CCN2 Mediates S1P-Induced Upregulation of COX2 Expression in Human Granulosa-Lutein Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antibodies and Reagents
2.2. Culture of Simian Virus 40 Large T Antigen-Immortalized Human Granulosa Cells (SVOG)
2.3. Culture of Primary Human Granulosa-Lutein (hGL) Cells
2.4. Small Interfering RNA (siRNA) Transfection
2.5. Reverse Transcription Quantitative Real-Time PCR (RT-qPCR)
2.6. Western Blot Analysis
2.7. Prostaglandin E2 Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Immunofluorescent Staining
2.9. Statistical Analysis
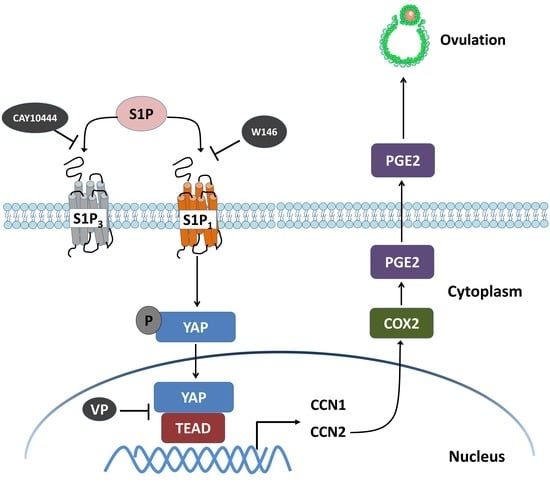
3. Results
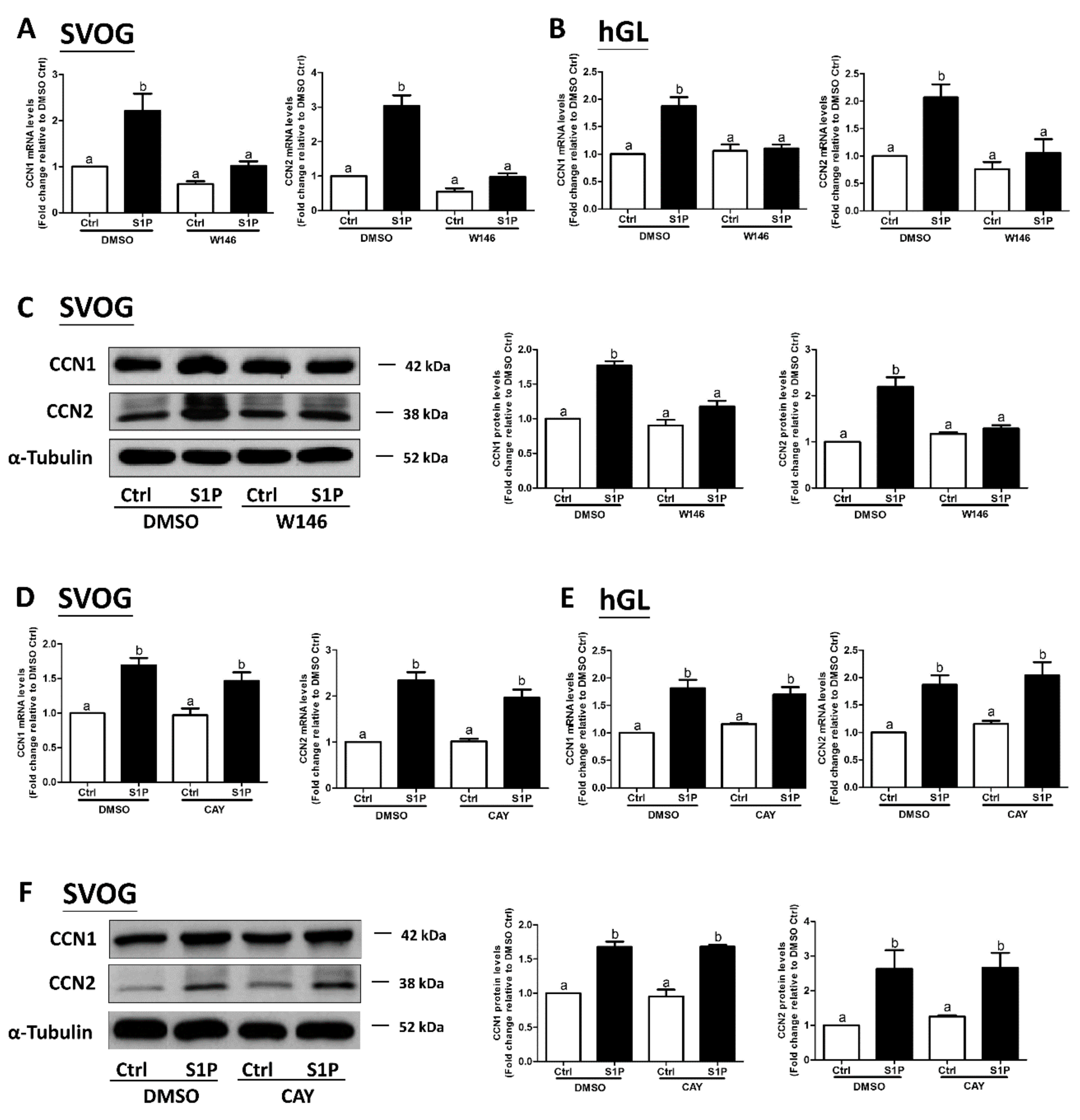
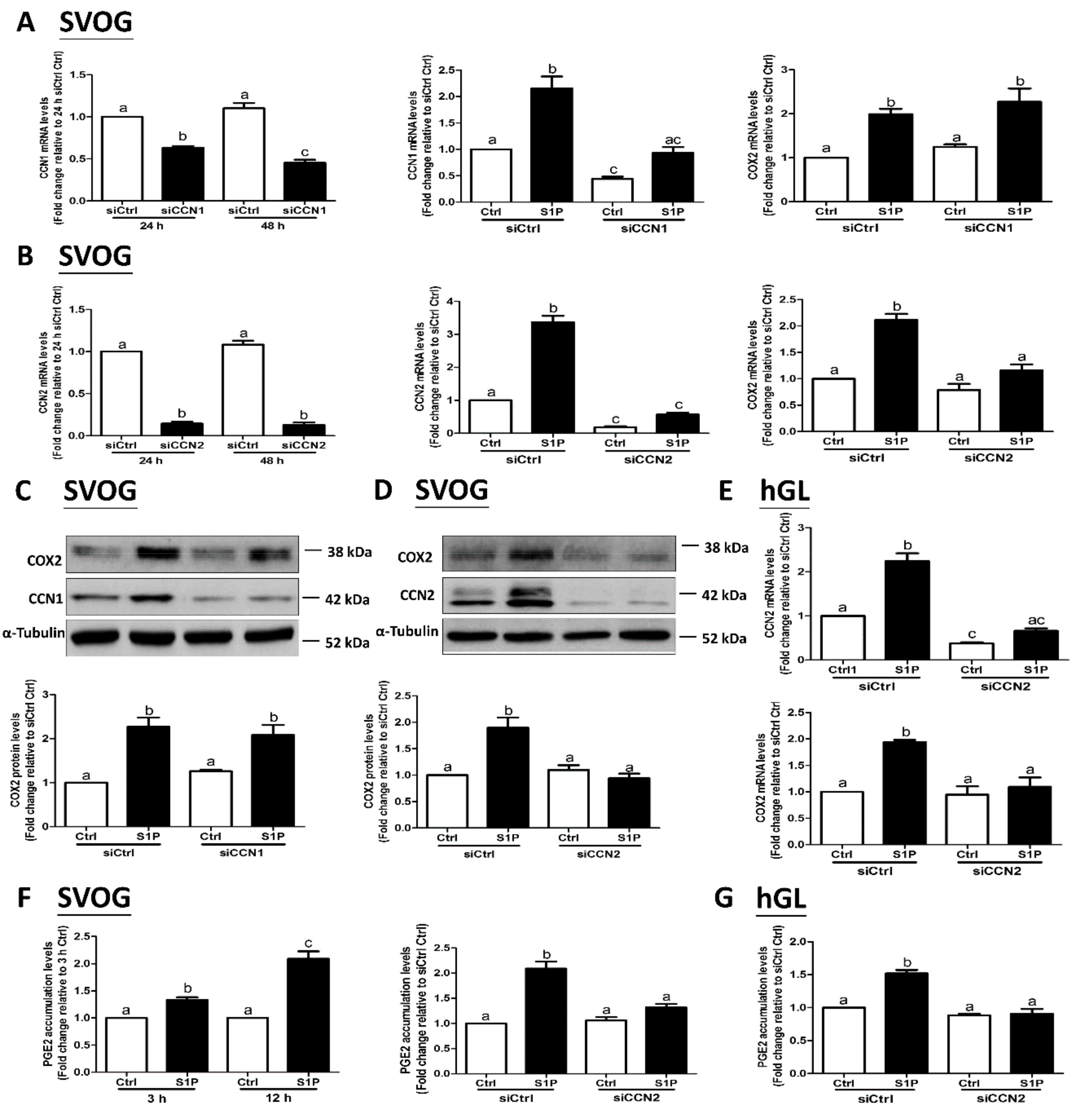
3.1. S1P Upregulates the Expression of CCN1 and CCN2 in hGL Cells
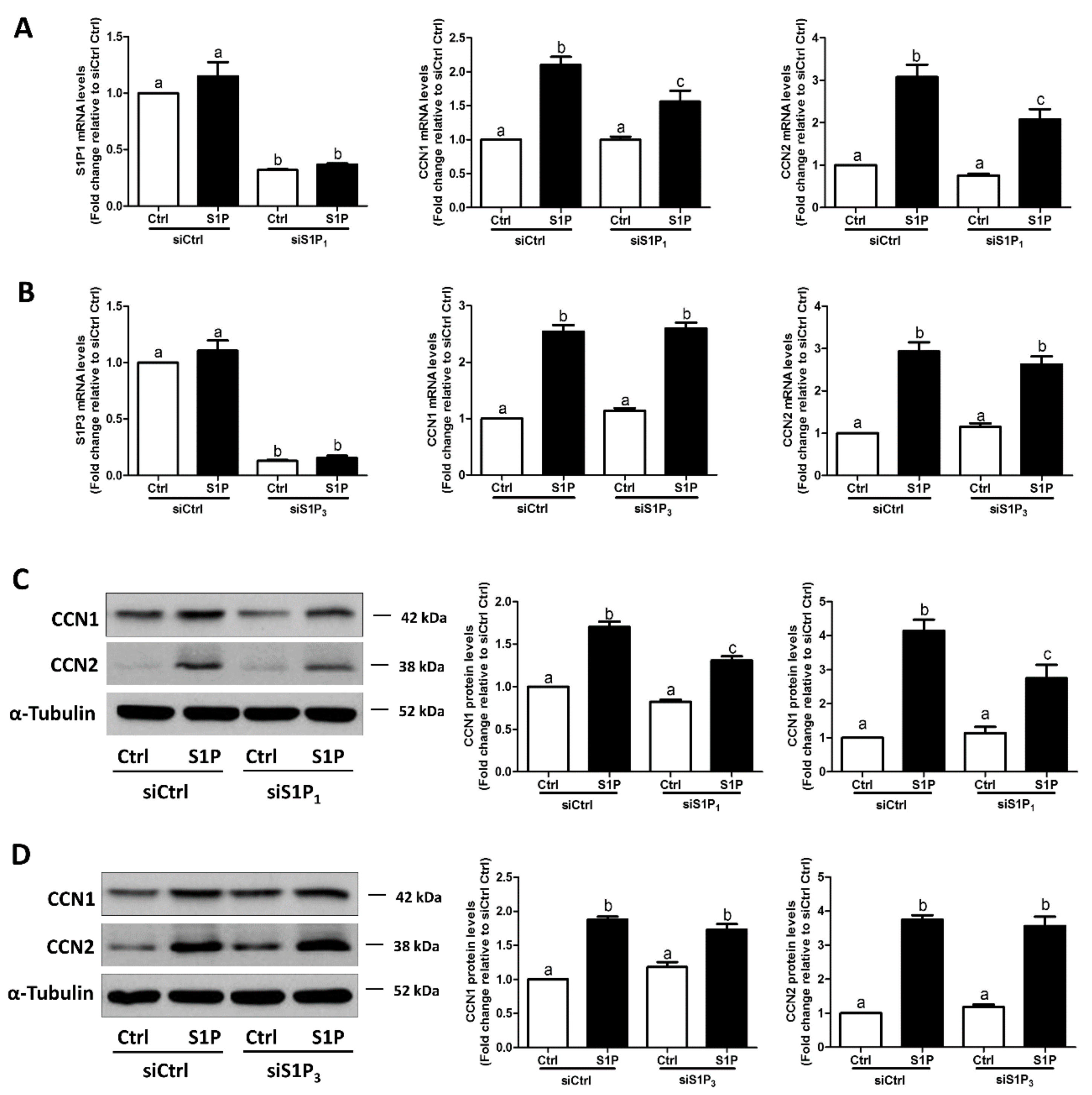
3.2. The S1P1 Receptor Mediates the S1P-Induced Upregulation of CCN1 and CCN2 Expression in hGL Cells
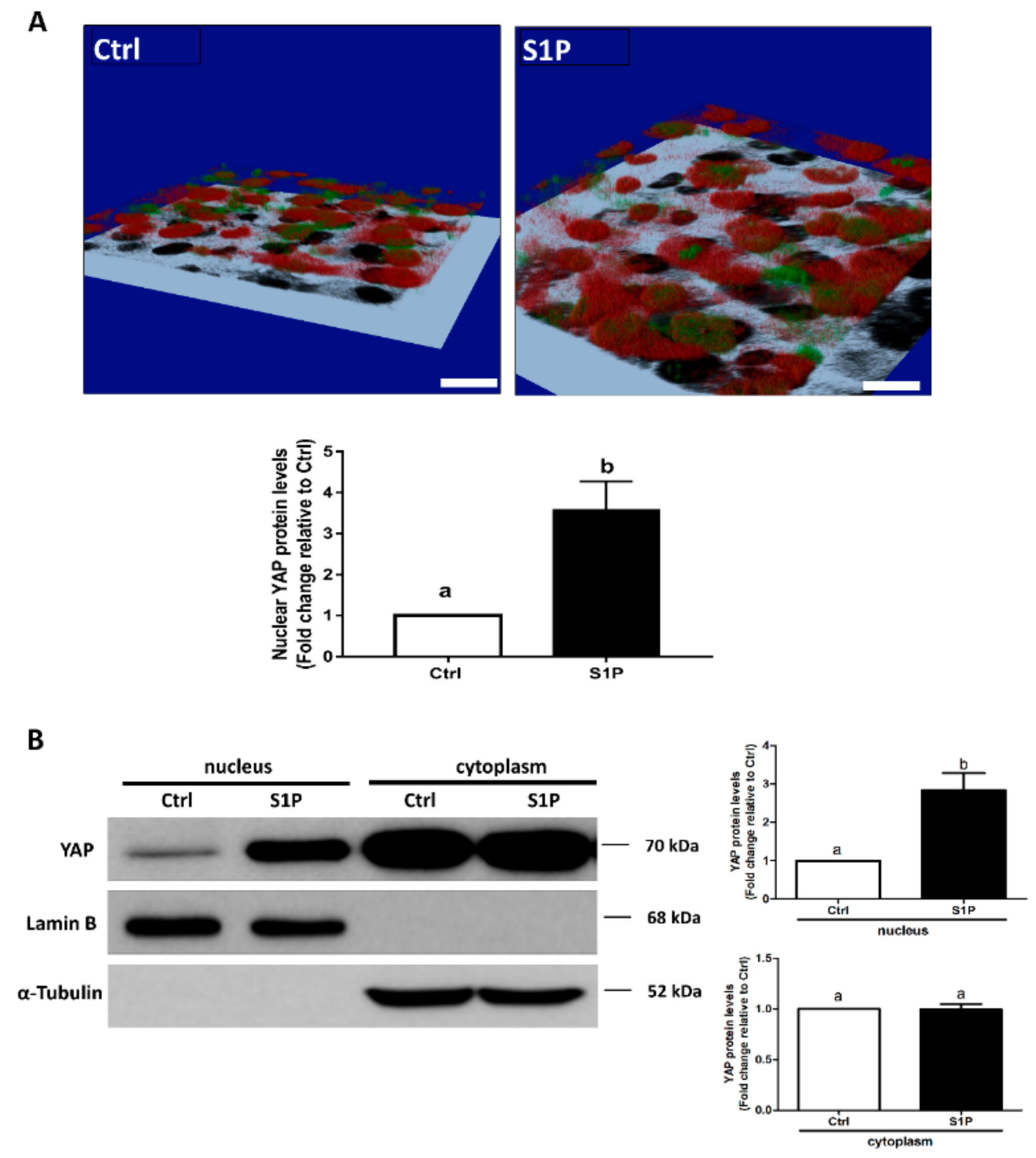
3.3. S1P Induces Nuclear Translocation of YAP in SVOG Cells
3.4. YAP Mediates the S1P-Induced Upregulation of CCN1, CCN2, and COX2 Expression
3.5. CCN2, But Not CCN1, Mediates the S1P-Induced Upregulation of COX2 and Increase in PGE2 Production in SVOG Cells
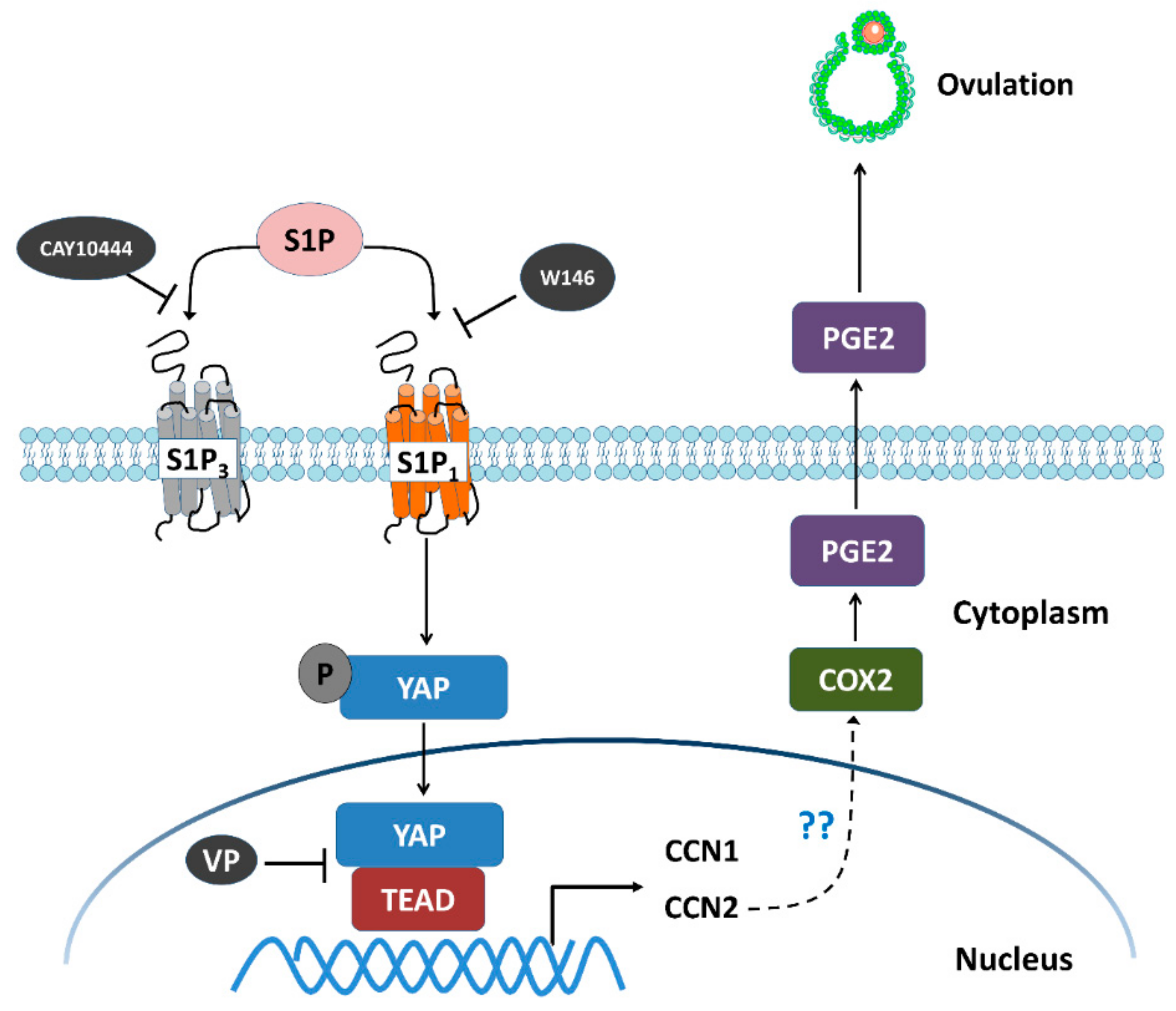
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Holbourn, K.P.; Acharya, K.R.; Perbal, B. The CCN family of proteins: Structure-function relationships. Trends Biochem. Sci. 2008, 33, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Kubota, S.; Takigawa, M. The CCN family acting throughout the body recent research developments. Biomol. Concepts 2013, 4, 477–494. [Google Scholar] [CrossRef] [PubMed]
- Perbal, B. CCN proteins: Multifunctional signalling regulators. Lancet 2004, 363, 62–64. [Google Scholar] [CrossRef]
- Winterhager, E.; Gellhaus, A. The role of the CCN family of proteins in female reproduction. Cell. Mol. Life Sci. 2014, 71, 2299–2311. [Google Scholar] [CrossRef] [PubMed]
- Gashaw, I.; Hastings, J.M.; Jackson, K.S.; Winterhager, E.; Fazleabas, A.T. Induced endometriosis in the baboon (Papio anubis) increases the expression of the proangiogenic factor CYR61 (CCN1) in eutopic and ectopic endometria. Biol. Reprod. 2006, 74, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Absenger, Y.; Hess-Stumpp, H.; Kreft, B.; Kratzschmar, J.; Haendler, B.; Schutze, N.; Regidor, P.A.; Winterhager, E. Cyr61, a deregulated gene in endometriosis. Mol. Hum. Reprod. 2004, 10, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tsang, P.C.; Pate, J.L.; Moses, M.A. A role for cysteine-rich 61 in the angiogenic switch during the estrous cycle in cows: Regulation by prostaglandin F2alpha. Biol. Reprod. 2011, 85, 261–268. [Google Scholar] [CrossRef]
- Yang, R.; Chen, Y.; Chen, D. Biological functions and role of CCN1/Cyr61 in embryogenesis and tumorigenesis in the female reproductive system (Review). Mol. Med. Rep. 2018, 17, 3–10. [Google Scholar] [CrossRef]
- Nagashima, T.; Kim, J.; Li, Q.; Lydon, J.P.; DeMayo, F.J.; Lyons, K.M.; Matzuk, M.M. Connective tissue growth factor is required for normal follicle development and ovulation. Mol. Endocrinol. 2011, 25, 1740–1759. [Google Scholar] [CrossRef]
- Chang, H.M.; Fang, Y.; Liu, P.P.; Cheng, J.C.; Yang, X.; Leung, P.C. Connective tissue growth factor mediates growth differentiation factor 8-induced increase of lysyl oxidase activity in human granulosa-lutein cells. Mol. Cell. Endocrinol. 2016, 434, 186–198. [Google Scholar] [CrossRef]
- Chang, H.M.; Cheng, J.C.; Liu, Y.; Klausen, C.; Xu, C.; Leung, P.C. Activin A-induced increase in LOX activity in human granulosa-lutein cells is mediated by CTGF. Reproduction 2016, 152, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.M.; Pan, H.H.; Cheng, J.C.; Zhu, Y.M.; Leung, P.C. Growth differentiation factor 8 suppresses cell proliferation by up-regulating CTGF expression in human granulosa cells. Mol. Cell Endocrinol. 2016, 422, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Badouel, C.; McNeill, H. SnapShot: The hippo signaling pathway. Cell 2011, 145, 484. [Google Scholar] [CrossRef] [PubMed]
- Halder, G.; Johnson, R.L. Hippo signaling: Growth control and beyond. Development 2011, 138, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Feldmann, G.; Huang, J.; Wu, S.; Zhang, N.; Comerford, S.A.; Gayyed, M.F.; Anders, R.A.; Maitra, A.; Pan, D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 2007, 130, 1120–1133. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.X.; Zhao, B.; Guan, K.L. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell 2015, 163, 811–828. [Google Scholar] [CrossRef]
- Hu, L.L.; Su, T.; Luo, R.C.; Zheng, Y.H.; Huang, J.; Zhong, Z.S.; Nie, J.; Zheng, L.P. Hippo pathway functions as a downstream effector of AKT signaling to regulate the activation of primordial follicles in mice. J. Cell. Physiol. 2019, 234, 1578–1587. [Google Scholar] [CrossRef]
- Li, J.; Zhou, F.; Zheng, T.; Pan, Z.; Liang, X.; Huang, J.; Zheng, L.; Zheng, Y. Ovarian Germline Stem Cells (OGSCs) and the Hippo Signaling Pathway Association with Physiological and Pathological Ovarian Aging in Mice. Cell. Physiol. Biochem. 2015, 36, 1712–1724. [Google Scholar] [CrossRef]
- Cheng, J.C.; Chang, H.M.; Liu, P.P.; Leung, P.C. Sphingosine-1-phosphate induces COX-2 expression and PGE2 production in human granulosa cells through a S1P1/3-mediated YAP signaling. Cell Signal. 2016, 28, 643–651. [Google Scholar] [CrossRef]
- Richards, J.S.; Russell, D.L.; Ochsner, S.; Espey, L.L. Ovulation: New dimensions and new regulators of the inflammatory-like response. Annu. Rev. Physiol. 2002, 64, 69–92. [Google Scholar] [CrossRef]
- Davis, B.J.; Lennard, D.E.; Lee, C.A.; Tiano, H.F.; Morham, S.G.; Wetsel, W.C.; Langenbach, R. Anovulation in Cyclooxygenase-2-Deficient Mice Is Restored by Prostaglandin E2 and Interleukin-1b. Endocrinology 1999, 140, 2685–2695. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.M.; Stouffer, R.L. Follicular administration of a cyclooxygenase inhibitor can prevent oocyte release without alteration of normal luteal function in rhesus monkeys. Hum. Reprod. 2002, 17, 2825–2831. [Google Scholar] [CrossRef] [PubMed]
- Hester, K.E.; Harper, M.J.; Duffy, D.M. Oral administration of the cyclooxygenase-2 (COX-2) inhibitor meloxicam blocks ovulation in non-human primates when administered to simulate emergency contraception. Hum. Reprod 2010, 25, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, S.; Milstien, S. Sphingosine-1-phosphate: An enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 2003, 4, 397–407. [Google Scholar] [CrossRef]
- Mendelson, K.; Evans, T.; Hla, T. Sphingosine 1-phosphate signalling. Development 2014, 141, 5–9. [Google Scholar] [CrossRef]
- Guo, L.; Ou, X.; Li, H.; Han, Z. Roles of Sphingosine-1-Phosphate in Reproduction. Reprod. Sci. 2014, 21, 550–554. [Google Scholar] [CrossRef]
- Becker, S.; von Otte, S.; Robenek, H.; Diedrich, K.; Nofer, J.R. Follicular fluid high-density lipoprotein-associated sphingosine 1-phosphate (S1P) promotes human granulosa lutein cell migration via S1P receptor type 3 and small G-protein RAC1. Biol. Reprod. 2011, 84, 604–612. [Google Scholar] [CrossRef]
- Nakahara, T.; Iwase, A.; Nakamura, T.; Kondo, M.; Bayasula Kobayashi, H.; Takikawa, S.; Manabe, S.; Goto, M.; Kotani, T.; et al. Sphingosine-1-phosphate inhibits H2O2-induced granulosa cell apoptosis via the PI3K/Akt signaling pathway. Fertil. Steril. 2012, 98, 1001–1008. [Google Scholar] [CrossRef]
- Fan, Q.; Cheng, Y.; Chang, H.M.; Deguchi, M.; Hsueh, A.J.; Leung, P.C.K. Sphingosine-1-phosphate promotes ovarian cancer cell proliferation by disrupting Hippo signaling. Oncotarget 2017, 8, 27166–27176. [Google Scholar] [CrossRef]
- Morita, Y.; Perez, G.I.; Paris, F.; Miranda, S.R.; Ehleiter, D.; Haimovitz-Friedman, A.; Fuks, Z.; Xie, Z.; Reed, J.C.; Schuchman, E.H.; et al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine -1-phosphate therapy. Nat. Med. 2000, 6, 1109–1114. [Google Scholar] [CrossRef]
- Lie , B.L.; Leung, E.; Leung, P.C.; Auersperg, N. Long-term growth and steroidogenic potential of human granulosa-lutein cells immor talized with SV40 large T antigen. Mol. Cell. Endocrinol. 1996, 120, 169–176. [Google Scholar] [PubMed]
- Bai, L.; Chang, H.M.; Cheng, J.C.; Chu, G.; Leung, P.C.K.; Yang, G. Lithium Chloride Increases COX-2 Expression and PGE2 Production in a Human Granulosa-Lutein SVOG Cell Line Via a GSK-3beta/beta-Catenin Signaling Pathway. Endocrinology 2017, 158, 2813–2825. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Chang, H.M.; Cheng, J.C.; Klausen, C.; Leung, P.C.; Yang, X. Transforming growth factor-beta1 increases lysyl oxidase expression by downregulating MIR29A in human granulosa lutein cells. Reproduction 2016, 152, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.C.; Fang, L.; Chang, H.M.; Sun, Y.P.; Leung, P.C. hCG-induced Sprouty2 mediates amphiregulin-stimulated COX-2/PGE2 up-regulation in human granulosa cells: A potential mechanism for the OHSS. Sci. Rep. 2016, 6, 31675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.M.; Wu, H.C.; Sun, Z.G.; Lian, F.; Leung, P.C.K. Neurotrophins and glial cell line-derived neurotrophic factor in the ovary: Physiological and pathophysiological implications. Hum. Reprod. Update 2019, 25, 224–242. [Google Scholar] [CrossRef]
- Shi, F.T.; Cheung, A.P.; Leung, P.C. Growth differentiation factor 9 enhances activin a-induced inhibin B production in human granulosa cells. Endocrinology 2009, 150, 3540–3546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, X. Lysophospholipid signaling in the function and pathology of the reproductive system. Hum. Reprod. Update 2008, 14, 519–536. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, Y.; Inazumi, T.; Tsuchiya, S. Roles of prostaglandin receptors in female reproduction. J. Biochem. 2015, 157, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Committee, O.P.B.G. ACOG practice bulletin #34. Management of infertility caused by ovulatory dysfunction. Obstet. Gynecol. 2002, 99, 347–358. [Google Scholar] [CrossRef]
- Niringiyumukiza, J.D.; Cai, H.; Xiang, W. Prostaglandin E2 involvement in mammalian female fertility: Ovulation, fertilization, embryo development and early implantation. Reprod. Biol. Endocrinol. 2018, 16, 43. [Google Scholar] [CrossRef]
- Fang, L.; Cheng, J.C.; Chang, H.M.; Sun, Y.P.; Leung, P.C. EGF-like growth factors induce COX-2-derived PGE2 production through ERK1/2 in human granulosa cells. J. Clin. Endocrinol. Metab. 2013, 98, 4932–4941. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Chang, H.M.; Cheng, J.C.; Leung, P.C.; Sun, Y.P. TGF-β1 induces COX-2 expression and PGE2 production in human granulosa cells through Smad signaling pathways. J. Clin. Endocrinol. Metab. 2014, 99, 1217–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.C.; Wang, E.Y.; Yi, Y.; Thakur, A.; Tsai, S.H.; Hoodless, P.A. S1P Stimulates Proliferation by Upregulating CTGF Expression through S1PR2-Mediated YAP Activation. Mol. Cancer Res. 2018, 16, 1543–1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinkel, R.; Trenkwalder, T.; Petersen, B.; Husada, W.; Gesenhues, F.; Lee, S.; Hannappel, E.; Bock-Marquette, I.; Theisen, D.; Leitner, L.; et al. MRTF-A controls vessel growth and maturation by increasing the expression of CCN1 and CCN2. Nat. Commun. 2014, 5, 3970. [Google Scholar] [CrossRef] [Green Version]
- Pu, N.; Gao, S.; Yin, H.; Li, J.A.; Wu, W.; Fang, Y.; Zhang, L.; Rong, Y.; Xu, X.; Wang, D.; et al. Cell-intrinsic PD-1 promotes proliferation in pancreatic cancer by targeting CYR61/CTGF via the hippo pathway. Cancer Lett. 2019, 460, 42–53. [Google Scholar] [CrossRef]
- Bonda, A.T.; Dziemidowicz, M.; Litvinovich, S.; Kożuch, M.; Hirnle, T.; Dmitruk, I.; Chyczewski, L.; Winnicka, M. Atrial expression of the CCN1 and CCN2 proteins in chronic heart failure. Folia Histochem. Cytobiol. 2012, 50, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Markiewicz, M.; Nakerakanti, S.S.; Kapanadze, B.; Ghatnekar, A.; Trojanowska, M. Connective tissue growth factor (CTGF/CCN2) mediates angiogenic effect of S1P in human dermal microvascular endothelial cells. Microcirculation 2011, 18, 1–11. [Google Scholar] [CrossRef]
- Leask, A.; Abraham, D.J. All in the CCN family: Essential matricellular signaling modulators emerge from the bunker. J. Cell Sci 2006, 119, 4803–4810. [Google Scholar] [CrossRef] [Green Version]



| Name | Forward (5’–3’) | Reverse (5’–3’) |
|---|---|---|
| CCN1 | AGCCTCGCATCCTATACAACC | TTCTTTCACAAGGCGGCACTC |
| CCN2 | GCGTGTGCACCGCCAAAGAT | CAGGGCTGGGCAGACGAACG |
| COX2 | CCCTTGGGTGTCAAAGGTAA | GCCCTCGCTTATGATCTGTC |
| S1P1 | TGCGGGAAGGGAGTATGTTT | CGATGGCGAGGAGACTGAAC |
| S1P3 | TGCAGCTTCATCGTCTTGGAG | GCCAATGAAAAAGTACATGCGG |
| GAPDH | GAGTCAACGGATTTGGTCGT | GACAAGCTTCCCGTTCTCAG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.-L.; Chang, H.-M.; Yi, Y.; Liu, Y.; Taylor, E.L.; Zheng, L.-P.; Leung, P.C.K. CCN2 Mediates S1P-Induced Upregulation of COX2 Expression in Human Granulosa-Lutein Cells. Cells 2019, 8, 1445. https://doi.org/10.3390/cells8111445
Hu L-L, Chang H-M, Yi Y, Liu Y, Taylor EL, Zheng L-P, Leung PCK. CCN2 Mediates S1P-Induced Upregulation of COX2 Expression in Human Granulosa-Lutein Cells. Cells. 2019; 8(11):1445. https://doi.org/10.3390/cells8111445
Chicago/Turabian StyleHu, Liao-Liao, Hsun-Ming Chang, Yuyin Yi, Yingtao Liu, Elizabeth L. Taylor, Li-Ping Zheng, and Peter C.K. Leung. 2019. "CCN2 Mediates S1P-Induced Upregulation of COX2 Expression in Human Granulosa-Lutein Cells" Cells 8, no. 11: 1445. https://doi.org/10.3390/cells8111445