Transgene-Free Cynomolgus Monkey iPSCs Generated under Chemically Defined Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Statement Addressing the Methods Used in This Study
2.2. Animals
2.3. Isolation and Cultivation of Macaque Primary Fibroblasts
2.4. Reprogramming NHP and Human Skin Fibroblasts and iPSC Maintenance
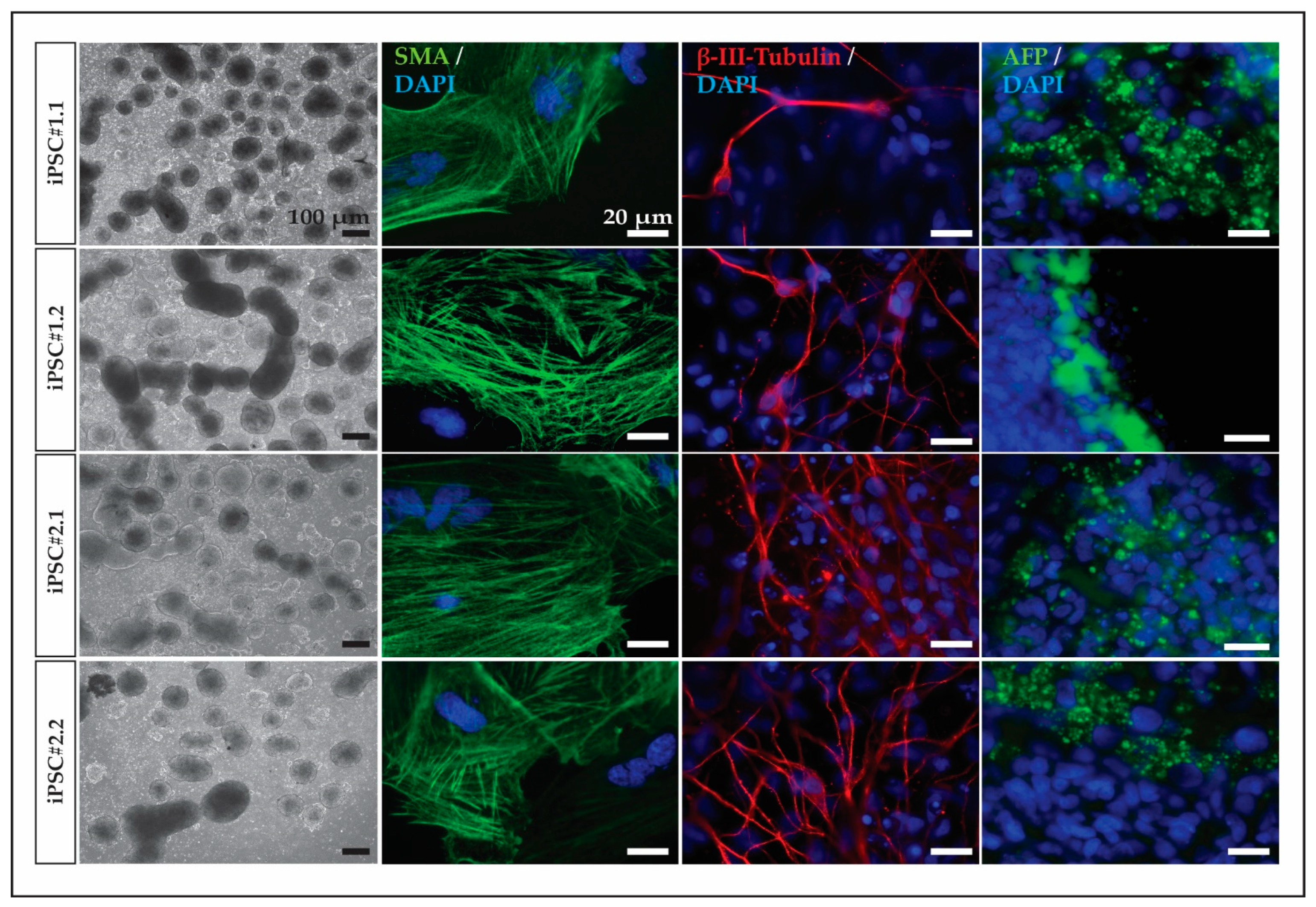
2.5. In Vitro Differentiation
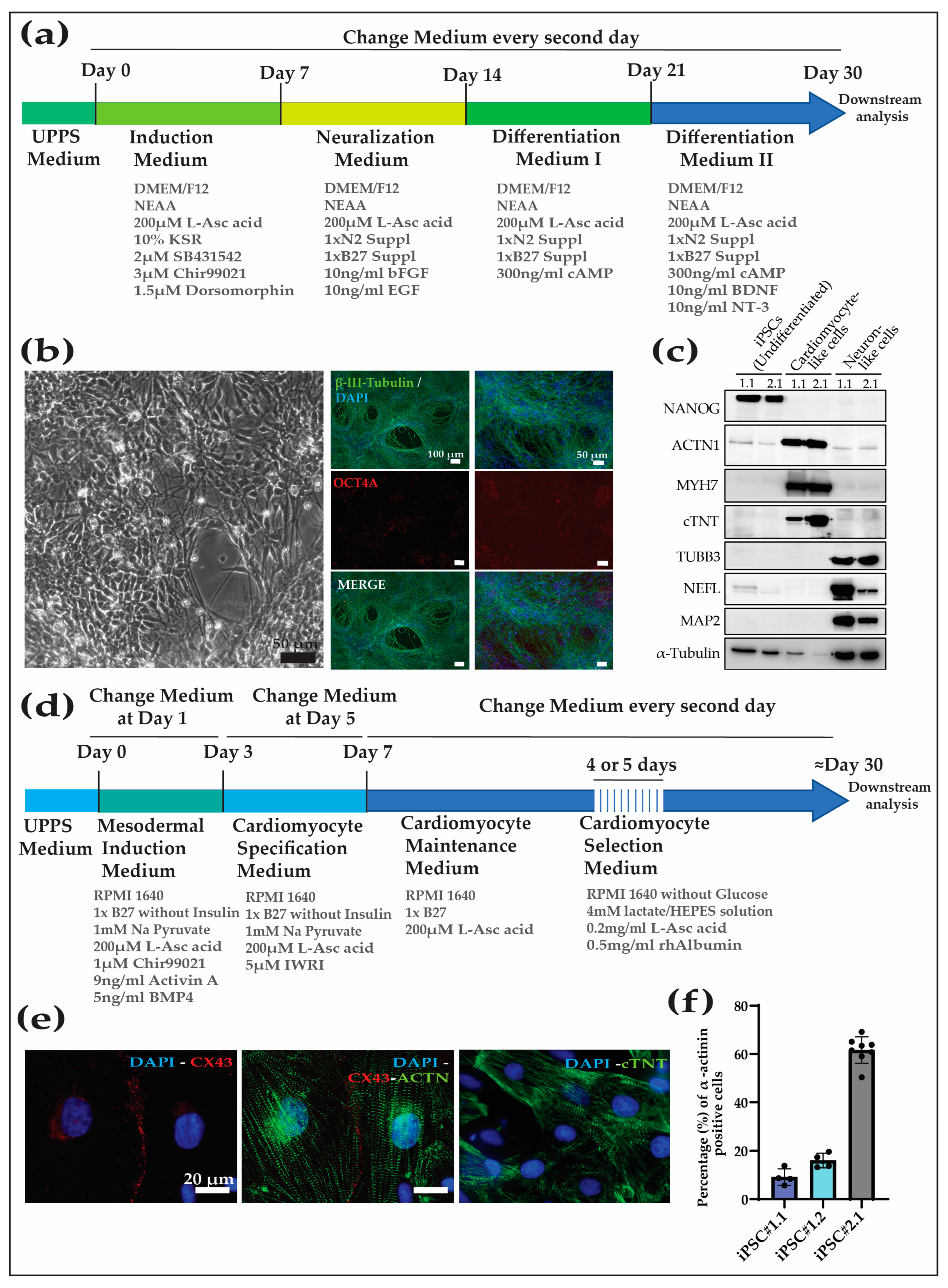
2.6. Cardiac Differentiation
2.7. Neural Differentiation
2.8. Immunostaining
2.9. Flow Cytometry
2.10. Nucleic Acid (DNA) Isolation and Polymerase Chain Reaction
2.11. Protein Extraction and Western Blot Analysis
3. Results
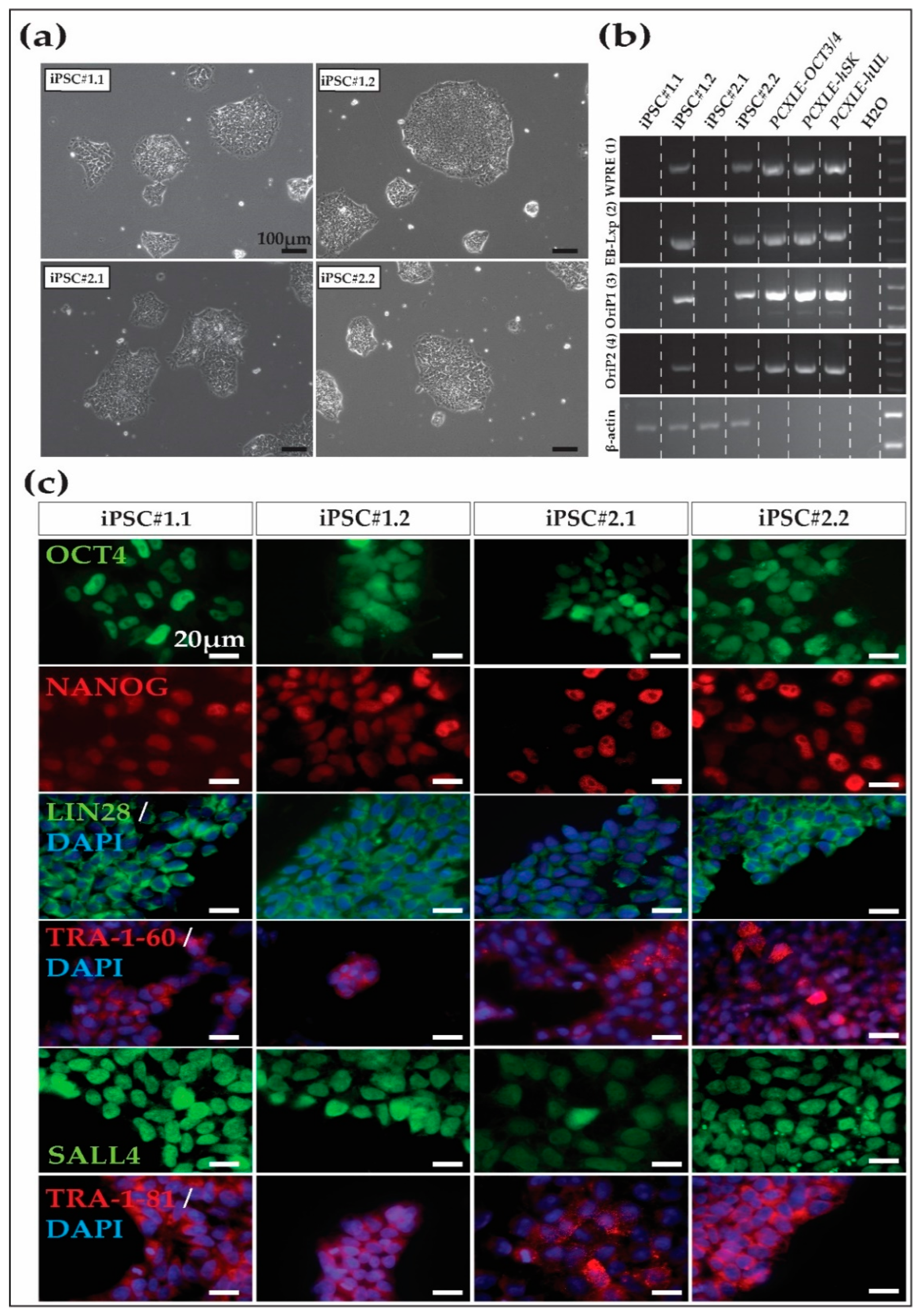
3.1. NHP and Human Fibroblast Reprogramming
3.2. Cynomolgus Macaque iPSC Characterization
3.3. NHP-iPSC-Derived Cardiomyocyte and Neurons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Deinsberger, J.; Reisinger, D.; Weber, B. Global trends in clinical trials involving pluripotent stem cells: A systematic multi-database analysis. NPJ Regen. Med. 2020, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Zhang, X.-J.; Renier, N.; Wu, Z.; Atkin, T.; Sun, Z.; Ozair, M.Z.; Tchieu, J.; Zimmer, B.; Fattahi, F.; et al. Combined small-molecule inhibition accelerates the derivation of functional, early-born, cortical neurons from human pluripotent stem cells. Nat. Biotechnol. 2017, 35, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, D.O.; Johnson, D.K.; Whitney, R.A. Nonhuman Primates in Biomedical Research, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Cox, L.A.; Olivier, M.; Spradling-Reeves, K.; Karere, G.M.; Comuzzie, A.G.; Vandeberg, J.L. Nonhuman Primates and Translational Research—Cardiovascular Disease. ILAR J. 2017, 58, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Polo, I.; Behr, R. Non-human primate pluripotent stem cells for the preclinical testing of regenerative therapies. Neural Regen. Res. 2022, 17, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Geuder, J.; Wange, L.E.; Janjic, A.; Radmer, J.; Janssen, P.; Bagnoli, J.W.; Müller, S.; Kaul, A.; Ohnuki, M.; Enard, W. A non-invasive method to generate induced pluripotent stem cells from primate urine. Sci. Rep. 2021, 11, 3516. [Google Scholar] [CrossRef] [PubMed]
- Stauske, M.; Polo, I.R.; Haas, W.; Knorr, D.Y.; Borchert, T.; Streckfuss-Bömeke, K.; Dressel, R.; Bartels, I.; Tiburcy, M.; Zimmermann, W.-H.; et al. Non-Human Primate iPSC Generation, Cultivation, Cardiac Differentiation under Chemically Defined Conditions. Cells 2020, 9, 1349. [Google Scholar] [CrossRef] [PubMed]
- Vermilyea, S.C.; Guthrie, S.; Meyer, M.; Smuga-Otto, K.; Braun, K.; Howden, S.; Thomson, J.A.; Zhang, S.-C.; Emborg, M.E.; Golos, T.G. Induced Pluripotent Stem Cell-Derived Dopaminergic Neurons from Adult Common Marmoset Fibroblasts. Stem Cells Dev. 2017, 26, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Anwised, P.; Moorawong, R.; Samruan, W.; Somredngan, S.; Srisutush, J.; Laowtammathron, C.; Aksoy, I.; Parnpai, R.; Savatier, P. An expedition in the jungle of pluripotent stem cells of non-human primates. Stem Cell Rep. 2023, 18, 2016–2037. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Yang, Z.; Rodrigues, A.D. Cynomolgus Monkey as an Emerging Animal Model to Study Drug Transporters: In Vitro, In Vivo, In Vitro-to-In Vivo Translation. Drug Metab. Dispos. 2022, 50, 299–319. [Google Scholar] [CrossRef] [PubMed]
- Cappon, G.D.; Bailey, G.P.; Buschmann, J.; Feuston, M.H.; Fisher, J.E.; Hew, K.W.; Hoberman, A.M.; Ooshima, Y.; Stump, D.G.; Hurtt, M.E. Juvenile animal toxicity study designs to support pediatric drug development. Birth Defects Res. B Dev. Reprod. Toxicol. 2009, 86, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Bailey, G.P.; Wise, L.D.; Buschmann, J.; Hurtt, M.; Fisher, J.E. Pre- and postnatal developmental toxicity study design for pharmaceuticals. Birth Defects Res. B Dev. Reprod. Toxicol. 2009, 86, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, K.; Uno, Y.; Utoh, M.; Yamazaki, H. Importance of cynomolgus monkeys in development of monoclonal antibody drugs. Drug Metab. Pharmacokinet. 2019, 34, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Polo, I.; Stauske, M.; Behr, R. Generation and cultivation of transgene-free macaque and baboon iPSCs under chemically defined conditions. In iPS CELLS; Turksen, K., Nagy, A., Eds.; Springer: New York, NY, USA, 2021; pp. 1–5. [Google Scholar]
- Rodriguez-Polo, I.; Stauske, M.; Becker, A.; Bartels, I.; Dressel, R.; Behr, R. Baboon induced pluripotent stem cell generation by piggyBac transposition of reprogramming factors. Primate Biol. 2019, 6, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Okita, K.; Matsumura, Y.; Sato, Y.; Okada, A.; Morizane, A.; Okamoto, S.; Hong, H.; Nakagawa, M.; Tanabe, K.; Tezuka, K.-I.; et al. A more efficient method to generate integration-free human iPS cells. Nat. Methods 2011, 8, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Tiburcy, M.; Hudson, J.E.; Balfanz, P.; Schlick, S.; Meyer, T.; Liao, M.-L.C.; Levent, E.; Raad, F.; Zeidler, S.; Wingender, E.; et al. Defined engineered human myocardium with advanced maturation for applications in heart failure modeling and repair. Circulation 2017, 135, 1832–1847. [Google Scholar] [CrossRef] [PubMed]
- Knorr, D.Y.; Polo, I.R.; Pies, H.S.; Schwedhelm-Domeyer, N.; Pauls, S.; Behr, R.; Heinrich, R. The cytokine receptor CRLF3 is a human neuroprotective EV-3 (Epo) receptor. Front. Mol. Neurosci. 2023, 16, 1154509. [Google Scholar] [CrossRef] [PubMed]
- Chellman, G.J.; Bussiere, J.L.; Makori, N.; Martin, P.L.; Ooshima, Y.; Weinbauer, G.F. Developmental and reproductive toxicology studies in nonhuman primates. Birth Defects Res. B Dev. Reprod. Toxicol. 2009, 86, 446–462. [Google Scholar] [CrossRef] [PubMed]
| Name | Company | Catalog # | Dilution | |
|---|---|---|---|---|
| Primary Antibodies | α-feto protein | Dako (Glostrup, Denmark) | A0008 | 1:100 |
| α-actinin | Sigma-Aldrich | A7811 | 1:1000 | |
| β-tubulin III | Sigma-Aldrich | T8660 | 1:1000 | |
| Connexin 43 (Cx43) | Abcam (Cambridge, UK) | ab11370 | 1:1000 | |
| α-actinin * | Miltenyi Biotec | 130-128-591 | 1:50 | |
| Cardiac troponin T (cTNT) | Miltenyi Biotec | 130-106-687 | 1:10 | |
| LIN28 | R&D systems (Minneapolis, MN, USA) | AF3757 | 1:300 | |
| NANOG | Cell Signalling (Danvers, MA, USA) | 4903 | 1:400 | |
| OCT4A | Cell Signalling | C52G3 | 1:1600 | |
| TRA-1-81 | Abcam | ab16289 | 1:200 | |
| SALL4 | Abcam | ab57577 | 1:200 | |
| Smooth muscle actin | Sigma-Aldrich | A2547 | 1:1000 | |
| TRA-1-60 | Abcam | ab16288 | 1:200 | |
| α-actinin # | Sigma-Aldrich | A7811 | 1:2000 | |
| α-tubulin # | Cell Signalling | mAb#3873 | 1:5000 | |
| MYH7 # | Sigma-Aldrich | HPA001239 | 1:1000 | |
| Nanog # | Cell Signalling | D73G4 | 1:1000 | |
| NEFL # | Sigma-Aldrich | N4142 | 1:2000 | |
| MAP2 # | Sigma-Aldrich | HPA012828 | 1:2000 | |
| B III tubulin # | Biolegend (San Diego, CA, USA) | Biolegend | 1:2000 | |
| cTNT # | Miltenyi Biotec | 130-106-687 | 1:1000 | |
| Secondary antibodies | Alexa555-goat-α-mouse IgG | Thermo Fisher | A21424 | 1:1000 |
| Alexa488-goat-α-mouse IgG | Thermo Fisher | A11029 | 1:1000 | |
| Alexa488-goat-α-mouse IgG/IgM | Thermo Fisher | A10680 | 1:1000 | |
| Alexa488-donkey-α-goat IgG | Thermo Fisher | A11055 | 1:1000 | |
| Alexa488-donkey-α-rabbit IgG | Thermo Fisher | A21206 | 1:1000 |
| Name | Sequence | Amp | T | C |
|---|---|---|---|---|
| WPRE (1) | for: 5′-GCT ATT GCT TCC CGT ATG GC-3′ | 470 | 54 | 32 |
| rev: 5′-CAA AGG GAG ATC CGA CTC GT-3′ | ||||
| EBNA-LoxP (2) | for: 5′-AAG AGG AGG GGT CCC GAG A-3′ | 555 | 61 | 32 |
| rev: 5′-GCC AAT GCA ACT TGG ACG TT-3′ | ||||
| OriP1 (3) | for: 5′-GGT TCA CTA CCC TCG TGG AAT-3′ | 592 | 57 | 32 |
| rev: 5′-CGG GGC AGT GCA TGT AAT-3′ | ||||
| OriP2 (4) | for: 5′-GGT GAC TGT GTG CAG CTT TG-3′ | 416 | 54 | 32 |
| rev: 5′-GGA GCT GAG TGA CGT GAC AA-3′ | ||||
| β-actin | for: 5′-GAC CTG ACT GAC TAC CTC ATG-3′ | 380 | 61 | 32 |
| rev: 5′-GGT AGT TTC GTG GAT GCC ACA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tereshchenko, Y.; Esiyok, N.; Garea-Rodríguez, E.; Repetto, D.; Behr, R.; Rodríguez-Polo, I. Transgene-Free Cynomolgus Monkey iPSCs Generated under Chemically Defined Conditions. Cells 2024, 13, 558. https://doi.org/10.3390/cells13060558
Tereshchenko Y, Esiyok N, Garea-Rodríguez E, Repetto D, Behr R, Rodríguez-Polo I. Transgene-Free Cynomolgus Monkey iPSCs Generated under Chemically Defined Conditions. Cells. 2024; 13(6):558. https://doi.org/10.3390/cells13060558
Chicago/Turabian StyleTereshchenko, Yuliia, Nesil Esiyok, Enrique Garea-Rodríguez, Daniele Repetto, Rüdiger Behr, and Ignacio Rodríguez-Polo. 2024. "Transgene-Free Cynomolgus Monkey iPSCs Generated under Chemically Defined Conditions" Cells 13, no. 6: 558. https://doi.org/10.3390/cells13060558






