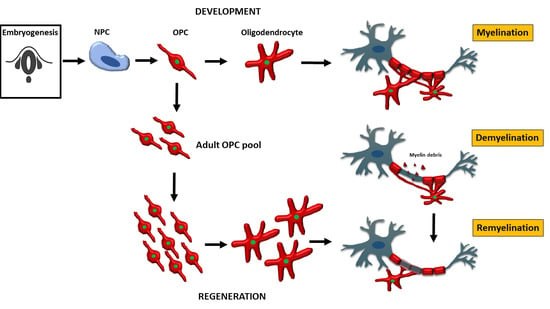
Oligodendrocytes in Development, Myelin Generation and Beyond
Abstract
:1. Introduction
2. Oligodendrocyte Progenitor Cells (OPC) and Oligodendrocytes in Development
2.1. OPC and Oligodendrocytes during Embryonic Development
2.2. Distribution of OPC and Oligodendrocytes within the CNS
2.3. Developmental Markers of OPC and Oligodendrocytes
2.4. Other Myelin-Producing Cells
3. Oligodendrocyte Function in Health
3.1. Myelination of Axons
3.2. Functions of the Myelin Sheath
3.3. Non-Myelinating Functions of Oligodendrocytes and OPC
4. Models to Study Oligodendrocytes and OPC
4.1. Rodent and Human In Vitro Models
4.2. In Vivo Models
4.3. Animal Models to study Oligodendrocytes and Remyelination in Multiple Sclerosis
5. Oligodendrocytes in Disease
5.1. Oligodendrocytes in Diseases with Myelin Pathology
5.2. Oligodendrocytes in Demyelination
5.3. Oligodendrocytes in Ageing
6. Oligodendrocytes in Myelin Regeneration
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Bradl, M.; Lassmann, H. Oligodendrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.J.M.; Ffrench-Constant, C. Regenerating CNS myelin-from mechanisms to experimental medicines. Nat. Rev. Neurosci 2017, 18, 753–769. [Google Scholar] [CrossRef] [PubMed]
- Nave, K.A. Myelination and support of axonal integrity by glia. Nature 2010, 468, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Virchow, R. Gesammelte Abhandlungen zur Wissenschaftlichen Medicin; Hamm, Frankfurt a.M. 1856. Available online: https://archive.org/details/b21462161 (accessed on 9 November 2019).
- Deiters, O.F.C. Untersuchungen über Gehirn und Rückenmark des Menschen und der Säugetiere; Braunschweig. 1865. Available online: https://archive.org/details/untersuchungen00deit/page/n8 (accessed on 9 November 2019).
- Golgi, C. Sulla Fina Anatomia Degli Organi Centrali del Sistema Nervoso. Milano, 1885. Available online: https://archive.org/details/b21978724/page/n8 (accessed on 9 November 2019).
- Virchow, R. Die Cellularpathologie in ihrer Begründung auf physiologische und pathologische Gewebelehre. Berlin, 1858. Available online: https://archive.org/details/diecellularpatho00virc/page/n8 (accessed on 9 November 2019).
- Del Rio-Hortega, P. La microglia y su transformacíon en células en bastoncito y cuerpos gránulo-adiposos. Trab. Lab. Invest. Biol. Madrid. 1920, 18, 37–82. [Google Scholar]
- Del Rio-Hortega, P. Arte y artificio de la ciencia histológica. Residencia Revista de la Residencia de Estudiantes IV. 1933, 4, 191–206. [Google Scholar]
- Hardy, R.; Reynolds, R. Proliferation and differentiation potential of rat forebrain oligodendroglial progenitors both in vitro and in vivo. Development 1991, 111, 1061–1080. [Google Scholar] [PubMed]
- Rexed, B. The cytoarchitectonic organization of the spinal cord in the cat. J. Comp. Neurol. 1952, 96, 415–495. [Google Scholar] [CrossRef] [PubMed]
- Nornes, H.O.; Das, G.D. Temporal pattern of neurogenesis in spinal cord of rat. I. An autoradiographic study — time and sites of origin and migration and settling patterns of neuroblasts. Brain Res. 1974, 73, 121–138. [Google Scholar] [CrossRef]
- Ginhoux, F.; Prinz, M. Origin of microglia: Current concepts and past controversies. Cold Spring Harb. Perspect. Biol. 2015, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Raff, M.C.; Miller, R.H.; Noble, M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature 1983, 303, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Pringle, N.P.; Richardson, W.D. A singularity of PDGF alpha-receptor expression in the dorsoventral axis of the neural tube may define the origin of the oligodendrocyte lineage. Development 1993, 117. [Google Scholar]
- Timsit, S.; Martinez, S.; Allinquant, B.; Peyron, F.; Puelles, L.; Zalc, B. Oligodendrocytes originate in a restricted zone of the embryonic ventral neural tube defined by DM-20 mRNA expression. J. Neurosci. 1995, 15, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Warf, B.C.; Fok-Seang, J.; Miller, R.H. Evidence for the ventral origin of oligodendrocyte precursors in the rat spinal cord. J. Neurosci. 1991, 11, 2477–2488. [Google Scholar] [CrossRef] [PubMed]
- Vallstedt, A.; Klos, J.M.; Ericson, J. Multiple dorsoventral origins of oligodendrocyte generation in the spinal cord and hindbrain. Neuron 2005, 45, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, M.; Richardson, W.D.; Kessaris, N. A subset of oligodendrocytes generated from radial glia in the dorsal spinal cord. Development 2005, 132, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.A.; Temple, S. A self-renewing multipotential stem cell in embryonic rat cerebral cortex. Nature 1994, 372, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Rogister, B.; Ben-Hur, T.; Dubois-Dalcq, M. From neural stem cells to myelinating oligodendrocytes. Mol. Cell. Neurosci. 1999, 14, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Spassky, N.; Goujet-Zalc, C.; Parmantier, E.; Olivier, C.; Martinez, S.; Ivanova, A.; Ikenaka, K.; Macklin, W.; Cerruti, I.; Zalc, B.; et al. Multiple restricted origin of oligodendrocytes. J. Neurosci. 1998, 18, 8331–8343. [Google Scholar] [CrossRef] [PubMed]
- Hajihosseini, M.; Tham, T.N.; Dubois-Dalcq, M. Origin of oligodendrocytes within the human spinal cord. J. Neurosci. 1996, 16, 7981–7994. [Google Scholar] [CrossRef] [PubMed]
- Kessaris, N.; Fogarty, M.; Iannarelli, P.; Grist, M.; Wegner, M.; Richardson, W.D. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat. Neurosci. 2006, 9, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.B.; Clarke, L.E.; Burzomato, V.; Kessaris, N.; Anderson, P.N.; Attwell, D.; Richardson, W.D. Dorsally and Ventrally Derived Oligodendrocytes Have Similar Electrical Properties but Myelinate Preferred Tracts. J. Neurosci. 2011, 31, 6809–6819. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Qi, Y.; Hu, X.; Tan, M.; Liu, Z.; Zhang, J.; Li, Q.; Sander, M.; Qiu, M. Generation of oligodendrocyte precursor cells from mouse dorsal spinal cord independent of Nkx6 regulation and Shh signaling. Neuron 2005, 45, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Barres, B.A.; Hart, I.K.; Coles, H.S.; Burne, J.F.; Voyvodic, J.T.; Richardson, W.D.; Raff, M.C. Cell death and control of cell survival in the oligodendrocyte lineage. Cell 1992, 70, 31–46. [Google Scholar] [CrossRef]
- Trapp, B.D.; Nishiyama, A.; Cheng, D.; Macklin, W. Differentiation and death of premyelinating oligodendrocytes in developing rodent brain. J. Cell Biol. 1997, 137, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Barres, B.A.; Raff, M.C. Axonal control of oligodendrocyte development. J. Cell Biol. 1999, 147, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Orduz, D.; Benamer, N.; Ortolani, D.; Coppola, E.; Vigier, L.; Pierani, A.; Angulo, M.C. Developmental cell death regulates lineage-related interneuron-oligodendroglia functional clusters and oligodendrocyte homeostasis. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, R.D.; Curtis, M.A.; Spalding, K.L.; Buchholz, B.A.; Fink, D.; Björk-Eriksson, T.; Nordborg, C.; Gage, F.H.; Druid, H.; Eriksson, P.S.; et al. Neocortical neurogenesis in humans is restricted to development. Proc. Natl. Acad. Sci. USA 2006, 103, 12564–12568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandeira, F.; Lent, R.; Herculano-Houzel, S. Changing numbers of neuronal and non-neuronal cells underlie postnatal brain growth in the rat. Proc. Natl. Acad. Sci. USA 2009, 106, 14108–14113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnabé-Heider, F.; Göritz, C.; Sabelström, H.; Takebayashi, H.; Pfrieger, F.W.; Meletis, K.; Frisén, J. Origin of New Glial Cells in Intact and Injured Adult Spinal Cord. Cell Stem Cell 2010, 7, 470–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inta, D.; Alfonso, J.; von Engelhardt, J.; Kreuzberg, M.M.; Meyer, A.H.; van Hooft, J.A.; Monyer, H. Neurogenesis and widespread forebrain migration of distinct GABAergic neurons from the postnatal subventricular zone. Proc. Natl. Acad. Sci. USA 2008, 105, 20994–20999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orentas, D.M.; Hayes, J.E.; Dyer, K.L.; Miller, R.H. Sonic hedgehog signaling is required during the appearance of spinal cord oligodendrocyte precursors. Development 1999, 126, 2419–2429. [Google Scholar] [PubMed]
- Van Straaten, H.W.M.; Hekking, J.W.M.; Beursgens, J.P.W.M.; Terwindt-Rouwenhorst, E.; Drukker, J. Effect of the notochord on proliferation and differentiation in the neural tube of the chick embryo. Development 1989, 107, 793–803. [Google Scholar] [CrossRef]
- Echelard, Y.; Epstein, D.J.; St-Jacques, B.; Shen, L.; Mohler, J.; McMahon, J.A.; McMahon, A.P. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 1993, 75, 1417–1430. [Google Scholar] [CrossRef]
- Pringle, N.P.; Yu, W.-P.; Guthrie, S.; Roelink, H.; Lumsden, A.; Peterson, A.C.; Richardson, W.D. Determination of Neuroepithelial Cell Fate: Induction of the Oligodendrocyte Lineage by Ventral Midline Cells and Sonic Hedgehog. Dev. Biol. 1996, 177, 30–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, M.K.; Hanken, J.; Gooneratne, M.L.; Pieau, C.; Raynaud, A.; Selwood, L.; Wright, G.M. There is no highly conserved embryonic stage in the vertebrates: Implications for current theories of evolution and development. Anat. Embryol. 1997, 196, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Roelink, H.; Augsburger, A.; Heemskerk, J.; Korzh, V.; Norlin, S.; Ruiz i Altaba, A.; Tanabe, Y.; Placzek, M.; Edlund, T.; Jessell, T.M.; et al. Floor plate and motor neuron induction by vhh-1, a vertebrate homolog of hedgehog expressed by the notochord. Cell 1994, 76, 761–775. [Google Scholar] [CrossRef]
- Nery, S.; Wichterle, H.; Fishell, G. Sonic hedgehog contributes to oligodendrocyte specification in the mammalian forebrain. Development 2001, 128, 527–540. [Google Scholar] [PubMed]
- Hashimoto, H.; Jiang, W.; Yoshimura, T.; Moon, K.H.; Bok, J.; Ikenaka, K. Strong sonic hedgehog signaling in the mouse ventral spinal cord is not required for oligodendrocyte precursor cell (OPC) generation but is necessary for correct timing of its generation. Neurochem. Int. 2018, 119, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Tiane, A.; Schepers, M.; Rombaut, B.; Hupperts, R.; Prickaerts, J.; Hellings, N.; van den Hove, D.; Vanmierlo, T. From OPC to Oligodendrocyte: An Epigenetic Journey. Cells 2019, 8, 1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barres, B.A.; Raff, M.C. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature 1993, 361, 258–260. [Google Scholar] [CrossRef] [PubMed]
- Hughes, E.G.; Orthmann-Murphy, J.L.; Langseth, A.J.; Bergles, D.E. Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nat. Neurosci. 2018, 21, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.M.; Purger, D.; Mount, C.W.; Goldstein, A.K.; Lin, G.L.; Wood, L.S.; Inema, I.; Miller, S.E.; Bieri, G.; Zuchero, J.B.; et al. Neuronal Activity Promotes Oligodendrogenesis and Adaptive Myelination in the Mammalian Brain. Science 2014, 344, 1252304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitew, S.; Gobius, I.; Fenlon, L.R.; McDougall, S.J.; Hawkes, D.; Xing, Y.L.; Bujalka, H.; Gundlach, A.L.; Richards, L.J.; Kilpatrick, T.J.; et al. Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner. Nat. Commun. 2018, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Dawson, M.R.L.; Polito, A.; Levine, J.M.; Reynolds, R. NG2-expressing glial progenitor cells: An abundant and widespread population of cycling cells in the adult rat CNS. Mol. Cell. Neurosci. 2003, 24, 476–488. [Google Scholar] [CrossRef]
- Hill, R.A.; Patel, K.D.; Medved, J.; Reiss, A.M.; Nishiyama, A. NG2 cells in white matter but not gray matter proliferate in response to PDGF. J. Neurosci. 2013, 33, 14558–14566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lentferink, D.H.; Jongsma, J.M.; Werkman, I.; Baron, W. Grey matter OPCs are less mature and less sensitive to IFNγ than white matter OPCs: consequences for remyelination. Sci. Rep. 2018, 8, 2113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimou, L.; Simon, C.; Kirchhoff, F.; Takebayashi, H.; Götz, M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J. Neurosci. 2008, 28, 10434–10442. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Fukaya, M.; Yang, J.K.; Rothstein, J.D.; Bergles, D.E. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron 2010, 68, 668–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivers, L.E.; Young, K.M.; Rizzi, M.; Jamen, F. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat. Neurosci. 2008, 11, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, E.G.; Kang, S.H.; Fukaya, M.; Bergles, D.E. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat. Neurosci. 2013, 16, 668–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lachapelle, F.; Gumpel, M.; Baulac, M.; Jacque, C.; Duc, P.; Baumann, N. Transplantation of CNS Fragments into the Brain of Shiverer Mutant Mice: Extensive Myelination by Implanted Oligodendrocytes. Dev. Neurosci. 1983, 6, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.H.; Niu, J.; Munji, R.; Davalos, D.; Chang, J.; Zhang, H.; Tien, A.C.; Kuo, C.J.; Chan, J.R.; Daneman, R.; et al. Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science 2016, 351, 379–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, J.; Tsai, H.H.; Hoi, K.K.; Huang, N.; Yu, G.; Kim, K.; Baranzini, S.E.; Xiao, L.; Chan, J.R.; Fancy, S.P.J. Aberrant oligodendroglial–vascular interactions disrupt the blood–brain barrier, triggering CNS inflammation. Nat. Neurosci. 2019, 22, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.H.; Payne, J.; Milner, L.; Zhang, H.; Orentas, D.M. Spinal cord oligodendrocytes develop from a limited number of migratory, highly proliferative precursors. J. Neurosci. Res. 1997, 50, 157–168. [Google Scholar] [CrossRef]
- Fok-Seang, J.; Miller, R.H. Distribution and differentiation of A2B5+ glial precursors in the developing rat spinal cord. J Neurosci Res 1994, 37, 219–235. [Google Scholar] [CrossRef] [PubMed]
- Raff, M.C.; Abney, E.R.; Miller, R.H. Two glial cell lineages diverge prenatally in rat optic nerve. Dev. Biol. 1984, 106, 53–60. [Google Scholar] [CrossRef]
- Hughes, S.M.; Lillien, L.E.; Raff, M.C.; Rohrer, H.; Sendtner, M. Ciliary neurotrophic factor induces type-2 astrocyte differentiation in culture. Nature 1988, 335, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Noble, M.; Murray, K.; Stroobant, P.; Waterfield, M.D.; Riddle, P. Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature 1988, 333, 560–562. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.J.; Ruit, K.G.; Wang, Y.X.; Parks, W.C.; Snider, W.D.; Deuel, T.F. PDGF A-chain gene is expressed by mammalian neurons during development and in maturity. Cell 1991, 64, 209–216. [Google Scholar] [CrossRef]
- Calver, A.R.; Hall, A.C.; Yu, W.P.; Walsh, F.S.; Heath, J.K.; Betsholtz, C.; Richardson, W.D. Oligodendrocyte population dynamics and the role of PDGF in vivo. Neuron 1998, 20, 869–882. [Google Scholar] [CrossRef] [Green Version]
- Sommer, I.; Schachner, M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: An immunocytological study in the central nervous system. Dev. Biol. 1981, 83, 311–327. [Google Scholar] [CrossRef]
- Braun, P.E.; Sandillon, F.; Edwards, A.; Matthieu, J.-M.; Privat, A. lmmunocytochemical Localization by Electron Microscopy of 2’,3’-Cyclic Nucleotide 3’-Phosphodiesterase in Developing Oligodendrocytes of Normal and Mutant Brain. J. Neurosci. 1988, 8, 3057–3066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherer, S.S.; Braun, P.E.; Grinspan, J.; Collarini, E.; Wang, D.Y.; Kamholz, J. Differential regulation of the 2′,3′-cyclic nucleotide 3′-phosphodiesterase gene during oligodendrocyte development. Neuron 1994, 12, 1363–1375. [Google Scholar] [CrossRef]
- Jakovcevski, I.; Filipovic, R.; Mo, Z.; Rakic, S.; Zecevic, N. Oligodendrocyte development and the onset of myelination in the human fetal brain. Front. Neuroanat. 2009, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunner, C.; Lassmann, H.; Waehneldt, T.V.; Matthieu, J.-M.; Linington, C. Differential Ultrastructural Localization of Myelin Basic Protein, Myelin/Oligodendroglial Glycoprotein, and 2′,3′-Cyclic Nucleotide 3′-Phosphodiesterase in the CNS of Adult Rats. J. Neurochem. 1989, 52, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Linnington, C.; Webb, M.; Woodhams, P.L. A novel myelin-associated glycoprotein defined by a mouse monoclonal antibody. J. Neuroimmunol. 1984, 6, 387–396. [Google Scholar] [CrossRef]
- Barbarese, E.; Barry, C.; Chou, C.J.; Goldstein, D.J.; Nakos, G.A.; Hyde-DeRuyscher, R.; Scheld, K.; Carson, J.H. Expression and Localization of Myelin Basic Protein in Oligodendrocytes and Transfected Fibroblasts. J. Neurochem. 1988, 51, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Michalski, J.P.; Anderson, C.; Beauvais, A.; de Repentigny, Y.; Kothary, R. The proteolipid protein promoter drives expression outside of the oligodendrocyte lineage during embryonic and early postnatal development. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trapp, B.D. Myelin-Associated Glycoprotein Location and Potential Functions. Ann. N. Y. Acad. Sci. 1990, 605, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Raff, M.C.; Mirsky, R.; Fields, K.L.; Lisak, R.P.; Dorfman, S.H.; Silberberg, D.H.; Gregson, N.A.; Leibowitz, S.; Kennedy, M.C. Galactocerebroside is a specific cell-surface antigenic marker for oligodendrocytes in culture. Nature 1978, 274, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Dubois-Dalcq, M.; Behar, T.; Hudson, L.; Lazzarini, R.A. Emergence of three myelin proteins in oligodendrocytes cultured without neurons. J. Cell Biol. 1986, 102, 384–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harlow, D.E.; Saul, K.E.; Culp, C.M.; Vesely, E.M.; Macklin, W.B. Expression of proteolipid protein gene in spinal cord stem cells and early oligodendrocyte progenitor cells is dispensable for normal cell migration and myelination. J. Neurosci. 2014, 34, 1333–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Wang, S.; Anderson, D.J. Identification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron 2000, 25, 331–343. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Anderson, D.J. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell 2002, 109, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Rowitch, D.H. Glial specification in the vertebrate neural tube. Nat. Rev. Neurosci. 2004, 5, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Wegener, A.; Deboux, C.; Bachelin, C.; Frah, M.; Kerninon, C.; Seilhean, D.; Weider, M.; Wegner, M.; Nait-Oumesmar, B. Gain of Olig2 function in oligodendrocyte progenitors promotes remyelination. Brain 2015, 138, 120–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, M.; Yue, T.; Ma, Z.; Wu, F.; Gow, A.; Lu, Q.R. Myelinogenesis and axonal recognition by oligodendrocytes in brain are uncoupled in Olig1-null mice. J. Neurosci. 2005, 25, 1354–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, J.; Bercury, K.K.; Ahrendsen, J.T.; Macklin, W.B. Olig1 function is required for oligodendrocyte differentiation in the mouse brain. J. Neurosci. 2015, 35, 4386–4402. [Google Scholar] [CrossRef] [PubMed]
- Arnett, H.A.; Fancy, S.P.J.; Alberta, J.A.; Zhao, C.; Plant, S.R.; Kaing, S.; Raine, C.S.; Rowitch, D.H.; Franklin, R.J.M.; Stiles, C.D. bHLH Transcription Factor Olig1 Is Required to Repair Demyelinated Lesions in the CNS. Science 2004, 306, 2111–2115. [Google Scholar] [CrossRef] [PubMed]
- Kuhlbrodt, K.; Herbarth, B.; Sock, E.; Hermans-Borgmeyer, I.; Wegner, M. Sox10, a novel transcriptional modulator in glial cells. J. Neurosci. 1998, 18, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Pozniak, C.D.; Langseth, A.J.; Dijkgraaf, G.J.P.; Choe, Y.; Werb, Z.; Pleasure, S.J. Sox10 directs neural stem cells toward the oligodendrocyte lineage by decreasing Suppressor of Fused expression. Proc. Natl. Acad. Sci. USA 2010, 107, 21795–21800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stolt, C.C.; Rehberg, S.; Ader, M.; Lommes, P.; Riethmacher, D.; Schachner, M.; Bartsch, U.; Wegner, M. Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev. 2002, 16, 165–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stolt, C.C.; Lommes, P.; Friedrich, R.P.; Wegner, M. Transcription factors Sox8 and Sox10 perform non-equivalent roles during oligodendrocyte development despite functional redundancy. Development 2004, 131, 2349–2358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, Y.; Cai, J.; Wu, Y.; Wu, R.; Lee, J.; Fu, H.; Rao, M.; Sussel, L.; Rubenstein, J.; Qiu, M. Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development 2001, 128, 2723–2733. [Google Scholar] [PubMed]
- Zhu, Q.; Zhao, X.; Zheng, K.; Li, H.; Huang, H.; Zhang, Z.; Mastracci, T.; Wegner, M.; Chen, Y.; Sussel, L.; et al. Genetic evidence that Nkx2.2 and Pdgfra are major determinants of the timing of oligodendrocyte differentiation in the developing CNS. Development 2014, 141, 548–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Bergles, D.E.; Nishiyama, A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development 2008, 135, 145–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodhoo, A.; Sommer, L. Development of the Schwann cell lineage: From the neural crest to the myelinated nerve. Glia 2008, 56, 1481–1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jessen, K.R.; Mirsky, R. The origin and development of glial cells in peripheral nerves. Nat. Rev. Neurosci. 2005, 6, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Salzer, J.L. Schwann cell myelination. Cold Spring Harb. Perspect. Biol. 2015, 7, a020529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harty, B.L.; Coelho, F.; Pease-Raissi, S.E.; Mogha, A.; Ackerman, S.D.; Herbert, A.L.; Gereau, R.W.; Golden, J.P.; Lyons, D.A.; Chan, J.R.; et al. Myelinating Schwann cells ensheath multiple axons in the absence of E3 ligase component Fbxw7. Nat. Commun. 2019, 10, 2976. [Google Scholar] [CrossRef] [PubMed]
- Kucenas, S.; Wang, W.-D.; Knapik, E.W.; Appel, B. A selective glial barrier at motor axon exit points prevents oligodendrocyte migration from the spinal cord. J. Neurosci. 2009, 29, 15187–15194. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Morris, A.D.; Welsh, T.G.; Kucenas, S. Contact-Mediated Inhibition Between Oligodendrocyte Progenitor Cells and Motor Exit Point Glia Establishes the Spinal Cord Transition Zone. PLoS Biol. 2014, 12, e1001961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontenas, L.; Welsh, T.G.; Piller, M.; Coughenour, P.; Gandhi, A.V.; Prober, D.A.; Kucenas, S. The Neuromodulator Adenosine Regulates Oligodendrocyte Migration at Motor Exit Point Transition Zones. Cell Rep. 2019, 27, 115–128.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raine, C.S. Morphology of Myelin and Myelination. In Myelin; Springer US: Boston, MA, USA, 1984; pp. 1–50. [Google Scholar]
- Rosenbluth, J. A brief history of myelinated nerve fibers: one hundred and fifty years of controversy. J. Neurocytol. 1999, 28, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Morell, P.; Quarles, R.H. The Myelin Sheath. 1999. Available online: https://www.ncbi.nlm.nih.gov/books/NBK27954/ (accessed on 9 November 2019).
- Baraban, M.; Koudelka, S.; Lyons, D.A. Ca2+ activity signatures of myelin sheath formation and growth in vivo. Nat. Neurosci. 2018, 21, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Nave, K.-A.; Werner, H.B. Myelination of the Nervous System: Mechanisms and Functions. Annu. Rev. Cell Dev. Biol. 2014, 30, 503–533. [Google Scholar] [CrossRef] [PubMed]
- Kirby, B.B.; Takada, N.; Latimer, A.J.; Shin, J.; Carney, T.J.; Kelsh, R.N.; Appel, B. In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat. Neurosci. 2006, 9, 1506–1511. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Trotter, J. Wrapping it up: The cell biology of myelination. Curr. Opin. Neurobiol. 2007, 17, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Geren, B.B.; Schmitt, F.O. The structure of the Schwann cell and its relation to the axon in certain invertebrate nerve fibers. Proc. Natl. Acad. Sci. USA 1954, 40, 863–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobottka, B.; Ziegler, U.; Kaech, A.; Becher, B.; Goebels, N. CNS live imaging reveals a new mechanism of myelination: The liquid croissant model. Glia 2011, 59, 1841–1849. [Google Scholar] [CrossRef] [PubMed]
- Snaidero, N.; Möbius, W.; Czopka, T.; Hekking, L.H.P.; Mathisen, C.; Verkleij, D.; Goebbels, S.; Edgar, J.; Merkler, D.; Lyons, D.A.; et al. Myelin Membrane Wrapping of CNS Axons by PI(3,4,5)P3-Dependent Polarized Growth at the Inner Tongue. Cell 2014, 156, 277–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friede, R.L. Control of myelin formation by axon caliber. (With a model of the control mechanism). J. Comp. Neurol. 1972, 144, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Windebank, A.J.; Wood, P.; Bunge, R.P.; Dyck, P.J. Myelination determines the caliber of dorsal root ganglion neurons in culture. J. Neurosci. 1985, 5, 1563–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Leach, M.K.; Redmond, S.A.; Chong, S.Y.C.; Mellon, S.H.; Tuck, S.J.; Feng, Z.-Q.; Corey, J.M.; Chan, J.R. A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat. Methods 2012, 9, 917–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez, I.; Hassinger, L.; Paskevich, P.A.; Shine, H.D.; Nixon, R.A. Oligodendroglia regulate the regional expansion of axon caliber and local accumulation of neurofilaments during development independently of myelin formation. J. Neurosci. 1996, 16, 5095–5105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez, I.; Hassinger, L.; Sihag, R.K.; Cleveland, D.W.; Mohan, P.; Nixon, R.A. Local Control of Neurofilament Accumulation during Radial Growth of Myelinating Axons in Vivo. J. Cell Biol. 2000, 151, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Nave, K.-A.; Salzer, J.L. Axonal regulation of myelination by neuregulin 1. Curr. Opin. Neurobiol. 2006, 16, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Waxman, S.G.; Ritchie, J.M. Molecular dissection of the myelinated axon. Ann. Neurol. 1993, 33, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Seidl, A.H. Regulation of conduction time along axons. Neuroscience 2014, 276, 126–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bercury, K.K.; Macklin, W.B. Dynamics and Mechanisms of CNS Myelination. Dev. Cell 2015, 32, 447–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Morrison, B.M.; Li, Y.; Lengacher, S.; Farah, M.H.; Hoffman, P.N.; Liu, Y.; Tsingalia, A.; Jin, L.; Zhang, P.-W.; et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 2012, 487, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Fünfschilling, U.; Supplie, L.M.; Mahad, D.; Boretius, S.; Saab, A.S.; Edgar, J.; Brinkmann, B.G.; Kassmann, C.M.; Tzvetanova, I.D.; Möbius, W.; et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 2012, 485, 517–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravera, S.; Bartolucci, M.; Calzia, D.; Aluigi, M.G.; Ramoino, P.; Morelli, A.; Panfoli, I. Tricarboxylic acid cycle-sustained oxidative phosphorylation in isolated myelin vesicles. Biochimie 2013, 95, 1991–1998. [Google Scholar] [CrossRef] [PubMed]
- Assinck, P.; Duncan, G.J.; Plemel, J.R.; Lee, M.J.; Stratton, J.A.; Manesh, S.B.; Liu, J.; Ramer, L.M.; Kang, S.H.; Bergles, D.E.; et al. Myelinogenic Plasticity of Oligodendrocyte Precursor Cells following Spinal Cord Contusion Injury. J. Neurosci. 2017, 37, 8635–8654. [Google Scholar] [CrossRef] [PubMed]
- Itoyama, Y.; Ohnishi, A.; Tateishi, J.; Kuroiwa, Y.; Webster, H.D. Spinal cord multiple sclerosis lesions in Japanese patients: Schwann cell remyelination occurs in areas that lack glial fibrillary acidic protein (GFAP). Acta Neuropathol. 1985, 65, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Baerwald, K.D.; Popko, B. Developing and mature oligodendrocytes respond differently to the immune cytokine interferon-gamma. J. Neurosci. Res. 1998, 52, 230–239. [Google Scholar] [CrossRef]
- Arnett, H.A.; Mason, J.; Marino, M.; Suzuki, K.; Matsushima, G.K.; Ting, J.P.-Y. TNFα promotes proliferation of oligodendrocyte progenitors and remyelination. Nat. Neurosci. 2001, 4, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Bonora, M.; De Marchi, E.; Patergnani, S.; Suski, J.M.; Celsi, F.; Bononi, A.; Giorgi, C.; Marchi, S.; Rimessi, A.; Duszyński, J.; et al. Tumor necrosis factor-α impairs oligodendroglial differentiation through a mitochondria-dependent process. Cell Death Differ. 2014, 21, 1198–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Z.; Wang, C.; Zepp, J.; Wu, L.; Sun, K.; Zhao, J.; Chandrasekharan, U.; DiCorleto, P.E.; Trapp, B.D.; Ransohoff, R.M.; et al. Act1 mediates IL-17–induced EAE pathogenesis selectively in NG2+ glial cells. Nat. Neurosci. 2013, 16, 1401–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falcão, A.M.; van Bruggen, D.; Marques, S.; Meijer, M.; Jäkel, S.; Agirre, E.; Samudyata; Floriddia, E.M.; Vanichkina, D.P.; ffrench-Constant, C.; et al. Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nat. Med. 2018, 24, 1837–1844. [Google Scholar]
- Kirby, L.; Jin, J.; Cardona, J.G.; Smith, M.D.; Martin, K.A.; Wang, J.; Strasburger, H.; Herbst, L.; Alexis, M.; Karnell, J.; et al. Oligodendrocyte precursor cells present antigen and are cytotoxic targets in inflammatory demyelination. Nat. Commun. 2019, 10, 3887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dincman, T.A.; Beare, J.E.; Ohri, S.S.; Whittemore, S.R. Isolation of cortical mouse oligodendrocyte precursor cells. J. Neurosci. Methods 2012, 209, 219–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emery, B.; Dugas, J.C. Purification of oligodendrocyte lineage cells from mouse cortices by immunopanning. Cold Spring Harb. Protoc. 2013, 2013, 854–868. [Google Scholar] [CrossRef] [PubMed]
- Flores-Obando, R.E.; Freidin, M.M.; Abrams, C.K. Rapid and specific immunomagnetic isolation of mouse primary oligodendrocytes. J. Vis. Exp. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Zheng, B.; Kimberly, S.L.; Cai, Z.; Rhodes, P.G.; Lin, R.C.S. Neuron-oligodendrocyte myelination co-culture derived from embryonic rat spinal cord and cerebral cortex. Brain Behav. 2012, 2, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Mei, F.; Fancy, S.P.J.; Shen, Y.A.A.; Niu, J.; Zhao, C.; Presley, B.; Miao, E.; Lee, S.; Mayoral, S.R.; Redmond, S.A.; et al. Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nat. Med. 2014, 20, 954–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, J.C.; Magal, E.; Muir, D.; Manthorpe, M.; Varon, S. CG-4, A new bipotential glial cell line from rat brain, is capable of differentiating in vitro into either mature oligodendrocytes or type-2 astrocytes. J. Neurosci. Res. 1992, 31, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Richter-Landsberg, C.; Heinrich, M. OLN-93: A new permanent oligodendroglia cell line derived from primary rat brain glial cultures. J. Neurosci. Res. 1996, 45, 161–173. [Google Scholar] [CrossRef]
- Jungl, M.; Kramer’, E.; Grzenkowskil, M.; Tang2, K.; Biakemore2, W.; Aguzzi3, A.; Khazaie4, K.; Chiichlia4, K.; Von Blankenfeld5, G.; Kettenmann5, H.; et al. Lines of Murine Oligodendroglial Precursor Cells Immortalized by an Activated neu Tvrosine Kinase Show Distinct Degrees of Interaction with Axons In Wtro and In Wvo. EJN 1995, 7, 1245–1265. [Google Scholar]
- Van Tilborg, E.; de Theije, C.G.M.; van Hal, M.; Wagenaar, N.; de Vries, L.S.; Benders, M.J.; Rowitch, D.H.; Nijboer, C.H. Origin and dynamics of oligodendrocytes in the developing brain: Implications for perinatal white matter injury. Glia 2018, 66, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Chanoumidou, K.; Mozafari, S.; Baron-Van Evercooren, A.; Kuhlmann, T. Stem cell derived oligodendrocytes to study myelin diseases. Glia 2019, glia.23733. [Google Scholar] [CrossRef] [PubMed]
- Medina-Rodríguez, E.M.; Arenzana, F.J.; Bribián, A.; De Castro, F. Protocol to isolate a large amount of functional oligodendrocyte precursor cells from the cerebral cortex of adult mice and humans. PLoS ONE 2013, 8, e81620. [Google Scholar]
- De Groot, J.A.; Montagne, L.; Janssen, I.; Ravid, R.; Van Der Valk, P.; Veerhuis, R. Isolation and characterization of adult microglial cells and oligodendrocytes derived from postmortem human brain tissue. Brain Res. Protoc. 2000, 5, 85–94. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Bates, J.; Li, X.; Schanz, S.; Chandler-Militello, D.; Levine, C.; Maherali, N.; Studer, L.; Hochedlinger, K.; Windrem, M.; et al. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell 2013, 12, 252–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrlich, M.; Mozafari, S.; Glatza, M.; Starost, L.; Velychko, S.; Hallmann, A.L.; Cui, Q.L.; Schambach, A.; Kim, K.P.; Bachelin, C.; et al. Rapid and efficient generation of oligodendrocytes from human induced pluripotent stem cells using transcription factors. Proc. Natl. Acad. Sci. USA 2017, 114, E2243–E2252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-León, J.A.; Kumar, M.; Boon, R.; Chau, D.; One, J.; Wolfs, E.; Eggermont, K.; Berckmans, P.; Gunhanlar, N.; de Vrij, F.; et al. SOX10 Single Transcription Factor-Based Fast and Efficient Generation of Oligodendrocytes from Human Pluripotent Stem Cells. Stem Cell Rep. 2018, 10, 655–672. [Google Scholar]
- Dittmer, M.; Young, A.; O’Hagan, T.; Eleftheriadis, G.; Bankhead, P.; Dombrowski, Y.; Medina, R.J.; Fitzgerald, D.C. Characterization of a murine mixed neuron-glia model and cellular responses to regulatory T cell-derived factors. Mol Brain 2018, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Harrer, M.D.; von Büdingen, H.C.; Stoppini, L.; Alliod, C.; Pouly, S.; Fischer, K.; Goebels, N. Live imaging of remyelination after antibody-mediated demyelination in an ex-vivo model for immune mediated CNS damage. Exp. Neurol. 2009, 216, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Marton, R.M.; Miura, Y.; Sloan, S.A.; Li, Q.; Revah, O.; Levy, R.J.; Huguenard, J.R.; Pașca, S.P. Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nat. Neurosci. 2019, 22, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Yasuda, A.; Renault-Mihara, F.; Suyama, S.; Katoh, H.; Inoue, T.; Inoue, Y.U.; Nagoshi, N.; Sato, M.; Nakamura, M.; et al. Sox10-Venus mice: A new tool for real-time labeling of neural crest lineage cells and oligodendrocytes. Mol. Brain 2010, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, X.; Chittajallu, R.; Belachew, S.; Anderson, S.; McBain, C.J.; Gallo, V. Expression of the green fluorescent protein in the oligodendrocyte lineage: A transgenic mouse for developmental and physiological studies. J. Neurosci. Res. 2002, 70, 529–545. [Google Scholar] [CrossRef] [PubMed]
- Mallon, B.S.; Shick, H.E.; Kidd, G.J.; Macklin, W.B. Proteolipid Promoter Activity Distinguishes Two Populations of NG2-Positive Cells throughout Neonatal Cortical Development. J. Neurosci. 2002, 22, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.G.; Czopka, T.; Ffrench-Constant, C.; Lyons, D.A. Individual axons regulate the myelinating potential of single oligodendrocytes in vivo. Development 2011, 138, 4443–4450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, J.; Park, H.-C.; Topczewska, J.M.; Mawdsley, D.J.; Appel, B. Neural cell fate analysis in zebrafish using olig2 BAC transgenics. Methods Cell Sci. 2003, 25, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nawaz, S.; Schweitzer, J.; Jahn, O.; Werner, H.B. Molecular evolution of myelin basic protein, an abundant structural myelin component. Glia 2013, 61, 1364–1377. [Google Scholar] [CrossRef] [PubMed]
- Early, J.J.; Cole, K.L.; Williamson, J.M.; Swire, M.; Kamadurai, H.; Muskavitch, M.; Lyons, D.A. An automated high-resolution in vivo screen in zebrafish to identify chemical regulators of myelination. Elife 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M. Animal models of multiple sclerosis: The good, the bad and the bottom line. Nat. Neurosci. 2012, 15, 1074–1077. [Google Scholar] [CrossRef] [PubMed]
- Croxford, A.L.; Kurschus, F.C.; Waisman, A. Mouse models for multiple sclerosis: Historical facts and future implications. Biochim. Biophys. Acta Mol. Basis Dis. 2011, 1812, 177–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blakemore, W.F. Pattern of remyelination in the CNS. Nature 1974, 249, 577–578. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.M. The effect of injections of lysophosphatidyl choline into white matter of the adult mouse spinal cord. J. Cell Sci. 1972, 10, 535–546. [Google Scholar] [PubMed]
- Kotter, M.R.; Setzu, A.; Sim, F.J.; Van Rooijen, N.; Franklin, R.J.M. Macrophage depletion impairs oligodendrocyte remyelination following lysolecithin-induced demyelination. Glia 2001, 35, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Kipp, M.; Clarner, T.; Dang, J.; Copray, S.; Beyer, C. The cuprizone animal model: New insights into an old story. Acta Neuropathol. 2009, 118, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, G.K.; Morell, P. The Neurotoxicant, Cuprizone, as a Model to Study Demyelination and Remyelination in the Central Nervous System. Brain Pathol. 2006, 11, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Sachs, H.H.; Bercury, K.K.; Popescu, D.C.; Narayanan, S.P.; Macklin, W.B. A new model of Cuprizone-Mediated demyelination/remyelination. ASN Neuro. 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, R.H.; Fruttiger, M.; Richardson, W.D.; Franklin, R.J.M. Platelet-derived growth factor regulates oligodendrocyte progenitor numbers in adult CNS and their response following CNS demyelination. Mol. Cell. Neurosci. 2004, 25, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Fancy, S.P.J.; Baranzini, S.E.; Zhao, C.; Yuk, D.I.; Irvine, K.A.; Kaing, S.; Sanai, N.; Franklin, R.J.M.; Rowitch, D.H. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 2009, 23, 1571–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rüther, B.J.; Scheld, M.; Dreymueller, D.; Clarner, T.; Kress, E.; Brandenburg, L.O.; Swartenbroekx, T.; Hoornaert, C.; Ponsaerts, P.; Fallier-Becker, P.; et al. Combination of cuprizone and experimental autoimmune encephalomyelitis to study inflammatory brain lesion formation and progression. Glia 2017, 65, 1900–1913. [Google Scholar] [CrossRef] [PubMed]
- Sampaio-Baptista, C.; Johansen-Berg, H. White Matter Plasticity in the Adult Brain. Neuron 2017, 96, 1239–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKenzie, I.A.; Ohayon, D.; Li, H.; Paes de Faria, J.; Emery, B.; Tohyama, K.; Richardson, W.D. Motor skill learning requires active central myelination. Science 2014, 346, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Butts, B.D.; Houde, C.; Mehmet, H. Maturation-dependent sensitivity of oligodendrocyte lineage cells to apoptosis: Implications for normal development and disease. Cell Death Differ. 2008, 15, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Juurlink, B.H.J.; Thorburne, S.K.; Hertz, L. Peroxide-scavenging deficit underlies oligodendrocyte susceptibility to oxidative stress. Glia 1998, 22, 371–378. [Google Scholar] [CrossRef]
- McTigue, D.M.; Tripathi, R.B. The life, death, and replacement of oligodendrocytes in the adult CNS. J. Neurochem. 2008, 107, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Matute, C.; Torre, I.; Pérez-Cerdá, F.; Pérez-Samartín, A.; Alberdi, E.; Etxebarria, E.; Arranz, A.M.; Ravid, R.; Rodríguez-Antigüedad, A.; Sánchez-Gómez, M.V.; et al. P2X 7 receptor blockade prevents ATP excitotoxicity in oligodendrocytes and ameliorates experimental autoimmune encephalomyelitis. J. Neurosci. 2007, 27, 9525–9533. [Google Scholar] [CrossRef] [PubMed]
- Matute, C.; Sánchez-Gómez, M.V.; Martínez-Millán, L.; Miledi, R. Glutamate receptor-mediated toxicity in optic nerve oligodendrocytes. Proc. Natl. Acad. Sci. USA 1997, 94, 8830–8835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traka, M.; Podojil, J.R.; McCarthy, D.P.; Miller, S.D.; Popko, B. Oligodendrocyte death results in immune-mediated CNS demyelination. Nat. Neurosci. 2016, 19, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Fancy, S.P.J.; Harrington, E.P.; Yuen, T.J.; Silbereis, J.C.; Zhao, C.; Baranzini, S.E.; Bruce, C.C.; Otero, J.J.; Huang, E.J.; Nusse, R.; et al. Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat. Neurosci. 2011, 14, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Torii, T.; Miyamoto, Y.; Yamauchi, J.; Tanoue, A. Pelizaeus-Merzbacher disease: Cellular pathogenesis and pharmacologic therapy. Pediatr. Int. 2014, 56, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Krämer, E.M.; Macchi, P.; Rathke-Hartlieb, S.; Trotter, J.; Nave, K.A.; Schulz, J.B. Overexpression of the myelin proteolipid protein leads to accumulation of cholesterol and proteolipid protein in endosomes/lysosomes: Implications for Pelizaeus-Merzbacher disease. J. Cell Biol. 2002, 157, 327–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAleese, K.E.; Walker, L.; Graham, S.; Moya, E.L.J.; Johnson, M.; Erskine, D.; Colloby, S.J.; Dey, M.; Martin-Ruiz, C.; Taylor, J.P.; et al. Parietal white matter lesions in Alzheimer’s disease are associated with cortical neurodegenerative pathology, but not with small vessel disease. Acta Neuropathol. 2017, 134, 459–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behrendt, G.; Baer, K.; Buffo, A.; Curtis, M.A.; Faull, R.L.; Rees, M.I.; Götz, M.; Dimou, L. Dynamic changes in myelin aberrations and oligodendrocyte generation in chronic amyloidosis in mice and men. Glia 2013, 61, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.K.; Mastrangelo, M.A.; Ryan, D.A.; Sudol, K.L.; Narrow, W.C.; Bowers, W.J. Early oligodendrocyte/myelin pathology in Alzheimer’s disease mice constitutes a novel therapeutic target. Am. J. Pathol. 2010, 177, 1422–1435. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, S.; Ahmed, S.H.; Chen, H.; Ku, G.; Goldberg, M.P.; Hsu, C.Y. Amyloid-beta peptides are cytotoxic to oligodendrocytes. J. Neurosci. 2001, 21, 1–5. [Google Scholar] [CrossRef]
- Fischer, F.U.; Wolf, D.; Scheurich, A.; Fellgiebel, A. Altered whole-brain white matter networks in preclinical Alzheimer’s disease. NeuroImage Clin. 2015, 8, 660–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braak, H.; Braak, E. Development of Alzheimer-related neurofibrillary changes in the neocortex inversely recapitulates cortical myelogenesis. Acta Neuropathol. 1996, 92, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Reisberg, B.; Franssen, E.H.; Souren, L.E.M.; Auer, S.R.; Akram, I.; Kenowsky, S. Evidence and mechanisms of retrogenesis in Alzheimer’s and other dementias: Management and treatment import. Am. J. Alzheimers. Dis. Other Demen. 2002, 17, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Fields, R.D. ScienceDirect-Trends in Neurosciences: White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2009, 31, 361–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgar, N.; Sibille, E. A putative functional role for oligodendrocytes in mood regulation. Transl. Psychiatry 2012, 2, e109. [Google Scholar] [CrossRef] [PubMed]
- Rajkowska, G. Cell pathology in bipolar disorder. Bipolar Disord. 2002, 4, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Vostrikov, V.M.; Uranova, N.A.; Orlovskaya, D.D. Deficit of perineuronal oligodendrocytes in the prefrontal cortex in schizophrenia and mood disorders. Schizophr. Res. 2007, 94, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Tkachev, D.; Mimmack, M.L.; Ryan, M.M.; Wayland, M.; Freeman, T.; Jones, P.B.; Starkey, M.; Webster, M.J.; Yolken, R.H.; Bahn, S. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet 2003, 362, 798–805. [Google Scholar] [CrossRef]
- Herring, N.R.; Konradi, C. Myelin, copper, and the cuprizone model of schizophrenia. Front. Biosci. Sch. 2011, 3 S, 23–40. [Google Scholar]
- Cathomas, F.; Azzinnari, D.; Bergamini, G.; Sigrist, H.; Buerge, M.; Hoop, V.; Wicki, B.; Goetze, L.; Soares, S.; Kukelova, D.; et al. Oligodendrocyte gene expression is reduced by and influences effects of chronic social stress in mice. Genes, Brain Behav. 2019, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S.; Taniguchi, M.; Koyama, Y.; Shimizu, S.; Tanaka, T.; Yasuno, F.; Yamamoto, A.; Iida, H.; Kudo, T.; Katayama, T.; et al. Association between chronic stress-induced structural abnormalities in Ranvier nodes and reduced oligodendrocyte activity in major depression. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, S. Demyelinating diseases. J. Clin. Pathol. 2006, 59, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Bauer, J.; Scheikl, T.; Zappulla, J.; Audebert, M.; Desbois, S.; Waisman, A.; Lassmann, H.; Liblau, R.S.; Mars, L.T. Cutting Edge: Multiple Sclerosis-Like Lesions Induced by Effector CD8 T Cells Recognizing a Sequestered Antigen on Oligodendrocytes. J. Immunol. 2008, 181, 1617–1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, I.D.; Radcliff, A.B. Inherited and acquired disorders of myelin: The underlying myelin pathology. Exp. Neurol. 2016, 283, 452–475. [Google Scholar] [CrossRef] [PubMed]
- Hesp, Z.C.; Goldstein, E.A.; Miranda, C.J.; Kaspar, B.K.; McTigue, D.M.; Kaspar, B.K.; McTigue, D.M. Chronic Oligodendrogenesis and Remyelination after Spinal Cord Injury in Mice and Rats. J. Neurosci. 2015, 35, 1274–1290. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.M.; Reynolds, R. Activation and Proliferation of Endogenous Oligodendrocyte Precursor Cells during Ethidium Bromide-Induced Demyelination. Exp. Neurol. 1999, 160, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Fancy, S.P.J.; Zhao, C.; Franklin, R.J.M. Increased expression of Nkx2.2 and Olig2 identifies reactive oligodendrocyte progenitor cells responding to demyelination in the adult CNS. Mol. Cell. Neurosci. 2004, 27, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Lo, E.H. An oligovascular niche: Cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J. Neurosci. 2009, 29, 4351–4355. [Google Scholar] [CrossRef] [PubMed]
- Hammond, T.R.; Gadea, A.; Dupree, J.; Kerninon, C.; Nait-Oumesmar, B.; Aguirre, A.; Gallo, V. Astrocyte-Derived Endothelin-1 Inhibits Remyelination through Notch Activation. Neuron 2014, 81, 588–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casaccia-Bonnefil, P.; Tikoo, R.; Kiyokawa, H.; Friedrich, V.; Chao, M.V.; Koff, A. Oligodendrocyte precursor differentiation is perturbed in the absence of the cyclin-dependent kinase inhibitor p27Kip1. Genes Dev. 1997, 11, 2335–2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magri, L.; Gacias, M.; Wu, M.; Swiss, V.A.; Janssen, W.G.; Casaccia, P. c-Myc-dependent transcriptional regulation of cell cycle and nucleosomal histones during oligodendrocyte differentiation. Neuroscience 2014, 276, 72–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magri, L.; Swiss, V.A.; Jablonska, B.; Lei, L.; Pedre, X.; Walsh, M.; Zhang, W.; Gallo, V.; Canoll, P.; Casaccia, P. E2F1 coregulates cell cycle genes and chromatin components during the transition of oligodendrocyte progenitors from proliferation to differentiation. J. Neurosci. 2014, 34, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Habes, M.; Erus, G.; Toledo, J.B.; Zhang, T.; Bryan, N.; Launer, L.J.; Rosseel, Y.; Janowitz, D.; Doshi, J.; Van Der Auwera, S.; et al. White matter hyperintensities and imaging patterns of brain ageing in the general population. Brain 2016, 139, 1164–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruckh, J.M.; Zhao, J.W.; Shadrach, J.L.; Van Wijngaarden, P.; Rao, T.N.; Wagers, A.J.; Franklin, R.J.M. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell 2012, 10, 96–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sim, F.J.; Zhao, C.; Penderis, J.; Franklin, R.J.M. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J. Neurosci. 2002, 22, 2451–2459. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Hill, R.A.; Dietrich, D.; Komitova, M.; Suzuki, R.; Nishiyama, A. Age-dependent fate and lineage restriction of single NG2 cells. Development 2011, 138, 745–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saher, G.; Brügger, B.; Lappe-Siefke, C.; Möbius, W.; Tozawa, R.I.; Wehr, M.C.; Wieland, F.; Ishibashi, S.; Nave, K.A. High cholesterol level is essential for myelin membrane growth. Nat. Neurosci. 2005, 8, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Thelen, K.M.; Falkai, P.; Bayer, T.A.; Lütjohann, D. Cholesterol synthesis rate in human hippocampus declines with aging. Neurosci. Lett. 2006, 403, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Thorburne, S.K.; Juurlink, B.H.J. Low Glutathione and High Iron Govern the Susceptibility of Oligodendroglial Precursors to Oxidative Stress. J. Neurochem. 1996, 67, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.M.; Choi, J. Age-associated decline in γ-glutamylcysteine synthetase gene expression in rats. Free Radic. Biol. Med. 2000, 28, 566–574. [Google Scholar] [CrossRef]
- Back, S.A.; Luo, N.L.; Borenstein, N.S.; Levine, J.M.; Volpe, J.J.; Kinney, H.C. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J. Neurosci. 2001, 21, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Shields, S.A.; Gilson, J.M.; Blakemore, W.F.; Franklin, R.J.M. Remyelination occurs as extensively but more slowly in old rats compared to young rats following gliotoxin-induced CNS demyelination. Glia 1999, 28, 77–83. [Google Scholar] [CrossRef]
- Duncan, I.D.; Brower, A.; Kondo, Y.; Curlee, J.F.; Schultz, R.D. Extensive remyelination of the CNS leads to functional recovery. Proc. Natl. Acad. Sci. USA 2009, 106, 6832–6836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruchot, J.; Weyers, V.; Göttle, P.; Förster, M.; Hartung, H.-P.; Küry, P.; Kremer, D. The Molecular Basis for Remyelination Failure in Multiple Sclerosis. Cells 2019, 8, 825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, M.S.; Djelloul, M.; Steiner, E.; Bernard, S.; Salehpour, M.; Possnert, G.; Brundin, L.; Frisén, J. Oligodendrocyte generation dynamics in multiple sclerosis. Nature 2019, 24, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Marques, S.; Zeisel, A.; Codeluppi, S.; van Bruggen, D.; Falcão, A.M.; Xiao, L.; Li, H.; Häring, M.; Hochgerner, H.; Romanov, R.A.; et al. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 2016, 352, 1326–1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viganò, F.; Möbius, W.; Götz, M.; Dimou, L. Transplantation reveals regional differences in oligodendrocyte differentiation in the adult brain. Nat. Neurosci. 2013, 16, 1370–1372. [Google Scholar] [CrossRef] [PubMed]
- Crawford, A.H.; Tripathi, R.B.; Foerster, S.; McKenzie, I.; Kougioumtzidou, E.; Grist, M.; Richardson, W.D.; Franklin, R.J.M. Pre-existing mature oligodendrocytes do not contribute to remyelination following toxin-induced spinal cord demyelination. Am. J. Pathol. 2016, 186, 511–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Targett, M.P.; Sussman, J.; Scolding, N.; O’Leary, M.T.; Compston, D.A.; Blakemore, W.F. Failure to achieve remyelination of demyelinated rat axons following transplantation of glial cells obtained from the adult human brain. Neuropathol. Appl. Neurobiol. 1996, 22, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Duncan, I.D.; Radcliff, A.B.; Heidari, M.; Kidd, G.; August, B.K.; Wierenga, L.A. The adult oligodendrocyte can participate in remyelination. Proc. Natl. Acad. Sci. USA 2018, 115, E11807–E11816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soundarapandian, M.M.; Selvaraj, V.; Lo, U.G.; Golub, M.S.; Feldman, D.H.; Pleasure, D.E.; Deng, W. Zfp488 promotes oligodendrocyte differentiation of neural progenitor cells in adult mice after demyelination. Sci. Rep. 2011, 1, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, G.J.; Plemel, J.R.; Assinck, P.; Manesh, S.B.; Muir, F.G.W.; Hirata, R.; Berson, M.; Liu, J.; Wegner, M.; Emery, B.; et al. Myelin regulatory factor drives remyelination in multiple sclerosis. Acta Neuropathol. 2017, 134, 403–422. [Google Scholar] [CrossRef] [PubMed]
- Dugas, J.C.; Ibrahim, A.; Barres, B.A. The T3-induced gene KLF9 regulates oligodendrocyte differentiation and myelin regeneration. Mol. Cell. Neurosci. 2012, 50, 45–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steelman, A.J.; Zhou, Y.; Koito, H.; Kim, S.J.; Payne, H.R.; Lu, Q.R.; Li, J. Activation of oligodendroglial Stat3 is required for efficient remyelination. Neurobiol. Dis. 2016, 91, 336–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhlmann, T.; Miron, V.; Cuo, Q.; Wegner, C.; Antel, J.; Bru« Ck, W.; Kuhlmann, T. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain 2008, 131, 1749–1758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, A.; Tourtellotte, W.W.; Rudick, R.; Trapp, B.D. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N. Engl. J. Med. 2002, 346, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.L.; Toews, A.; Hostettler, J.D.; Morell, P.; Suzuki, K.; Goldman, J.E.; Matsushima, G.K. Oligodendrocytes and Progenitors Become Progressively Depleted within Chronically Demyelinated Lesions. Am. J. Pathol. 2004, 164, 1673–1682. [Google Scholar] [CrossRef] [Green Version]
- Boyd, A.; Zhang, H.; Williams, A. Insufficient OPC migration into demyelinated lesions is a cause of poor remyelination in MS and mouse models. Acta Neuropathol. 2013, 125, 841–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bieber, A.J.; Kerr, S.; Rodriguez, M. Efficient central nervous system remyelination requires T cells. Ann. Neurol. 2003, 53, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Miron, V.E.; Boyd, A.; Zhao, J.-W.; Yuen, T.J.; Ruckh, J.M.; Shadrach, J.L.; van Wijngaarden, P.; Wagers, A.J.; Williams, A.; Franklin, R.J.M.; et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci. 2013, 16, 1211–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dombrowski, Y.; O’Hagan, T.; Dittmer, M.; Penalva, R.; Mayoral, S.R.; Bankhead, P.; Fleville, S.; Eleftheriadis, G.; Zhao, C.; Naughton, M.; et al. Regulatory T cells promote myelin regeneration in the central nervous system. Nat. Neurosci. 2017, 20, 674–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| In vitro Animal Models | In vitro Human Models | In Vivo Animal Models |
|---|---|---|
| OPC/OL cultures [128,129,130] | OPC derived from biopsies [138] or post mortem CNS [139] | Reporter mice (e.g., Sox10-Venus mice, CNP-EGFP mice, PLP-EGFP mice) [147,148,149] |
| Immortalized oligodendroglial cells lines (e.g., CG-4, OLN-93, Oli-neu) [133,134,135] | iOL derived from human iPSC [137,141,142,143] | Reporter zebrafish (e.g., Tg(sox10:mRFP), Tg(olig2:EGFP), Tg(mbp:EGFP)) [103,150,151] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuhn, S.; Gritti, L.; Crooks, D.; Dombrowski, Y. Oligodendrocytes in Development, Myelin Generation and Beyond. Cells 2019, 8, 1424. https://doi.org/10.3390/cells8111424
Kuhn S, Gritti L, Crooks D, Dombrowski Y. Oligodendrocytes in Development, Myelin Generation and Beyond. Cells. 2019; 8(11):1424. https://doi.org/10.3390/cells8111424
Chicago/Turabian StyleKuhn, Sarah, Laura Gritti, Daniel Crooks, and Yvonne Dombrowski. 2019. "Oligodendrocytes in Development, Myelin Generation and Beyond" Cells 8, no. 11: 1424. https://doi.org/10.3390/cells8111424