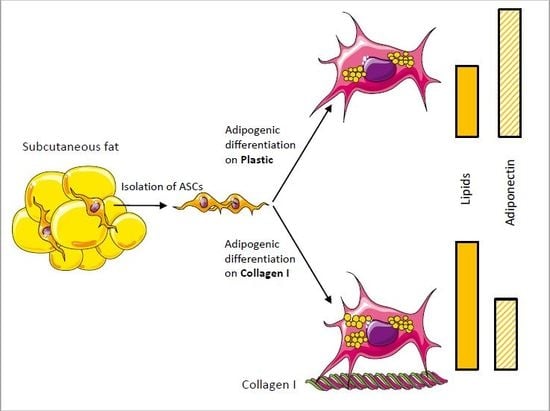
Collagen I Promotes Adipocytogenesis in Adipose-Derived Stem Cells In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Isolation and Characterization of ASCs
2.3. Flow Cytometry
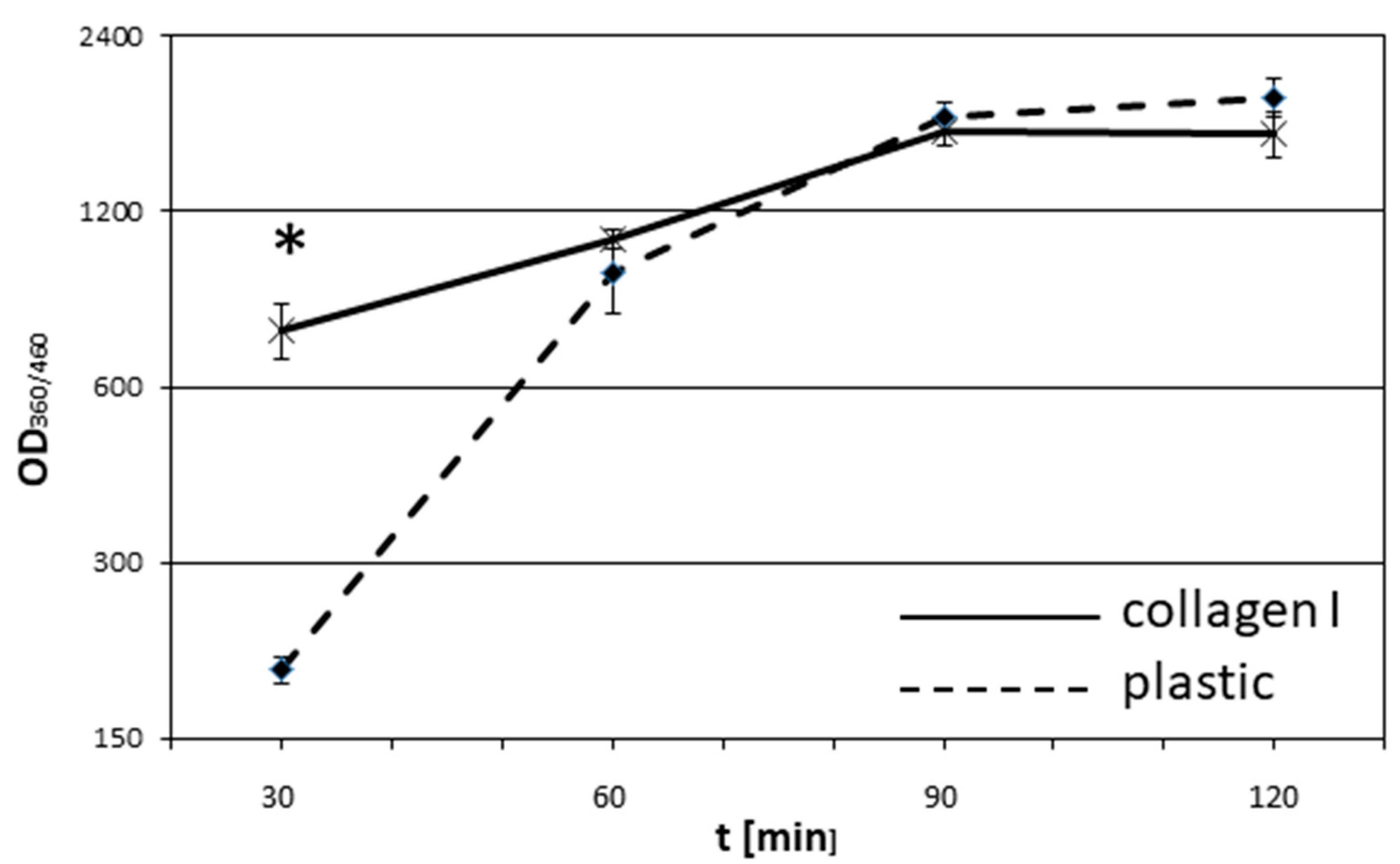
2.4. Adhesion Assay
2.5. Induction of Adipogenesis
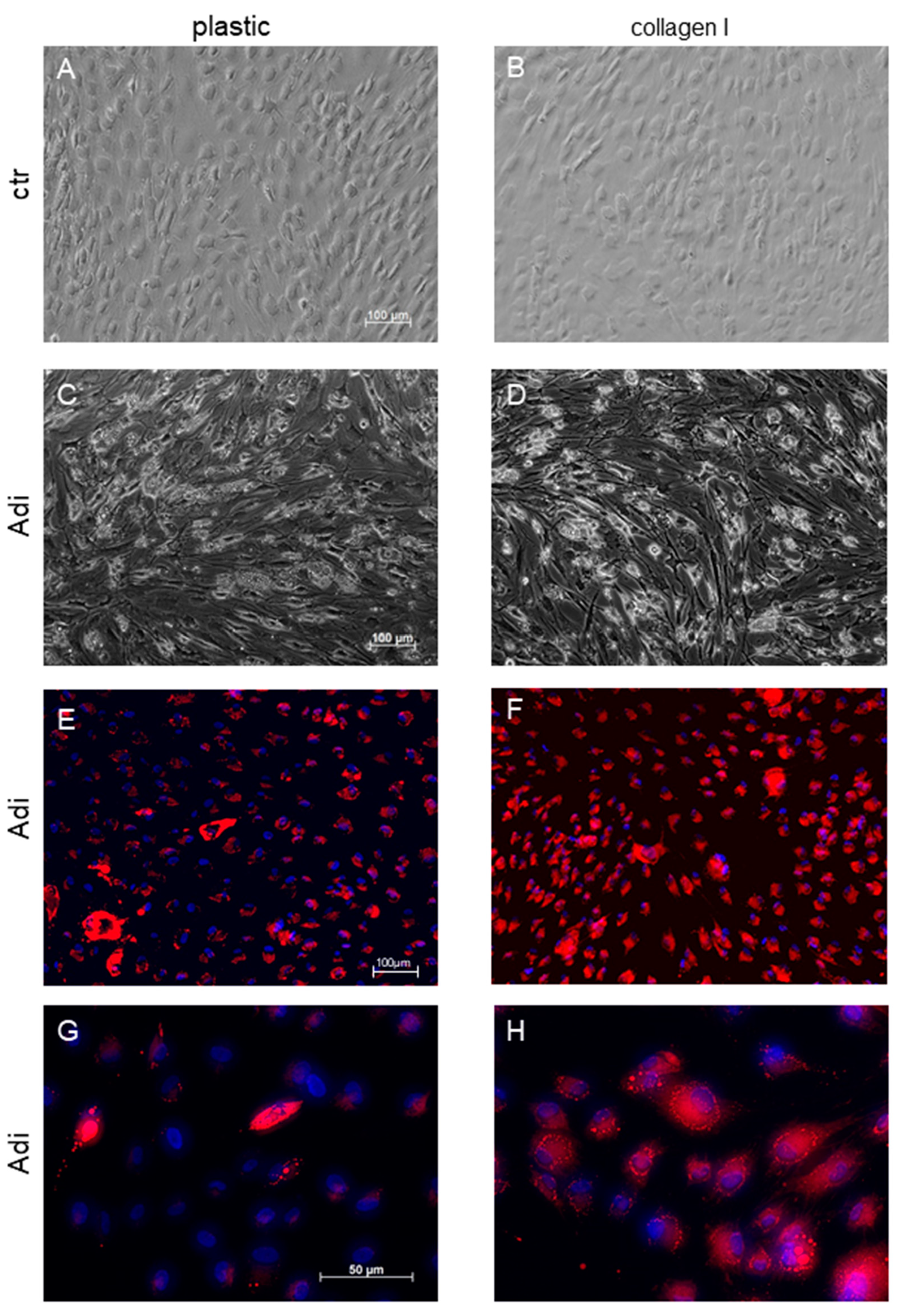
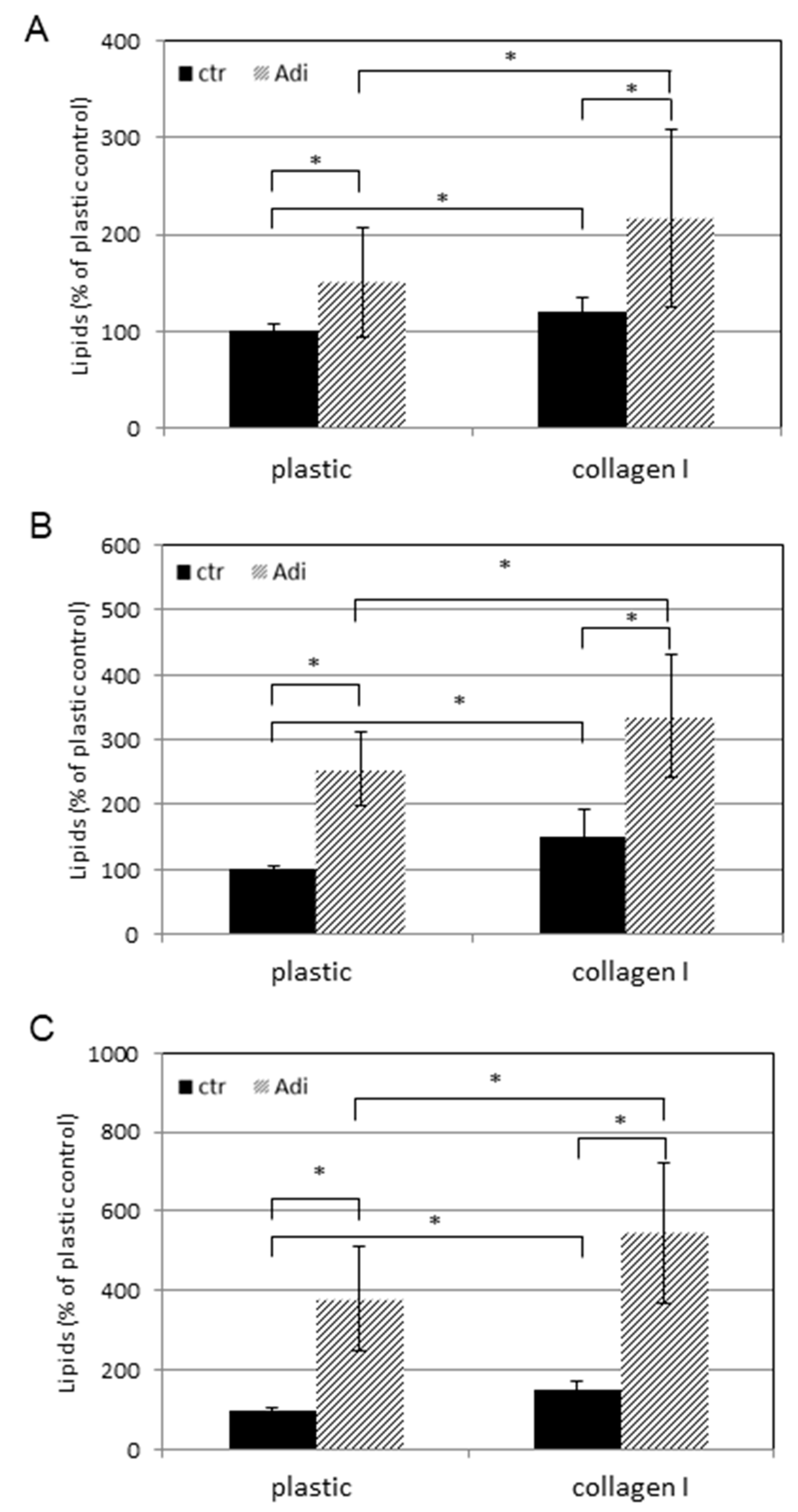
2.6. Detection of Lipids
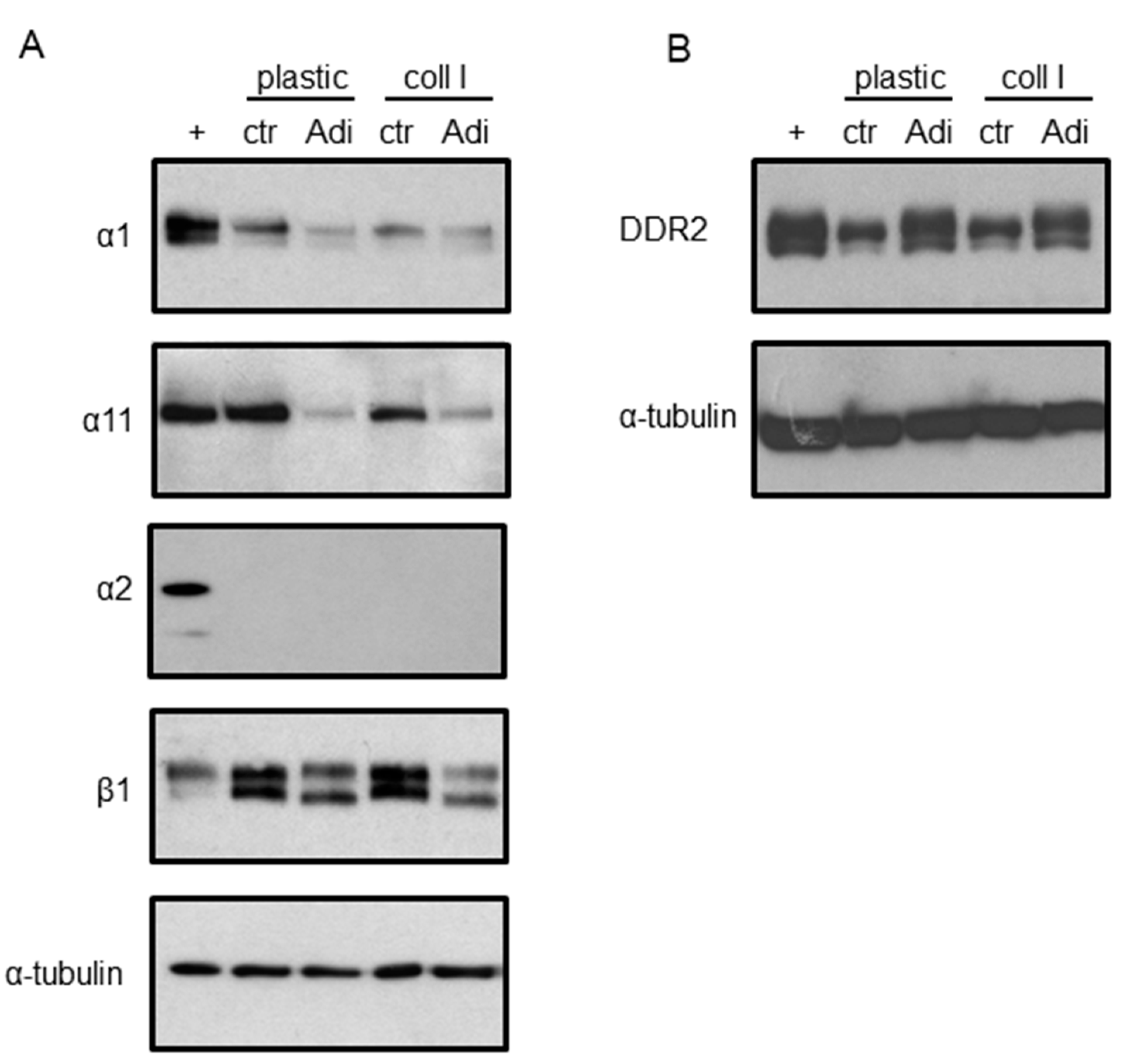
2.7. Western Blot
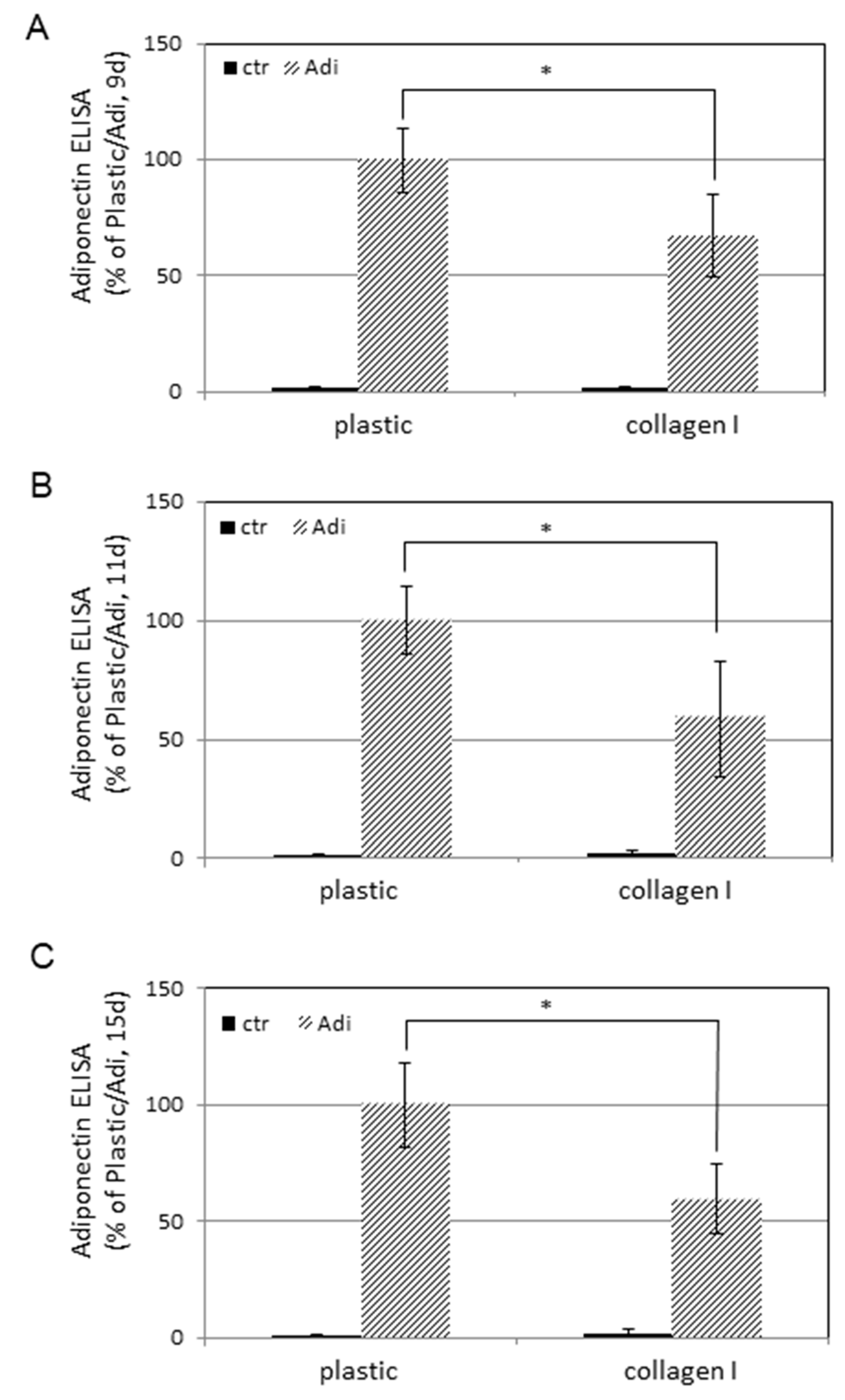
2.8. Adiponectin ELISA
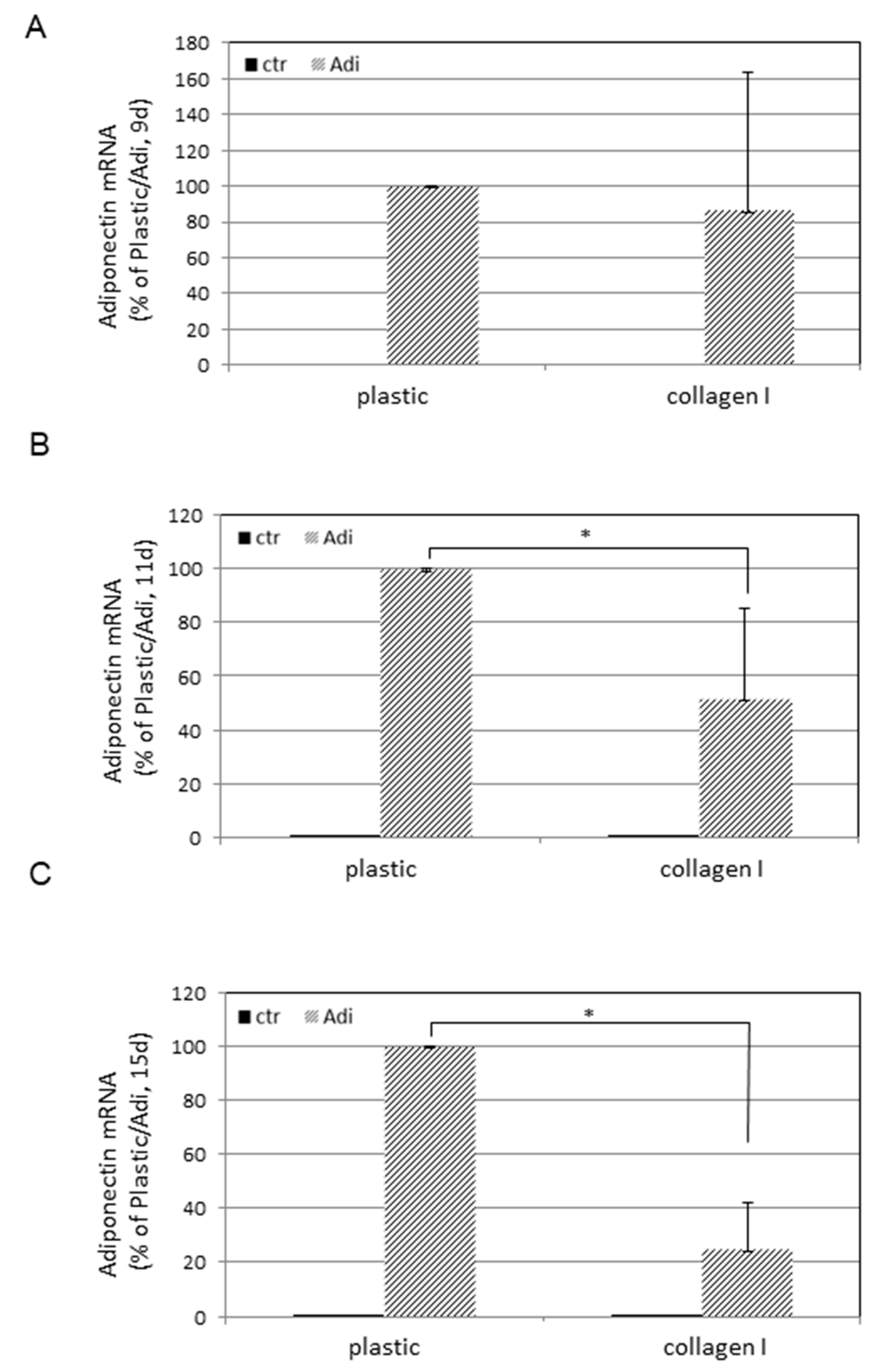
2.9. Real-Time RT-PCR Analysis
2.10. Statistics
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Baer, P.C.; Bereiter-Hahn, J.; Missler, C.; Brzoska, M.; Schubert, R.; Gauer, S.; Geiger, H. Conditioned medium from renal tubular epithelial cells initiates differentiation of human mesenchymal stem cells. Cell Prolif. 2009, 42, 29–37. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Adams, A.M.; Arruda, E.M.; Larkin, L.M. Use of adipose-derived stem cells to fabricate scaffoldless tissue-engineered neural conduits in vitro. Neuroscience 2012, 201, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Miranville, A.; Heeschen, C.; Sengenes, C.; Curat, C.A.; Busse, R.; Bouloumie, A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation 2004, 110, 349–355. [Google Scholar] [CrossRef]
- Hicok, K.C.; Du Laney, T.V.; Zhou, Y.S.; Halvorsen, Y.D.; Hitt, D.C.; Cooper, L.F.; Gimble, J.M. Human adipose-derived adult stem cells produce osteoid in vivo. Tissue Eng. 2004, 10, 371–380. [Google Scholar] [CrossRef]
- Erickson, G.R.; Gimble, J.M.; Franklin, D.M.; Rice, H.E.; Awad, H.; Guilak, F. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem. Biophis. Res. Commun. 2002, 290, 763–769. [Google Scholar] [CrossRef]
- Song, Y.H.; Gehmert, S.; Sadat, S.; Pinkernell, K.; Bai, X.; Matthias, N.; Alt, E. VEGF is critical for spontaneous differentiation of stem cells into cardiomyocytes. Biochem. Biophis. Res. Commun. 2007, 354, 999–1003. [Google Scholar] [CrossRef]
- Banas, A.; Teratani, T.; Yamamoto, Y.; Tokuhara, M.; Takeshita, F.; Quinn, G.; Okochi, H.; Ochiya, T. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology 2007, 46, 219–228. [Google Scholar] [CrossRef]
- Timper, K.; Seboek, D.; Eberhardt, M.; Linscheid, P.; Christ-Crain, M.; Keller, U.; Muller, B.; Zulewski, H. Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochem. Biophis. Res. Commun. 2006, 341, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Aldag, C.; Nogueira Teixeira, D.; Leventhal, P.S. Skin rejuvenation using cosmetic products containing growth factors, cytokines, and matrikines: A review of the literature. Clin. Cosmet. Investig. Dermatol. 2016, 9, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L. Skin ageing and its treatment. J. Pathol. 2007, 211, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; You, E.; Min, N.Y.; Lee, K.H.; Kim, H.K.; Rhee, S. Discoidin domain receptor 2 regulates the adhesion of fibroblasts to 3D collagen matrices. Int. J. Mol. Med. 2013, 31, 1113–1118. [Google Scholar] [CrossRef]
- Vogel, W.; Gish, G.D.; Alves, F.; Pawson, T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol. Cell 1997, 1, 13–23. [Google Scholar] [CrossRef]
- Vogel, W. Discoidin domain receptors: Structural relations and functional implications. FASEB J. 1999, 13, S77–S82. [Google Scholar] [CrossRef]
- Antuna-Puente, B.; Feve, B.; Fellahi, S.; Bastard, J.P. Adipokines: The missing link between insulin resistance and obesity. Diabetes Metab. 2008, 34, 2–11. [Google Scholar] [CrossRef]
- Fasshauer, M.; Bluher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Tsatsanis, C.; Zacharioudaki, V.; Androulidaki, A.; Dermitzaki, E.; Charalampopoulos, I.; Minas, V.; Gravanis, A.; Margioris, A.N. Peripheral factors in the metabolic syndrome: The pivotal role of adiponectin. Ann. N. Y. Acad. Sci. 2006, 1083, 185–195. [Google Scholar] [CrossRef]
- Kroeze, K.L.; Jurgens, W.J.; Doulabi, B.Z.; van Milligen, F.J.; Scheper, R.J.; Gibbs, S. Chemokine-mediated migration of skin-derived stem cells: Predominant role for CCL5/RANTES. J. Investig. Dermatol. 2009, 129, 1569–1581. [Google Scholar] [CrossRef] [PubMed]
- Knies, Y.; Bernd, A.; Kaufmann, R.; Bereiter-Hahn, J.; Kippenberger, S. Mechanical stretch induces clustering of beta1-integrins and facilitates adhesion. Exp. Dermatol. 2006, 15, 347–355. [Google Scholar] [CrossRef]
- Kippenberger, S.; Loitsch, S.; Muller, J.; Guschel, M.; Kaufmann, R.; Bernd, A. Ligation of the beta4 integrin triggers adhesion behavior of human keratinocytes by an “inside-out” mechanism. J. Investig. Dermatol. 2004, 123, 444–451. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Seltmann, H.; Hiroi, N.; Chen, W.; Young, M.; Oeff, M.; Scherbaum, W.A.; Orfanos, C.E.; McCann, S.M.; Bornstein, S.R. Corticotropin-releasing hormone: An autocrine hormone that promotes lipogenesis in human sebocytes. Proc. Natl. Acad. Sci. USA 2002, 99, 7148–7153. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Plant, A.L.; Bhadriraju, K.; Spurlin, T.A.; Elliott, J.T. Cell response to matrix mechanics: Focus on collagen. BBA-Mol. Cell Res. 2009, 1793, 893–902. [Google Scholar] [CrossRef]
- Fisher, G.J.; Varani, J.; Voorhees, J.J. Looking older: Fibroblast collapse and therapeutic implications. Arch. Dermatol. 2008, 144, 666–672. [Google Scholar] [CrossRef]
- Kippenberger, S.; Bernd, A.; Loitsch, S.; Guschel, M.; Muller, J.; Bereiter-Hahn, J.; Kaufmann, R. Signaling of mechanical stretch in human keratinocytes via MAP kinases. J. Investig. Dermatol. 2000, 114, 408–412. [Google Scholar] [CrossRef]
- Sepe, A.; Tchkonia, T.; Thomou, T.; Zamboni, M.; Kirkland, J.L. Aging and regional differences in fat cell progenitors - a mini-review. Gerontology 2011, 57, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Lowe, L.; Hamilton, T.A.; Fisher, G.J.; Voorhees, J.J.; Kang, S. Long-term treatment of photoaged human skin with topical retinoic acid improves epidermal cell atypia and thickens the collagen band in papillary dermis. J. Am. Acad. Dermatol. 2005, 53, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.E.; Russman, A.N.; Majmudar, G.; Singer, R.S.; Hamilton, T.A.; Voorhees, J.J. Restoration of collagen formation in photodamaged human skin by tretinoin (retinoic acid). N. Engl. J. Med. 1993, 329, 530–535. [Google Scholar] [CrossRef]
- Kafi, R.; Kwak, H.S.; Schumacher, W.E.; Cho, S.; Hanft, V.N.; Hamilton, T.A.; King, A.L.; Neal, J.D.; Varani, J.; Fisher, G.J.; et al. Improvement of naturally aged skin with vitamin A (retinol). Arch. Dermatol. 2007, 143, 606–612. [Google Scholar] [CrossRef]
- Orringer, J.S.; Kang, S.; Johnson, T.M.; Karimipour, D.J.; Hamilton, T.; Hammerberg, C.; Voorhees, J.J.; Fisher, G.J. Connective tissue remodeling induced by carbon dioxide laser resurfacing of photodamaged human skin. Arch. Dermatol. 2004, 140, 1326–1332. [Google Scholar] [CrossRef]
- Marinelli, E.; Montanari Vergallo, G.; Reale, G.; di Luca, A.; Catarinozzi, I.; Napoletano, S.; Zaami, S. The role of fillers in aesthetic medicine: Medico-legal aspects. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4628–4634. [Google Scholar] [PubMed]
- Ye, R.; Scherer, P.E. Adiponectin, driver or passenger on the road to insulin sensitivity? Mol. Metab. 2013, 2, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Jian, L.; Su, Y.X.; Deng, H.C. Adiponectin-induced inhibition of intrinsic and extrinsic apoptotic pathways protects pancreatic beta-cells against apoptosis. Horm. Metab. Res. 2013, 45, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Kataria, M.A.; Saini, V.; Yadav, A. Role of leptin and adiponectin in insulin resistance. Clin. Chim. Acta 2013, 417, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Nio, Y.; Maki, T.; Kobayashi, M.; Takazawa, T.; Iwabu, M.; Okada-Iwabu, M.; Kawamoto, S.; Kubota, N.; Kubota, T.; et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 2007, 13, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K.; et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophis. Res. Commun. 1999, 257, 79–83. [Google Scholar] [CrossRef]
- Hoffstedt, J.; Arvidsson, E.; Sjolin, E.; Wahlen, K.; Arner, P. Adipose tissue adiponectin production and adiponectin serum concentration in human obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2004, 89, 1391–1396. [Google Scholar] [CrossRef]
- Leitinger, B. Transmembrane collagen receptors. Annu. Rev. Cell Dev. Biol. 2011, 27, 265–290. [Google Scholar] [CrossRef]
| Surface Marker | Positive Cells [%] | ||
|---|---|---|---|
| Donor 1 | Donor 2 | Donor 3 | |
| CD31/PECAM-1 | 0.75 ± 0.29 | 1.30 ± 0.19 | 0.83 ± 0.02 |
| CD34 | 53.94 ± 7.09 | 63.98 ± 13.67 | 78.00 ± 1.29 |
| CD45 | 0.85 ± 0.10 | 1.10 ± 0.13 | 0.97± 0.11 |
| CD54/ICAM-1 | 1.36 ± 0.36 | 1.88 ± 0.57 | 2.76 ± 1.06 |
| CD90/Thy-1 | 99.96 ± 0.03 | 99.88 ± 0.11 | 99.09 ± 1.39 |
| CD105/Endoglin | 91.07 ± 4.92 | 90.82 ± 11.62 | 60.17 ± 5.36 |
| CD166/ALCAM | 98.93 ± 0.82 | 97.83 ± 1.80 | 99.12 ± 0.11 |
| HLA-ABC | 99.74 ± 0.17 | 99.88 ± 0.05 | 99.48 ± 0.27 |
| HLA-DR | 0.78 ± 0.46 | 0.72 ± 0.11 | 0.56 ± 0.10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zöller, N.; Schreiner, S.; Petry, L.; Hoffmann, S.; Steinhorst, K.; Kleemann, J.; Jäger, M.; Kaufmann, R.; Meissner, M.; Kippenberger, S. Collagen I Promotes Adipocytogenesis in Adipose-Derived Stem Cells In Vitro. Cells 2019, 8, 302. https://doi.org/10.3390/cells8040302
Zöller N, Schreiner S, Petry L, Hoffmann S, Steinhorst K, Kleemann J, Jäger M, Kaufmann R, Meissner M, Kippenberger S. Collagen I Promotes Adipocytogenesis in Adipose-Derived Stem Cells In Vitro. Cells. 2019; 8(4):302. https://doi.org/10.3390/cells8040302
Chicago/Turabian StyleZöller, Nadja, Sarah Schreiner, Laura Petry, Stephanie Hoffmann, Katja Steinhorst, Johannes Kleemann, Manuel Jäger, Roland Kaufmann, Markus Meissner, and Stefan Kippenberger. 2019. "Collagen I Promotes Adipocytogenesis in Adipose-Derived Stem Cells In Vitro" Cells 8, no. 4: 302. https://doi.org/10.3390/cells8040302