The Lymphatic Headmaster of the Mast Cell-Related Splanchnic Inflammation in Portal Hypertension
Abstract
:1. Portal Hypertension and Splanchnic Lymphatic Pathology
2. The Mast Cell as Mediator of the Splanchnic Lymphatic Pathology in Portal Hypertension
3. The Mast Cell and the Lymphatic Gut-Liver Axis
3.1. Mechanotransduction, Liver Disease and Mast Cells
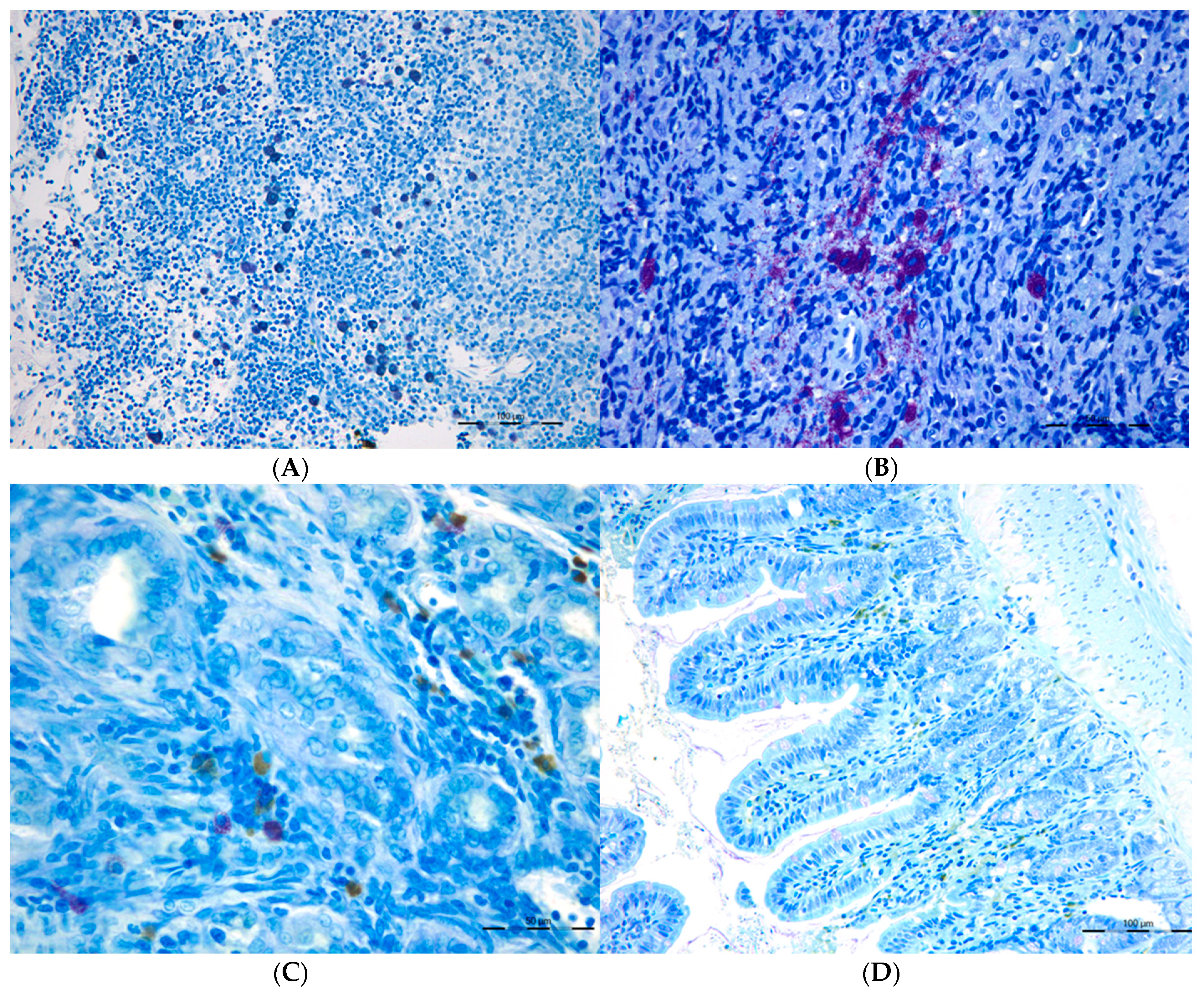
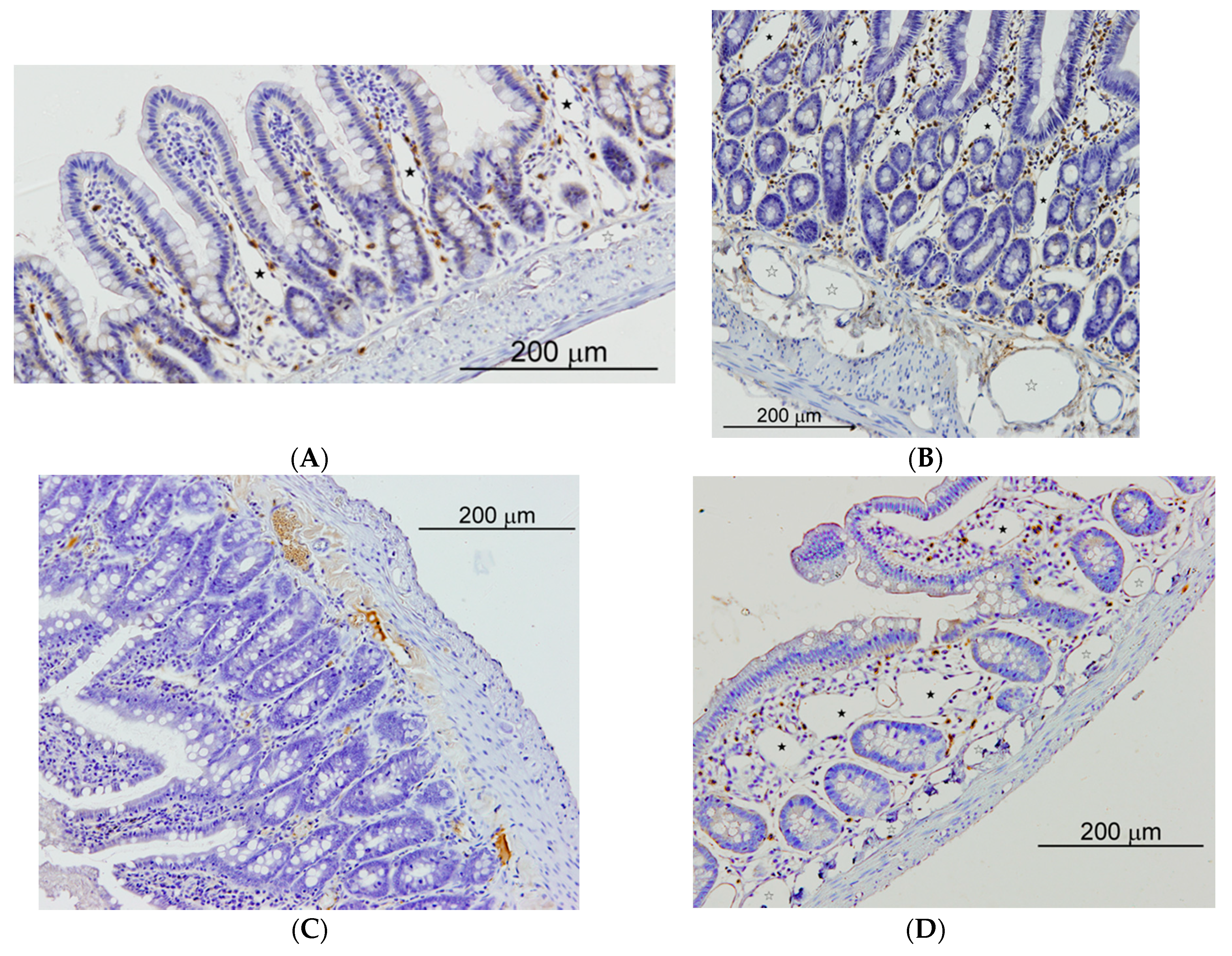
3.2. Splanchnic Mast Cells and Lymphangiogenesis
3.3. Mast Cells Compensating Chronic Liver Disease
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bloom, S.; Kemp, W.; Lubel, J. Portal hypertension: Pathophysiology, diagnosis and management. Intern. Med. J. 2015, 45, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, R.G.; Stasi, C. Recent Advancements in Diagnosis and Therapy of Liver Cirrhosis. Curr. Drug Targets 2016, 17, 1804–1817. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Kimura, T.; Fujimori, N.; Nagaya, T.; Komatsu, M.; Tanaka, E. Current status, problems, and perspectives of non-alcoholic fatty liver disease research. World J. Gastroenterol. 2019, 25, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Viggiano, T.R.; Gostout, C.J. Portal hypertensive intestinal vasculopathy: A review of the clinical, endoscopic, and histopathologic features. Am. J. Gastroenterol. 1992, 87, 944–954. [Google Scholar] [PubMed]
- Aller, M.A.; Nava, M.P.; Cuellar, C.; Chivato, T.; Arias, J.L.; Sanchez-Patan, F.; de Vicente, F.; Alvarez, E.; Arias, J. Evolutive phases of experimental prehepatic portal hypertension. J. Gastroenterol. Hepatol. 2007, 22, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Aller, M.A.; Arias, J.L.; Cruz, A.; Arias, J. Inflammation: A way to understanding the evolution of portal hypertension. Theor. Biol. Med. Model. 2007, 4, 44. [Google Scholar] [CrossRef] [PubMed]
- Aller, M.A.; Arias, N.; Blanco-Rivero, J.; Arias, J.L.; Arias, J. Hepatic encephalopathy: Sometimes more portal than hepatic. J. Gastroenterol. Hepatol. 2019, 34, 490–494. [Google Scholar] [CrossRef]
- Siebenhaar, F.; Redegeld, F.A.; Bischoff, S.C.; Gibbs, B.F.; Maurer, M. Mast Cells as Drivers of Disease and Therapeutic Targets. Trends Immunol. 2018, 39, 151–162. [Google Scholar] [CrossRef]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2016, 6, 620. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Patan, F.; Anchuelo, R.; Vara, E.; Garcia, C.; Saavedra, Y.; Vergara, P.; Cuellar, C.; Rodero, M.; Aller, M.A.; Arias, J. Prophylaxis with ketotifen in rats with portal hypertension: Involvement of mast cell and eicosanoids. Hepatobiliary Pancreat. Dis. Int. 2008, 7, 383–394. [Google Scholar]
- Sánchez-Patán, F.; Aller, M.A.; Cuellar, C.; Rodero, M.; Corcuera, M.T.; Nava, M.P.; Gómez, F.; Blanco, M.D.; Guerrero, S.; Anchuelo, R.; et al. Mast cell inhibition by ketotifen reduces splanchnic inflammatory response in a portal hypertension model in rats. Exp. Toxicol. Pathol. 2008, 60, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Nishikawa, Y.; Yamamoto, Y.; Doi, Y.; Tokairin, T.; Yoshioka, T.; Omori, Y.; Watanabe, A.; Takahashi, N.; Yoshioka, T.; et al. Mast cell leukemia with rapidly progressing portal hypertension. Pathol. Int. 2009, 59, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Diez-Arias, J.A.; Aller, M.A.; Palma, M.D.; Arias, J.L.; Muñiz, E.; Sánchez, M.; Arias, J. Increased duodenal mucosa infiltration by mast cells in rats with portal hypertension. Dig. Surg. 2001, 18, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Trezena, A.G.; da Silva, Z.L.; Oliveira-Filho, R.M.; Damazo, A.S.; Straus, A.H.; Takahashi, H.K.; Oliani, S.M.; de Lima, W.T. Differential regulation of the release of tumor necrosis factor-alpha and of eicosanoids by mast cells in rat airways after antigen challenge. Mediat. Inflamm. 2003, 12, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Benedé, S.; Cody, E.; Agashe, C.; Berin, M.C. Immune Characterization of Bone Marrow-Derived Models of Mucosal and Connective Tissue Mast Cells. Allergy Asthma Immunol. Res. 2018, 10, 268–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farha, S.; Sharp, J.; Asosingh, K.; Park, M.; Comhair, S.A.; Tang, W.H.; Thomas, J.; Farver, C.; Hsieh, F.; Loyd, J.E.; et al. Mast cell number, phenotype, and function in human pulmonary arterial hypertension. Pulm. Circ. 2012, 2, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Welle, M. Development, significance, and heterogeneity of mast cells with particular regard to the mast cell-specific proteases chymase and tryptase. J. Leukoc. Biol. 1997, 61, 233–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galli, S.J.; Borregaard, N.; Wynn, T.A. Phenotypic and functional plasticity of cells of innate immunity: Macrophages, mast cells and neutrophils. Nat. Immunol. 2011, 12, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Kalesnikoff, J.; Grimbaldeston, M.A.; Piliponsky, A.M.; Williams, C.M.; Tsai, M. Mast cells as “tunable” effector and immunoregulatory cells: Recent advances. Annu. Rev. Immunol. 2005, 23, 749–786. [Google Scholar] [CrossRef] [PubMed]
- Abraldes, J.G.; Pasarín, M.; García-Pagán, J.C. Animal models of portal hypertension. World J. Gastroenterol. 2006, 12, 6577–6584. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, Y.; Kawasaki, H.; Terada, T. Stromal mast cells and nerve fibers in various chronic liver diseases: Relevance to hepatic fibrosis. Am. J. Gastroenterol. 1999, 94, 1923–1932. [Google Scholar] [CrossRef] [PubMed]
- Aller, M.A.; Arias, J.L.; Arias, J. The mast cell integrates the splanchnic and systemic inflammatory response in portal hypertension. J. Transl. Med. 2007, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Roos, A.B.; Mori, M.; Gura, H.K.; Lorentz, A.; Bjermer, L.; Hoffmann, H.J.; Erjefält, J.S.; Stampfli, M.R. Increased IL-17RA and IL-17RC in End-Stage COPD and the Contribution to Mast Cell Secretion of FGF-2 and VEGF. Respir. Res. 2017, 18, 48. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Loffredo, S.; Galdiero, M.R.; Marone, G.; Cristinziano, L.; Granata, F.; Marone, G. Innate effector cells in angiogenesis and lymphangiogenesis. Curr. Opin. Immunol. 2018, 53, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Stanojević, S.; Kuštrimović, N.; Mitić, K.; Vujić, V.; Dimitrijević, M. Role of Mast Cells and C-Sensory Fibers in Concanavalin A-Induced Paw Edema in Two Rat Strains. Inflammation 2015, 38, 1434–1449. [Google Scholar] [CrossRef] [PubMed]
- Cifarelli, V.; Eichmann, A. The Intestinal Lymphatic System: Functions and Metabolic Implications. Cell. Mol. Gastroenterol. Hepatol. 2018, 7, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Eelen, G.; de Zeeuw, P.; Treps, L.; Harjes, U.; Wong, B.W.; Carmeliet, P. Endothelial Cell Metabolism. Physiol. Rev. 2018, 98, 3–58. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.U.; Chung, S.W.; Al-Hilal, T.A.; Alam, F.; Park, J.; Mahmud, F.; Jeong, J.H.; Kim, S.Y.; Byun, Y. A heparin conjugate, LHbisD4, inhibits lymphangiogenesis and attenuates lymph node metastasis by blocking VEGF-C signaling pathway. Biomaterials 2017, 139, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.W.; Zecchin, A.; García-Caballero, M.; Carmeliet, P. Emerging Concepts in Organ-Specific Lymphatic Vessels and Metabolic Regulation of Lymphatic Development. Dev. Cell 2018, 45, 289–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solari, E.; Marcozzi, C.; Negrini, D.; Moriondo, A. Fluid Osmolarity Acutely and Differentially Modulates Lymphatic Vessels Intrinsic Contractions and Lymph Flow. Front. Physiol. 2018, 9, 871. [Google Scholar] [CrossRef] [PubMed]
- Eelen, G.; de Zeeuw, P.; Simons, M.; Carmeliet, P. Endothelial cell metabolism in normal and diseased vasculature. Circ. Res. 2015, 116, 1231–1244. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Jiang, J.; Qin, P.; Wang, Q.; Hu, J.; Li, Z. Mast cells are important regulator of acupoint sensitization via the secretion of tryptase, 5-hydroxytryptamine, and histamine. PLoS ONE 2018, 13, e0194022. [Google Scholar] [CrossRef] [PubMed]
- Phang, J.M.; Liu, W.; Hancock, C. Bridging epigenetics and metabolism: Role of non-essential amino acids. Epigenetics 2013, 8, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Stoll, S.; Wang, C.; Qiu, H. DNA Methylation and Histone Modification in Hypertension. Int. J. Mol. Sci. 2018, 19, 1174. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Loganathan, G.; Mokshagundam, S.; Hughes, M.G.; Williams, S.K.; Balamurugan, A.N. Endothelial cell regulation through epigenetic mechanisms: Depicting parallels and its clinical application within an intra-islet microenvironment. Diabetes Res. Clin. Pract. 2018, 143, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.M.; Barandiaran Cornejo, J.F. Organ preservation review: History of organ preservation. Curr. Opin. Organ. Transpl. 2015, 20, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.M.; Mendoza, R.; Raghavendra, A.J.; Podila, R.; Brown, J.M. Contribution of engineered nanomaterials physicochemical properties to mast cell degranulation. Sci. Rep. 2017, 7, 43570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kok, B.; Abraldes, J.G. Child-Pugh Classification: Time to Abandon? Semin. Liver Dis. 2019, 39, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Ronald, J.; Wang, Q.; Choi, S.S.; Suhocki, P.V.; Hall, M.D.; Smith, T.P.; Kim, C.Y. Albumin-bilirubin grade versus MELD score for predicting survival after transjugular intrahepatic portosystemic shunt (TIPS) creation. Diagn. Interv. Imaging 2018, 99, 163–168. [Google Scholar] [CrossRef]
- Aller, M.Á.; Martínez, V.; Arias, A.; Nava, M.P.; Cuervas-Mons, V.; Vergara, P.; Arias, J. Mast cell-mediated splanchnic cholestatic inflammation. Clin. Res. Hepatol. Gastroenterol. 2019. [Google Scholar] [CrossRef]
- Odegaard, J.I.; Chawla, A. The immune system as a sensor of the metabolic state. Immunity 2013, 38, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.A.; Khan, M.; Smith, B.T.; Cimini, A.M.; Gilg, A.G.; Orak, J.; Singh, I.; Singh, A.K. Endotoxin induces structure-function alterations of rat liver peroxisomes: Kupffer cells released factors as possible modulators. Hepatology 2000, 31, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.L.; Morales, J.L.; Gonzalez, F.J.; Peters, J.M. Peroxisome proliferator-activated receptor-β/δ modulates mast cell phenotype. Immunology 2017, 150, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Brenner, D.A. Liver inflammation and fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Jeong, W.K.; Baik, S.K. Invasive and non-invasive diagnosis of cirrhosis and portal hypertension. World J. Gastroenterol. 2014, 20, 4300–4315. [Google Scholar] [CrossRef]
- Piano, S.; Tonon, M.; Angeli, P. Management of ascites and hepatorenal syndrome. Hepatol. Int. 2018, 12, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, Z.; Knolle, P.A. Liver macrophages in healthy and diseased liver. Pflugers Arch. 2017, 469, 553–560. [Google Scholar] [CrossRef]
- Maurer, M.; Köberle, M.; Metz, M.; Biedermann, T. Mast cells—Promotors of health and modulators of disease. J. Allergy Clin. Immunol. 2019. [Google Scholar] [CrossRef]
- Dudeck, A.; Köberle, M.; Goldmann, O.; Meyer, N.; Dudeck, J.; Lemmens, S.; Rohde, M.; Roldán, N.G.; Dietze-Schwonberg, K.; Orinska, Z.; et al. Mast cells as protectors of health. J. Allergy Clin. Immunol. 2018. [Google Scholar] [CrossRef]
- Chen, L.; Brenner, D.A.; Kisseleva, T. Combatting Fibrosis: Exosome-Based Therapies in the Regression of Liver Fibrosis. Hepatol. Commun. 2018, 3, 180–192. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, S.; Kim, J.; Jung, Y. Liver-Derived Exosomes and Their Implications in Liver Pathobiology. Int. J. Mol. Sci. 2018, 19, 3715. [Google Scholar] [CrossRef] [PubMed]
- Islinger, M.; Voelkl, A.; Fahimi, H.D.; Schrader, M. The peroxisome: An update on mysteries 2.0. Histochem. Cell Biol. 2018, 150, 443–471. [Google Scholar] [CrossRef]
- Deori, N.M.; Kale, A.; Maurya, P.K.; Nagotu, S. Peroxisomes: Role in cellular ageing and age related disorders. Biogerontology 2018, 19, 303–324. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.K.; Cho, H.W.; Song, S.E.; Song, D.K. Catalase and nonalcoholic fatty liver disease. Pflugers Arch. 2018, 470, 1721–1737. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.K.S.; Peixoto, C.A. Role of peroxisome proliferator-activated receptors in non-alcoholic fatty liver disease inflammation. Cell. Mol. Life Sci. 2018, 75, 2951–2961. [Google Scholar] [CrossRef] [PubMed]
- Baes, M.; Van Veldhoven, P.P. Hepatic dysfunction in peroxisomal disorders. Biochim. Biophys. Acta 2016, 1863, 956–970. [Google Scholar] [CrossRef]
- Dichlberger, A.; Schlager, S.; Kovanen, P.T.; Schneider, W.J. Lipid droplets in activated mast cells—A significant source of triglyceride-derived arachidonic acid for eicosanoid production. Eur. J. Pharmacol. 2016, 785, 59–69. [Google Scholar] [CrossRef]
- Botta, M.; Audano, M.; Sahebkar, A.; Sirtori, C.R.; Mitro, N.; Ruscica, M. PPAR Agonists and Metabolic Syndrome: An Established Role? Int. J. Mol. Sci. 2018, 19, 1197. [Google Scholar] [CrossRef]
- Mast, F.D.; Rachubinski, R.A.; Aitchison, J.D. Signaling dynamics and peroxisomes. Curr. Opin. Cell Biol. 2015, 35, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Demarquoy, J.; Le Borgne, F. Crosstalk between mitochondria and peroxisomes. World J. Biol. Chem. 2015, 6, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Ahuir, A.; Manzanares-Estreder, S.; Proft, M. Pro- and Antioxidant Functions of the Peroxisome-Mitochondria Connection and Its Impact on Aging and Disease. Oxidative Med. Cell. Longev. 2017, 2017, 9860841. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.H.; Lee, G.P.; Jeong, W.I.; Do, S.H.; Yang, H.J.; Yuan, D.W.; Park, H.Y.; Kim, K.J.; Jeong, K.S. Alterations of mast cells and TGF-beta1 on the silymarin treatment for CCl(4)-induced hepatic fibrosis. World J. Gastroenterol. 2005, 11, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Colgan, S.P.; Campbell, E.L. Oxygen metabolism and innate immune responses in the gut. J. Appl. Physiol. 2017, 123, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Worthington, J.J.; Reimann, F.; Gribble, F.M. Enteroendocrine cells-sensory sentinels of the intestinal environment and orchestrators of mucosal immunity. Mucosal. Immunol. 2018, 11, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Patán, F.; Aller, M.A.; Corcuera, M.T.; Vara, E.; Casado, I.; Gómez, F.; García, C.; Alonso, M.J.; Arias, J. Chronic inflammatory portal hypertensive enteropathy in the rat. Cirugía Española 2006, 80, 162–167. [Google Scholar] [CrossRef]
- Allaire, J.M.; Morampudi, V.; Crowley, S.M.; Stahl, M.; Yu, H.; Bhullar, K.; Knodler, L.A.; Bressler, B.; Jacobson, K.; Vallance, B.A. Frontline defenders: goblet cell mediators dictate host-microbe interactions in the intestinal tract during health and disease. Am. J. Physiol. Gastrointest Liver Physiol. 2018, 314, G360–G377. [Google Scholar] [CrossRef]
- Reichen, J.; Lebrec, D. The future treatment of portal hypertension. Best Pract. Res. Clin. Gastroenterol. 2007, 21, 191–202. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aller, M.-A.; Blanco-Rivero, J.; Arias, N.; Santamaria, L.; Arias, J. The Lymphatic Headmaster of the Mast Cell-Related Splanchnic Inflammation in Portal Hypertension. Cells 2019, 8, 658. https://doi.org/10.3390/cells8070658
Aller M-A, Blanco-Rivero J, Arias N, Santamaria L, Arias J. The Lymphatic Headmaster of the Mast Cell-Related Splanchnic Inflammation in Portal Hypertension. Cells. 2019; 8(7):658. https://doi.org/10.3390/cells8070658
Chicago/Turabian StyleAller, Maria-Angeles, Javier Blanco-Rivero, Natalia Arias, Luis Santamaria, and Jaime Arias. 2019. "The Lymphatic Headmaster of the Mast Cell-Related Splanchnic Inflammation in Portal Hypertension" Cells 8, no. 7: 658. https://doi.org/10.3390/cells8070658



