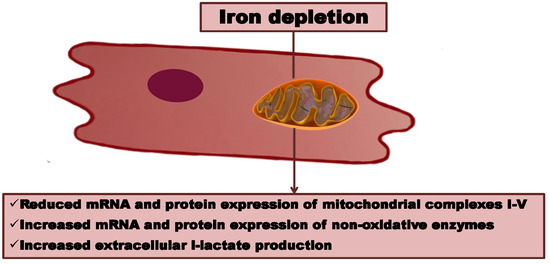
Iron Depletion Affects Genes Encoding Mitochondrial Electron Transport Chain and Genes of NonOxidative Metabolism, Pyruvate Kinase and Lactate Dehydrogenase, in Primary Human Cardiac Myocytes Cultured upon Mechanical Stretch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture Conditions
2.2. Experimental Schedule
2.3. Iron Content in Cells
2.4. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-QPCR)
2.5. Western Blotting
2.6. l-Lactate Concentration Measurement
2.7. Statistical Analysis
3. Results
3.1. Changes in Intracellular Iron due to the Addition of Iron Chelator, Deferroxamine (DFO), or Iron Salt, Ammonium Ferric Citrate (AFC), to Culture Media
3.2. Effects of Differing Iron Availability either in Static Conditions or upon Mechanical Stretch on the Expression of Genes of Mitochondrial Complexes in Primary Human Cardiac Myocytes
3.3. Effects of Differing Iron Availability either in Static Conditions or upon Mechanical Stretch on the Expression of Genes of Non-Oxidative Metabolism, Pyruvate Kinase and Lactate Dehydrogenase, in Primary Human Cardiac Myocytes
3.4. Effects of Differing Iron Availability in Static Conditions or upon Mechanical Stretch on the Concentration of l-Lactate Secreted to Culture Medium by Primary Human Cardiac Myocytes
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HCM | human cardiac myocytes |
| PKM2 | pyruvate kinase |
| LDHA | lactate dehydrogenase A |
| ATP | adenosine triphosphate |
| ID | iron deficiency |
| HF | heart failure |
| DFO | deferroxamine |
| AFC | ammonium ferric citrate |
| RIPA | radioimmunoprecipitation assay |
| RT-qPCR | reverse transcription-quantitative polymerase chain reaction |
| cDNA | complementary deoxyribonucleic acid |
| rRNA | ribosomal ribonuclein acid |
| BCA | bicinchoninic acid |
| OXPHOS | oxidative phosphorylation |
References
- Mettauer, B.; Zoll, J.; Garnier, A.; Ventura-Clapier, R. Heart failure: A model of cardiac and skeletal muscle energetic failure. Pflügers Arch.—Eur. J. Physiol. 2006, 452, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Ingwall, J.S. Energy metabolism in heart failure and remodelling. Cardiovasc. Res. 2008, 81, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ardehali, H.; Sabbah, H.N.; Burke, M.A.; Sarma, S.; Liu, P.P.; Cleland, J.G.F.; Maggioni, A.; Fonarow, G.C.; Abel, E.D.; Campia, U.; et al. Targeting myocardial substrate metabolism in heart failure: Potential for new therapies. Eur. J. Heart Fail. 2012, 14, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Fairbanks, E.; Beutler, V. Chapter 38 Iron deficiency (p.1018-1071). In Williams Hematology, 6th ed.; McGraw-Hill Medical: New York, NY, USA, 2001. [Google Scholar]
- Dunn, L.L.; Rahmanto, Y.S.; Richardson, D.R. Iron uptake and metabolism in the new millennium. Trends Cell Biol. 2007, 17, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.; Granner, D.; Rodwell, V. Harper’s Illustrated Biochemistry, 27th ed.; The McGraw-Hill Companies: New York, NY, USA, 2006. [Google Scholar]
- Jankowska, E.A.; Rozentryt, P.; Witkowska, A.; Nowak, J.; Hartmann, O.; Ponikowska, B.; Borodulin-Nadzieja, L.; Banasiak, W.; Polonski, L.; Filippatos, G.; et al. Iron deficiency: An ominous sign in patients with systolic chronic heart failure. Eur. Heart J. 2010, 31, 1872–1880. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, E.A.; Malyszko, J.; Ardehali, H.; Koc-Zorawska, E.; Banasiak, W.; von Haehling, S.; Macdougall, I.C.; Weiss, G.; McMurray, J.J.V.; Anker, S.D.; et al. Iron status in patients with chronic heart failure. Eur. Heart J. 2013, 34, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Comin Colet, J.; Filippatos, G.; Willenheimer, R.; Dickstein, K.; Drexler, H.; Lüscher, T.F.; Bart, B.; Banasiak, W.; Niegowska, J.; et al. FAIR-HF trial investigators ferric carboxymaltose in patients with heart failure and iron deficiency. N. Engl. J. Med. 2009, 361, 2436–2448. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; van Veldhuisen, D.J.; Comin-Colet, J.; Ertl, G.; Komajda, M.; Mareev, V.; McDonagh, T.; Parkhomenko, A.; Tavazzi, L.; Levesque, V.; et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur. Heart J. 2015, 36, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.J.; Lee, T.-J.; Park, J.-W.; Kwon, T.K. Desferrioxamine, an iron chelator, enhances HIF-1α accumulation via cyclooxygenase-2 signaling pathway. Biochem. Biophys. Res. Commun. 2006, 343, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Parkes, J.G.; Hussain, R.A.; Olivieri, N.F.; Templeton, D.M. Effects of iron loading on uptake, speciation, and chelation of iron in cultured myocardial cells. J. Lab. Clin. Med. 1993, 122, 36–47. [Google Scholar] [PubMed]
- Hoepken, H.H.; Korten, T.; Robinson, S.R.; Dringen, R. Iron accumulation, iron-mediated toxicity and altered levels of ferritin and transferrin receptor in cultured astrocytes during incubation with ferric ammonium citrate. J. Neurochem. 2004, 88, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Chandio, Z.A.; Talpur, F.N.; Khan, H.; Afridi, H.I.; Khaskheli, G.Q. Determination of cadmium and zinc in vegetables with online FAAS after simultaneous pre-concentration with 1,5-diphenylthiocarbazone immobilised on naphthalene. Food Addit. Contam. Part A 2013, 30, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Kobak, K.; Kasztura, M.; Dziegala, M.; Bania, J.; Kapuśniak, V.; Banasiak, W.; Ponikowski, P.; Jankowska, E.A. Iron limitation promotes the atrophy of skeletal myocytes, whereas iron supplementation prevents this process in the hypoxic conditions. Int. J. Mol. Med. 2018, 41. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M. The bicinchoninic acid (BCA) assay for protein quantitation. In Basic Protein and Peptide Protocols; Humana Press: New York, NJ, USA, 1994; pp. 5–8. [Google Scholar]
- Kim, M.-S.; Kim, J.-S.; Cheon, C.-I.; Cho, D.-H.; Park, J.-H.; Kim, K.-I.; Lee, K.-Y.; Song, E.-S. Increased expression of the F1F0 ATP synthase in response to iron in heart mitochondria. BMB Rep. 2008, 41, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Link, G.; Saada, A.; Pinson, A.; Konijn, A.M.; Hershko, C. Mitochondrial respiratory enzymes are a major target of iron toxicity in rat heart cells. J. Lab. Clin. Med. 1998, 131, 466–474. [Google Scholar] [CrossRef]
- Huang, J.; Jones, D.; Luo, B.; Sanderson, M.; Soto, J.; Abel, E.D.; Cooksey, R.C.; McClain, D.A. Iron overload and diabetes risk: A shift from glucose to fatty acid oxidation and increased hepatic glucose production in a mouse model of hereditary hemochromatosis. Diabetes 2011, 60, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Haddad, S.; Wang, Y.; Galy, B.; Korf-Klingebiel, M.; Hirsch, V.; Baru, A.M.; Rostami, F.; Reboll, M.R.; Heineke, J.; Flögel, U.; et al. Iron-regulatory proteins secure iron availability in cardiomyocytes to prevent heart failure. Eur. Heart J. 2016, 38, ehw333. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Barrientos, T.; Mao, L.; Rockman, H.A.; Sauve, A.A.; Andrews, N.C. Lethal cardiomyopathy in mice lacking transferrin receptor in the heart. Cell Rep. 2015, 13, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Lakhal-Littleton, S.; Wolna, M.; Chung, Y.J.; Christian, H.C.; Heather, L.C.; Brescia, M.; Ball, V.; Diaz, R.; Santos, A.; Biggs, D.; et al. An essential cell-autonomous role for hepcidin in cardiac iron homeostasis. Elife 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Melenovsky, V.; Petrak, J.; Mracek, T.; Benes, J.; Borlaug, B.A.; Nuskova, H.; Pluhacek, T.; Spatenka, J.; Kovalcikova, J.; Drahota, Z.; et al. Myocardial iron content and mitochondrial function in human heart failure: A direct tissue analysis. Eur. J. Heart Fail. 2017, 19, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Baudino, T.A.; Carver, W.; Giles, W.; Borg, T.K. Cardiac fibroblasts: Friend or foe? AJP Hear. Circ. Physiol. 2006, 291, H1015–H1026. [Google Scholar] [CrossRef] [PubMed]
- Okonko, D.O.; Grzeslo, A.; Witkowski, T.; Mandal, A.K.J.; Slater, R.M.; Roughton, M.; Foldes, G.; Thum, T.; Majda, J.; Banasiak, W.; et al. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency. J. Am. Coll. Cardiol. 2008, 51, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.P.; Bartlett, F.R.; Penston, H.S.; O’Leary, J.; Pollock, N.; Kaprielian, R.; Chapman, C.M. Intravenous iron alone for the treatment of anemia in patients with chronic heart failure. J. Am. Coll. Cardiol. 2006, 48, 1225–1227. [Google Scholar] [CrossRef] [PubMed]
- Toblli, J.E.; Lombraña, A.; Duarte, P.; Di Gennaro, F. Intravenous iron reduces NT-pro-brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J. Am. Coll. Cardiol. 2007, 50, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Usmanov, R.I.; Zueva, E.B.; Silverberg, D.S.; Shaked, M. Intravenous iron without erythropoietin for the treatment of iron deficiency anemia in patients with moderate to severe congestive heart failure and chronic kidney insufficiency. J. Nephrol. 2008, 21, 236–242. [Google Scholar] [PubMed]
- Gaber, R.; Kotb, N.A.; Ghazy, M.; Nagy, H.M.; Salama, M.; Elhendy, A. Tissue doppler and strain rate imaging detect improvement of myocardial function in iron deficient patients with congestive heart failure after iron replacement therapy. Echocardiography 2012, 29, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.B.; Knutson, M.D.; Paler-Martinez, A.; Lee, S.; Xu, Y.; Viteri, F.E.; Ames, B.N. Iron deficiency and iron excess damage mitochondria and mitochondrial DNA in rats. Proc. Natl. Acad. Sci. USA 2002, 99, 2264–2269. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Antigen | Dilution | Manufacturer | Ref. Number |
|---|---|---|---|
| LDH | 1:1000 | Santa Cruz Biotechnology | sc-33781 |
| PKM2 | 1:750 | Invitrogen | PA5-13980 |
| Total OXPHOS cocktail 1 | 1:500 | Abcam | ab110411 |
| Beta-actin HRP | 1:5000 | Santa Cruz Biotechnology | sc-1616 HRP |
| Rabbit IgG HRP | 1:40,000 | Jackson ImmunoResearch | 111-035-045 |
| Mouse IgG HRP | 1:40,000 | Sigma-Aldrich 2 | A 9917 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziegala, M.; Kobak, K.A.; Kasztura, M.; Bania, J.; Josiak, K.; Banasiak, W.; Ponikowski, P.; Jankowska, E.A. Iron Depletion Affects Genes Encoding Mitochondrial Electron Transport Chain and Genes of NonOxidative Metabolism, Pyruvate Kinase and Lactate Dehydrogenase, in Primary Human Cardiac Myocytes Cultured upon Mechanical Stretch. Cells 2018, 7, 175. https://doi.org/10.3390/cells7100175
Dziegala M, Kobak KA, Kasztura M, Bania J, Josiak K, Banasiak W, Ponikowski P, Jankowska EA. Iron Depletion Affects Genes Encoding Mitochondrial Electron Transport Chain and Genes of NonOxidative Metabolism, Pyruvate Kinase and Lactate Dehydrogenase, in Primary Human Cardiac Myocytes Cultured upon Mechanical Stretch. Cells. 2018; 7(10):175. https://doi.org/10.3390/cells7100175
Chicago/Turabian StyleDziegala, Magdalena, Kamil A. Kobak, Monika Kasztura, Jacek Bania, Krystian Josiak, Waldemar Banasiak, Piotr Ponikowski, and Ewa A. Jankowska. 2018. "Iron Depletion Affects Genes Encoding Mitochondrial Electron Transport Chain and Genes of NonOxidative Metabolism, Pyruvate Kinase and Lactate Dehydrogenase, in Primary Human Cardiac Myocytes Cultured upon Mechanical Stretch" Cells 7, no. 10: 175. https://doi.org/10.3390/cells7100175