatwall kinase: A new pathway in the control of the cell cycle. Oncogene 2012, 32, 537. [Google Scholar] [CrossRef] [PubMed]
- Dohadwala, M.; da Cruz e Silva, E.F.; Hall, F.L.; Williams, R.T.; Carbonaro-Hall, D.; Nairn, A.C.; Greengard, P.; Berndt, N. Phosphorylation and inactivation of protein phosphatase 1 by cyclin-dependent kinases. Proc. Natl. Acad. Sci. USA 1994, 91, 6408. [Google Scholar] [CrossRef] [PubMed]
- Mochida, S.; Ikeo, S.; Gannon, J.; Hunt, T. Regulated activity of PP2A-B55δ is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. Embo J. 2009, 28, 2777–2785. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Lee, S.Y.; Choi, Y.; Greengard, P.; Nairn, A.C. Cell Cycle-Dependent Phosphorylation of Mammalian Protein Phosphatase 1 by cdc2 Kinase. Proc. Natl. Acad. Sci. USA 1997, 94, 2168–2173. [Google Scholar] [CrossRef]
- Grallert, A.; Boke, E.; Hagting, A.; Hodgson, B.; Connolly, Y.; Griffiths, J.R.; Smith, D.L.; Pines, J.; Hagan, I.M. A PP1-PP2A phosphatase relay controls mitotic progression. Nature 2015, 517, 94–98. [Google Scholar] [CrossRef]
- Laurell, E.; Beck, K.; Krupina, K.; Theerthagiri, G.; Bodenmiller, B.; Horvath, P.; Aebersold, R.; Antonin, W.; Kutay, U. Phosphorylation of Nup98 by Multiple Kinases Is Crucial for NPC Disassembly during Mitotic Entry. Cell 2011, 144, 539–550. [Google Scholar] [CrossRef] [Green Version]
- Linder, M.I.; Köhler, M.; Boersema, P.; Weberruss, M.; Wandke, C.; Marino, J.; Ashiono, C.; Picotti, P.; Antonin, W.; Kutay, U. Mitotic Disassembly of Nuclear Pore Complexes Involves CDK1- and PLK1-Mediated Phosphorylation of Key Interconnecting Nucleoporins. Dev. Cell 2017, 43, 141. [Google Scholar] [CrossRef]
- Dephoure, N.; Zhou, C.; Villén, J.; Beausoleil, S.A.; Bakalarski, C.E.; Elledge, S.J.; Gygi, S.P. A quantitative atlas of mitotic phosphorylation. Proc. Natl. Acad. Sci. USA 2008, 105, 10762–10767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, J.V.; Vermeulen, M.; Santamaria, A.; Kumar, C.; Miller, M.L.; Jensen, L.J.; Gnad, F.; Cox, J.; Jensen, T.S.; Nigg, E.A.; et al. Quantitative Phosphoproteomics Reveals Widespread Full Phosphorylation Site Occupancy During Mitosis. Sci. Signal. 2010, 3, ra3. [Google Scholar] [CrossRef] [PubMed]
- Kettenbach, A.N.; Schweppe, D.K.; Faherty, B.K.; Pechenick, D.; Pletnev, A.A.; Gerber, S.A. Quantitative phosphoproteomics identifies substrates and functional modules of Aurora and Polo-like kinase activities in mitotic cells. Sci. Signal. 2011, 4, rs5. [Google Scholar] [CrossRef] [PubMed]
- Laurell, E.; Kutay, U. Dismantling the NPC permeability barrier at the onset of mitosis. Cell Cycle 2011, 10, 2243–2245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martino, L.; Morchoisne-Bolhy, S.; Cheerambathur, D.K.; Van Hove, L.; Dumont, J.; Joly, N.; Desai, A.; Doye, V.; Pintard, L. Channel Nucleoporins Recruit PLK-1 to Nuclear Pore Complexes to Direct Nuclear Envelope Breakdown in C. elegans. Dev. Cell 2017, 43, 157–171. [Google Scholar] [CrossRef] [PubMed]
- De Castro, I.J.; Gil, R.S.; Ligammari, L.; Di Giacinto, M.L.; Vagnarelli, P. CDK1 and PLK1 coordinate the disassembly and reassembly of the nuclear envelope in vertebrate mitosis. Oncotarget 2018, 9, 7763–7773. [Google Scholar] [CrossRef] [PubMed]
- Peter, M.; Nakagawa, J.; Doree, M.; Labbe, J.C.; Nigg, E.A. In Vitro Disassemblyof of the Nuclear Lamina and M Phase-Specific Phosphorylation of Lamins by cdc2 Kinase. Cell 1990, 61, 591–602. [Google Scholar] [CrossRef]
- Heald, R.; McKeon, F. Mutations of phosphorylation sites in lamin A that prevent nuclear lamina disassembly in mitosis. Cell 1990, 61, 579–589. [Google Scholar] [CrossRef]
- Collas, P.; Thompson, L.; Fields, A.P.; Poccia, D.L.; Courvalin, J. Protein Kinase C-mediated Interphase Lamin B Phosphorylation and Solubilization. J. Biol. Chem. 1997, 272, 21274–21280. [Google Scholar] [CrossRef] [Green Version]
- Goss, V.L.; Hocevar, B.A.; Thompson, L.; Stratton, C.A.; Burns, D.J.; Fields, A.P. Identification of Nuclear BII Protein Kinase C as a Mitotic Lamin Kinase. J. Biol. Chem. 1994, 269, 19074–19080. [Google Scholar]
- Gorjanacz, M.; Klerkx, E.P.F.; Galy, V.; Santarella, R.; Lopez-Iglesias, C.; Askjaer, P.; Mattaj, I.W. Caenorhabditis elegans BAF-1 and its kinase VRK-1 participate directly in post-mitotic nuclear envelope assembly. Embo J. 2007, 26, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Champion, L.; Pawar, S.; Luithle, N.; Ungricht, R.; Kutay, U. Dissociation of membrane–chromatin contacts is required for proper chromosome segregation in mitosis. Mol. Biol. Cell 2019, 30, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Álvarez, A.; Cooper, J.P. Chromosomes Orchestrate Their Own Liberation: Nuclear Envelope Disassembly. Trends Cell Biol. 2016, 27, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Timofeev, O.; Cizmecioglu, O.; Settele, F.; Kempf, T.; Hoffmann, I. Cdc25 Phosphatases Are Required for Timely Assembly of CDK1-Cyclin B at the G2/M Transition. J. Biol. Chem. 2010, 285, 16978–16990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanda, R.; Eguchi-Kasai, K.; Hayata, I. Phosphatase Inhibitors and Premature Chromosome Condensation in Human Peripheral Lymphocytes at Different Cell-Cycle Phases. Somat. Cell Mol. Genet. 1999, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cornforth, M.N.; Bedford, J.S. High-resolution measurement of breaks in prematurely condensed chromosomes by differential staining. Chromosoma 1983, 88, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Srebniak, M.I.; Trapp, G.G.; Wawrzkiewicz, A.K.; Kaźmierczak, W.; Wiczkowski, A.K. The Usefulness of Calyculin A for Cytogenetic Prenatal Diagnosis. J. Histochem. Cytochem. 2005, 53, 391–394. [Google Scholar] [CrossRef]
- Wieser, S.; Pines, J. The biochemistry of mitosis. Cold Spring Harb. Perspect. Biol. 2015, 7, a015776. [Google Scholar] [CrossRef]
- Gelens, L.; Qian, J.; Bollen, M.; Saurin, A.T. The Importance of Kinase–Phosphatase Integration: Lessons from Mitosis. Trends Cell Biol. 2018, 28, 6–21. [Google Scholar] [CrossRef]
- Trimborn, M.; Bell, S.M.; Felix, C.; Rashid, Y.; Jafri, H.; Griffiths, P.D.; Neumann, L.M.; Krebs, A.; Reis, A.; Sperling, K.; et al. Mutations in microcephalin cause aberrant regulation of chromosome condensation. Am. J. Hum. Genet. 2004, 75, 261–266. [Google Scholar] [CrossRef]
- Platani, M.; Santarella-Mellwig, R.; Posch, M.; Walczak, R.; Swedlow, J.R.; Mattaj, I.W. The Nup107-160 nucleoporin complex promotes mitotic events via control of the localization state of the chromosome passenger complex. Mol. Biol. Cell 2009, 20, 5260–5275. [Google Scholar] [CrossRef] [PubMed]
- Salina, D.; Enarson, P.; Rattner, J.B.; Burke, B. Nup358 integrates nuclear envelope breakdown with kinetochore assembly. J. Cell Biol. 2003, 162, 991–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belgareh, N.; Rabut, G.; Baï, S.W.; van Overbeek, M.; Beaudouin, J.; Daigle, N.; Zatsepina, O.V.; Pasteau, F.; Labas, V.; Fromont-Racine, M.; et al. An evolutionarily conserved NPC subcomplex, which redistributes in part to kinetochores in mammalian cells. J. Cell Biol. 2001, 154, 1147–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Lee, K.K.; Segura-Totten, M.; Neufeld, E.; Wilson, K.L.; Gruenbaum, Y. MAN1 and emerin have overlapping function(s) essential for chromosome segregation and cell division in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2003, 100, 4598. [Google Scholar] [CrossRef] [PubMed]
- Chatel, G.; Fahrenkrog, B. Nucleoporins: Leaving the nuclear pore complex for a successful mitosis. Cell. Signal. 2011, 23, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, R.A.; Gudise, S.; Hallberg, E. Microtubule-associated nuclear envelope proteins in interphase and mitosis. Biochem. Soc. Trans. 2011, 39, 1786. [Google Scholar] [CrossRef] [PubMed]
- Hezwani, M.; Fahrenkrog, B. The functional versatility of the nuclear pore complex proteins. Semin. Cell Dev. Biol. 2017, 68, 2–9. [Google Scholar] [CrossRef]
- De Magistris, P.; Antonin, W. The Dynamic Nature of the Nuclear Envelope. Curr. Biol. 2018, 28, R497. [Google Scholar] [CrossRef]
- Murray, A.W.; Solomon, M.J.; Kirschner, M.W. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature 1989, 339, 280–286. [Google Scholar] [CrossRef]
- Skoufias, D.A.; Indorato, R.; Lacroix, F.; Panopoulos, A.; Margolis, R.L. Mitosis persists in the absence of Cdk1 activity when proteolysis or protein phosphatase activity is suppressed. J. Cell Biol. 2007, 179, 671–685. [Google Scholar] [CrossRef] [Green Version]
- Ungricht, R.; Kutay, U. Mechanisms and functions of nuclear envelope remodelling. Nat. Rev. Mol. Cell Biol. 2017, 18, 229. [Google Scholar] [CrossRef]
- Steen, R.L.; Martins, S.B.; Taskén, K.; Collas, P. Recruitment of protein phosphatase 1 to the nuclear envelope by A-kinase anchoring protein AKAP149 is a prerequisite for nuclear lamina assembly. J. Cell Biol. 2000, 150, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Afonso, O.; Matos, I.; Pereira, A.J.; Aguiar, P.; Lampson, M.A.; Maiato, H. Feedback control of chromosome separation by a midzone Aurora B gradient. Science 2014, 345, 332–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moriuchi, T.; Kuroda, M.; Kusumoto, F.; Osumi, T.; Hirose, F. Lamin A reassembly at the end of mitosis is regulated by its SUMO-interacting motif. Exp. Cell Res. 2016, 342, 83–94. [Google Scholar] [CrossRef]
- Hached, K.; Goguet, P.; Charrasse, S.; Vigneron, S.; Sacristan, M.P.; Lorca, T.; Castro, A. ENSA and ARPP19 differentially control cell cycle progression and development. J. Cell Biol. 2019, 218, 541–558. [Google Scholar] [CrossRef] [PubMed]
- Rasala, B.A.; Ramos, C.; Harel, A.; Forbes, D.J. Capture of AT-rich chromatin by ELYS recruits POM121 and NDC1 to initiate nuclear pore assembly. Mol. Biol. Cell 2008, 19, 3982–3996. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, Y.; Zhang, P.; Jeffrey, P.D.; Shi, Y. Structure of a Protein Phosphatase 2A Holoenzyme: Insights into B55-Mediated Tau Dephosphorylation. Mol. Cell 2008, 31, 873–885. [Google Scholar] [CrossRef] [Green Version]
- Galy, V.; Askjaer, P.; Franz, C.; López-Iglesias, C.; Mattaj, I.W. MEL-28, a Novel Nuclear-Envelope and Kinetochore Protein Essential for Zygotic Nuclear-Envelope Assembly in C. elegans. Curr. Biol. 2006, 16, 1748–1756. [Google Scholar] [CrossRef]
- Rasala, B.A.; Orjalo, A.V.; Shen, Z.; Briggs, S.; Forbes, D.J. ELYS is a dual nucleoporin/kinetochore protein required for nuclear pore assembly and proper cell division. Proc. Natl. Acad. Sci. USA 2006, 103, 17801–17806. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, A.G.; Piano, F. MEL-28 Is Downstream of the Ran Cycle and Is Required for Nuclear-Envelope Function and Chromatin Maintenance. Curr. Biol. 2006, 16, 1757–1763. [Google Scholar] [CrossRef] [Green Version]
- Franz, C.; Walczak, R.; Yavuz, S.; Santarella, R.; Gentzel, M.; Askjaer, P.; Galy, V.; Hetzer, M.; Mattaj, I.W.; Antonin, W. MEL-28/ELYS is required for the recruitment of nucleoporins to chromatin and postmitotic nuclear pore complex assembly. Embo Rep. 2007, 8, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Hattersley, N.; Cheerambathur, D.; Moyle, M.; Stefanutti, M.; Richardson, A.; Lee, K.; Dumont, J.; Oegema, K.; Desai, A. A Nucleoporin Docks Protein Phosphatase 1 to Direct Meiotic Chromosome Segregation and Nuclear Assembly. Dev. Cell 2016, 38, 463–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clever, M.; Funakoshi, T.; Mimura, Y.; Takagi, M.; Imamoto, N. The nucleoporin ELYS/Mel28 regulates nuclear envelope subdomain formation in HeLa cells. Nucleus 2012, 3, 187–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinkle-Mulcahy, L.; Andersen, J.; Lam, Y.W.; Moorhead, G.; Mann, M.; Lamond, A.I. Repo-Man recruits PP1γ to chromatin and is essential for cell viability. J. Cell Biol. 2006, 172, 679. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, B.; Lorenz, M.; Moreno-Andrés, D.; Bodenhöfer, M.; De Magistris, P.; Astrinidis, S.; Schooley, A.; Flötenmeyer, M.; Leptihn, S.; Antonin, W. Nup153 Recruits the Nup107-160 Complex to the Inner Nuclear Membrane for Interphasic Nuclear Pore Complex Assembly. Dev. Cell 2015, 33, 717–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cundell, M.J.; Hutter, L.H.; Nunes Bastos, R.; Poser, E.; Holder, J.; Mohammed, S.; Novak, B.; Barr, F.A. A PP2A-B55 recognition signal controls substrate dephosphorylation kinetics during mitotic exit. J. Cell Biol. 2016, 214, 539–554. [Google Scholar] [CrossRef] [Green Version]
- De Castro, I.J.; Budzak, J.; Di Giacinto, M.L.; Ligammari, L.; Gokhan, E.; Spanos, C.; Moralli, D.; Richardson, C.; de Las Heras, J.I.; Salatino, S.; et al. Repo-Man/PP1 regulates heterochromatin formation in interphase. Nat. Commun. 2017, 8, 14048. [Google Scholar] [CrossRef]
- Asencio, C.; Davidson, I.; Santarella-Mellwig, R.; Ly-Hartig, T.; Mall, M.; Wallenfang, M.; Mattaj, I.; Gorjánácz, M. Coordination of Kinase and Phosphatase Activities by Lem4 Enables Nuclear Envelope Reassembly during Mitosis. Cell 2012, 150, 122–135. [Google Scholar] [CrossRef] [Green Version]
- Nichols, R.J.; Wiebe, M.S.; Traktman, P. The Vaccinia-related Kinases Phosphorylate the N′ Terminus of BAF, Regulating Its Interaction with DNA and Its Retention in the Nucleus. Mol. Biol. Cell 2006, 17, 2451–2464. [Google Scholar] [CrossRef]
- Samwer, M.; Schneider, M.W.G.; Hoefler, R.; Schmalhorst, P.S.; Jude, J.G.; Zuber, J.; Gerlich, D.W. DNA Cross-Bridging Shapes a Single Nucleus from a Set of Mitotic Chromosomes. Cell 2017, 170, 972.e23. [Google Scholar] [CrossRef]
- Haraguchi, T.; Kojidani, T.; Koujin, T.; Shimi, T.; Osakada, H.; Mori, C.; Yamamoto, A.; Hiraoka, Y. Live cell imaging and electron microscopy reveal dynamic processes of BAF-directed nuclear envelope assembly. J. Cell. Sci. 2008, 121, 2540. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Semenova, E.; Maric, D.; Craigie, R. Dephosphorylation of barrier-to-autointegration factor by protein phosphatase 4 and its role in cell mitosis. J. Biol. Chem. 2014, 289, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.J.; Hetzer, M.W. Nuclear envelope formation by chromatin-mediated reorganization of the endoplasmic reticulum. Nat. Cell Biol. 2007, 9, 1160. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Ladinsky, M.S.; Kirchhausen, T. Formation of the postmitotic nuclear envelope from extended ER cisternae precedes nuclear pore assembly. J. Cell Biol. 2011, 194, 425. [Google Scholar] [CrossRef] [PubMed]
- Olmos, Y.; Hodgson, L.; Mantell, J.; Verkade, P.; Carlton, J.G. ESCRT-III controls nuclear envelope reformation. Nature 2015, 522, 236–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olmos, Y.; Carlton, J.G. The ESCRT machinery: New roles at new holes. Curr. Opin. Cell Biol. 2016, 38, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Scourfield, E.J.; Martin-Serrano, J. Growing functions of the ESCRT machinery in cell biology and viral replication. Biochem. Soc. Trans. 2017, 45, 613. [Google Scholar] [CrossRef]
- Vietri, M.; Schink, K.O.; Campsteijn, C.; Wegner, C.S.; Schultz, S.W.; Christ, L.; Thoresen, S.B.; Brech, A.; Raiborg, C.; Stenmark, H. Spastin and ESCRT-III coordinate mitotic spindle disassembly and nuclear envelope sealing. Nature 2015, 522, 231–235. [Google Scholar] [CrossRef]
- Gu, M.; LaJoie, D.; Chen, O.S.; von Appen, A.; Ladinsky, M.S.; Redd, M.J.; Nikolova, L.; Bjorkman, P.J.; Sundquist, W.I.; Ullman, K.S.; et al. LEM2 recruits CHMP7 for ESCRT-mediated nuclear envelope closure in fission yeast and human cells. Proc. Natl. Acad. Sci. USA 2017, 114, E2175. [Google Scholar] [CrossRef]
- Olmos, Y.; Perdrix-Rosell, A.; Carlton, J.G. Membrane Binding by CHMP7 Coordinates ESCRT-III-Dependent Nuclear Envelope Reformation. Curr. Biol. 2016, 26, 2635–2641. [Google Scholar] [CrossRef] [Green Version]
- McCullough, J.; Frost, A.; Sundquist, W.I. Structures, Functions, and Dynamics of ESCRT-III/Vps4 Membrane Remodeling and Fission Complexes. Annu. Rev. Cell Dev. Biol. 2018, 34, 85–109. [Google Scholar] [CrossRef] [PubMed]
- Cimini, D.; Howell, B.; Maddox, P.; Khodjakov, A.; Degrassi, F.; Salmon, E.D. Merotelic Kinetochore Orientation Is a Major Mechanism of Aneuploidy in Mitotic Mammalian Tissue Cells. J. Cell Biol. 2001, 153, 517. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Kwon, M.; Mannino, M.; Yang, N.; Renda, F.; Khodjakov, A.; Pellman, D. Nuclear envelope assembly defects link mitotic errors to chromothripsis. Nature 2018, 561, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Hatch, E.M.; Fischer, A.H.; Deerinck, T.J.; Hetzer, M.W. Catastrophic nuclear envelope collapse in cancer cell micronuclei. Cell 2013, 154, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Luijten, M.N.H.; Lee, J.X.T.; Crasta, K.C. Mutational game changer: Chromothripsis and its emerging relevance to cancer. Mutat. Res./Rev. Mutat. Res. 2018, 777, 29–51. [Google Scholar] [CrossRef] [PubMed]
- Crasta, K.; Ganem, N.J.; Dagher, R.; Lantermann, A.B.; Ivanova, E.V.; Pan, Y.; Nezi, L.; Protopopov, A.; Chowdhury, D.; Pellman, D. DNA breaks and chromosome pulverization from errors in mitosis. Nature 2012, 482, 53. [Google Scholar] [CrossRef] [PubMed]
- Hatch, E.; Hetzer, M. Linking Micronuclei to Chromosome Fragmentation. Cell 2015, 161, 1502–1504. [Google Scholar] [CrossRef] [Green Version]
- Loïodice, I.; Alves, A.; Rabut, G.; Van Overbeek, M.; Ellenberg, J.; Sibarita, J.; Doye, V. The entire Nup107-160 complex, including three new members, is targeted as one entity to kinetochores in mitosis. Mol. Biol. Cell 2004, 15, 3333–3344. [Google Scholar] [CrossRef]
- Güttinger, S.; Laurell, E.; Kutay, U. Orchestrating nuclear envelope disassembly and reassembly during mitosis. Nat. Rev. Mol. Cell Biol. 2009, 10, 178. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Ikram, S.; Bibi, N.; Mir, A. Hutchinson–Gilford Progeria Syndrome: A Premature Aging Disease. Mol. Neurobiol. 2018, 55, 4417–4427. [Google Scholar] [CrossRef]
- Piekarowicz, K.; Machowska, M.; Dzianisava, V.; Rzepecki, R. Hutchinson-Gilford Progeria Syndrome-Current Status and Prospects for Gene Therapy Treatment. Cells 2019, 8, 88. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Abbas, A.; Irianto, J.; Ivanovska, I.L.; Xia, Y.; Tewari, M.; Discher, D.E. Progerin phosphorylation in interphase is lower and less mechanosensitive than lamin-A,C in iPS-derived mesenchymal stem cells. Nucleus 2018, 9, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Multani, P.S.; Sun, K.; Vincent, F.; de Pablo, Y.; Ghosh, S.; Gupta, R.; Lee, H.; Lee, H.; Smith, M.A.; et al. Nuclear envelope dispersion triggered by deregulated Cdk5 precedes neuronal death. Mol. Biol. Cell 2011, 22, 1452–1462. [Google Scholar] [CrossRef] [PubMed]
- Torvaldson, E.; Kochin, V.; Eriksson, J.E. Phosphorylation of lamins determine their structural properties and signaling functions. Nucleus 2015, 6, 166–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huguet, F.; Flynn, S.; Vagnarelli, P. The Role of Phosphatases in Nuclear Envelope Disassembly and Reassembly and Their Relevance to Pathologies. Cells 2019, 8, 687. https://doi.org/10.3390/cells8070687
Huguet F, Flynn S, Vagnarelli P. The Role of Phosphatases in Nuclear Envelope Disassembly and Reassembly and Their Relevance to Pathologies. Cells. 2019; 8(7):687. https://doi.org/10.3390/cells8070687
Chicago/Turabian StyleHuguet, Florentin, Shane Flynn, and Paola Vagnarelli. 2019. "The Role of Phosphatases in Nuclear Envelope Disassembly and Reassembly and Their Relevance to Pathologies" Cells 8, no. 7: 687. https://doi.org/10.3390/cells8070687




