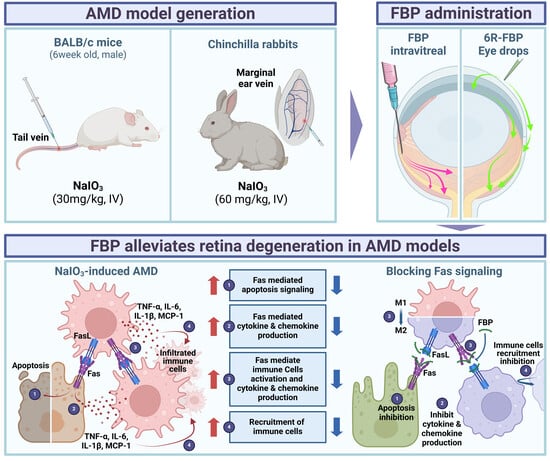
Eye Drop with Fas-Blocking Peptide Attenuates Age-Related Macular Degeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Peptides
2.2. Retinal Pigment Epithelial Cell Culture
2.3. Cell Viability Assay
2.4. In Vitro Real-Time PCR Analysis
2.5. In Vitro Western Blot Analysis
2.6. In Vitro Apoptosis Analysis
2.7. Animal Studies
2.8. In Vivo Delivery of Peptides
2.9. In Vivo Real-Time PCR Analysis
2.10. H&E Staining and TUNEL Assay
2.11. NaIO3-Induced Retinal Degeneration Rabbit Model and FBP Administration
2.12. Fundus Imaging in NaIO3-Induced Retinal Degeneration Rabbit Model
2.13. OCT Imaging in NaIO3-Induced Retinal Degeneration Rabbits
2.14. H&E Staining in NaIO3-Induced Retinal Degeneration Rabbits
2.15. Statistical Analysis
3. Results
3.1. FBP Reduces Inflammation and Apoptosis in NaIO3-Induced Oxidative Stress in ARPE-19 Cells
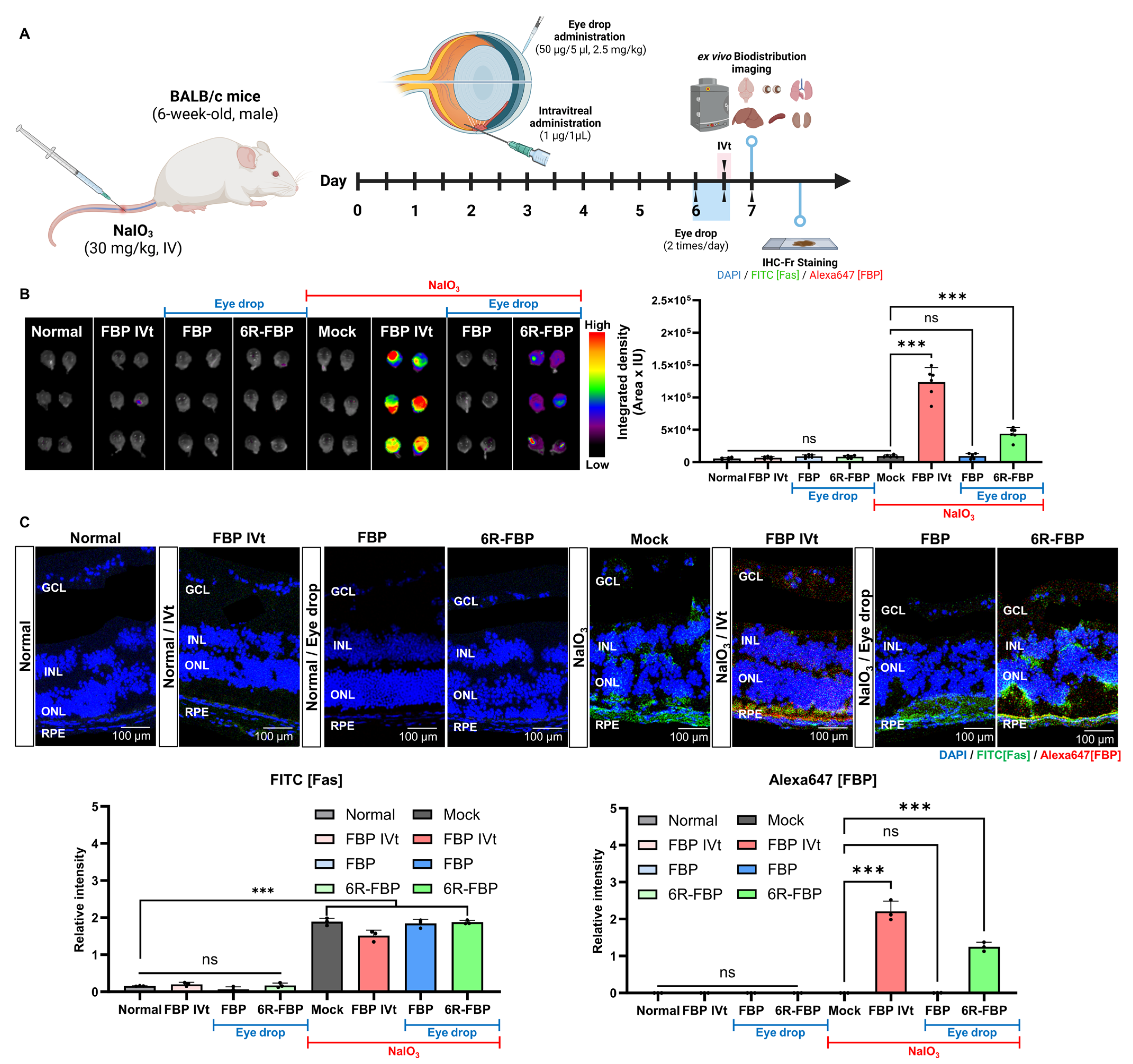
3.2. Evaluation of FBP Delivery via Eye Drops in a Murine Model of NaIO3-Induced Retinal Degeneration
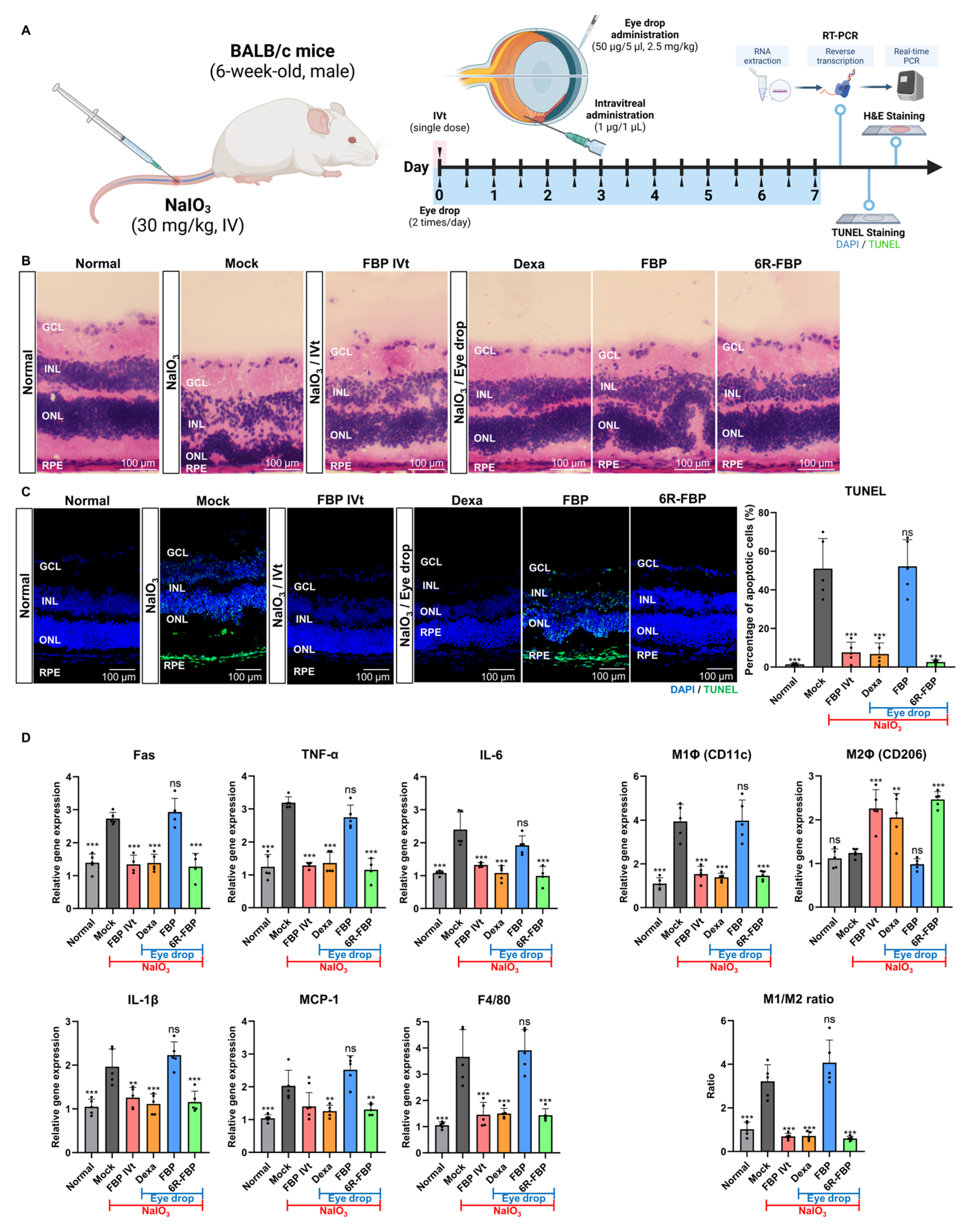
3.3. Therapeutic Efficacy of 6R-FBP Eye Drops in Attenuating Retinal Degeneration in a NaIO3-Induced Retinal Degeneration Mouse Model
3.4. FBP Attenuated Retinal Degeneration in the NaIO3-Induced Retinal Degeneration Rabbit Model
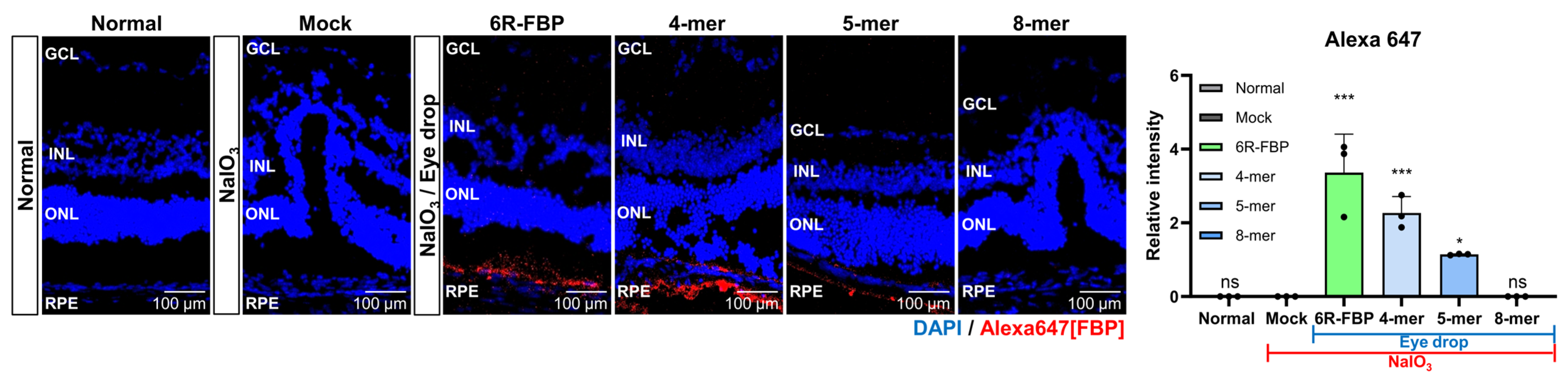
3.5. Impact of Peptide Size on the Efficacy of Eye Drop in the NaIO3-Induced Retinal Degeneration Mouse Model
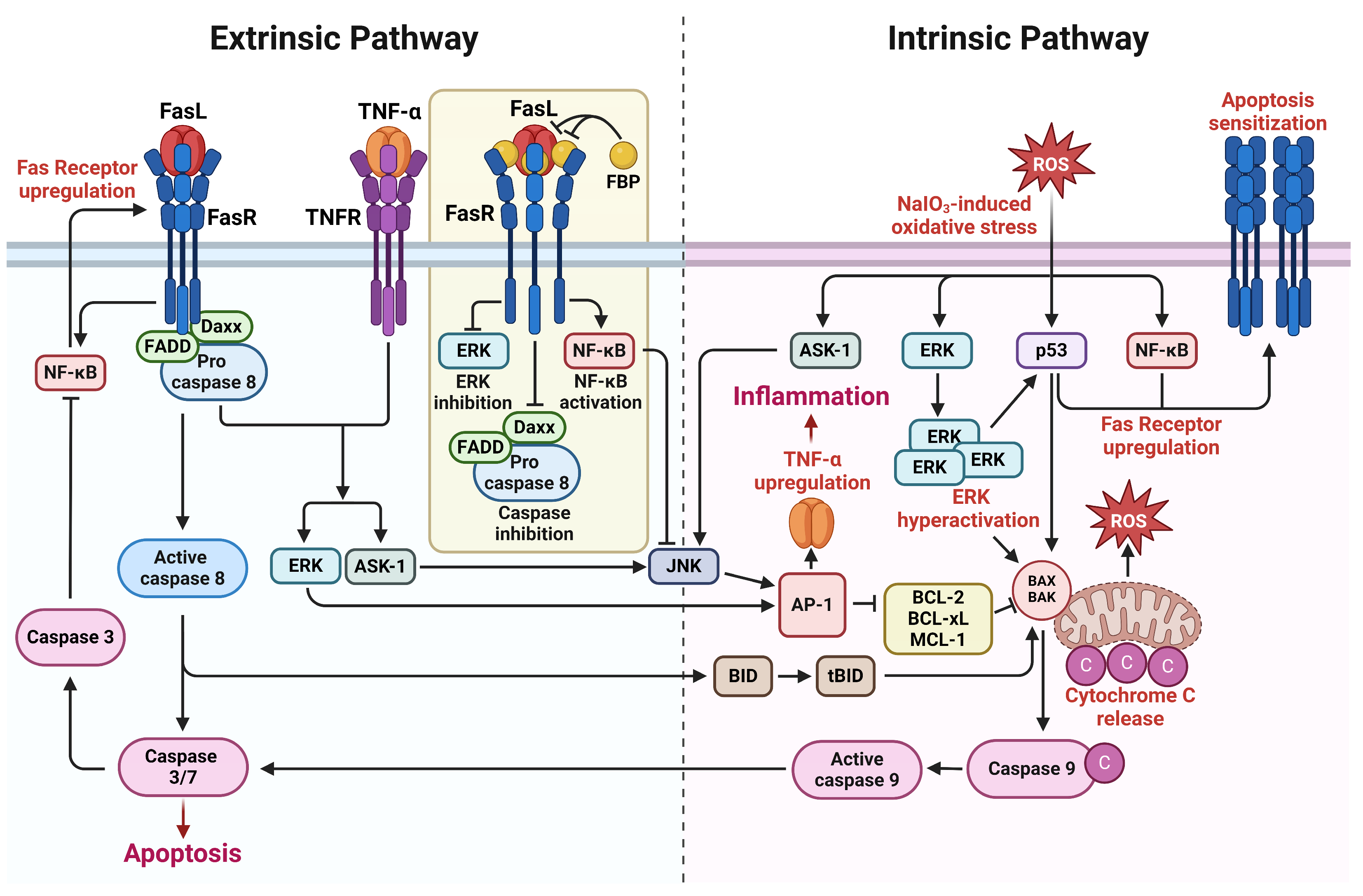
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Pennington, K.L.; DeAngelis, M.M. Epidemiology of age-related macular degeneration (AMD): Associations with cardiovascular disease phenotypes and lipid factors. Eye Vis. 2016, 3, 34. [Google Scholar] [CrossRef]
- Shingavi, M. Quantification of wet Age Related Macular Degeneration in Optical Coherence Tomography Angiography images. INFOCOMP J. Comput. Sci. 2020, 19. [Google Scholar]
- Rubner, R.; Li, K.V.; Canto-Soler, M.V. Progress of clinical therapies for dry age-related macular degeneration. Int. J. Ophthalmol. 2022, 15, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Stahl, A. The diagnosis and treatment of age-related macular degeneration. Dtsch. Ärzteblatt Int. 2020, 117, 513. [Google Scholar] [CrossRef]
- Thomas, C.N.; Sim, D.A.; Lee, W.H.; Alfahad, N.; Dick, A.D.; Denniston, A.K.; Hill, L.J. Emerging therapies and their delivery for treating age-related macular degeneration. Br. J. Pharmacol. 2022, 179, 1908–1937. [Google Scholar] [CrossRef]
- Ferris, F.L.; Fine, S.L.; Hyman, L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch. Ophthalmol. 1984, 102, 1640–1642. [Google Scholar] [CrossRef]
- Morris, B.; Imrie, F.; Armbrecht, A.M.; Dhillon, B. Age-related macular degeneration and recent developments: New hope for old eyes? Postgrad. Med. J. 2007, 83, 301–307. [Google Scholar] [CrossRef]
- Attarde, A.; Riad, T.S.; Zhang, Z.; Ahir, M.; Fu, Y. Characterization of Vascular Morphology of Neovascular Age-Related Macular Degeneration by Indocyanine Green Angiography. J. Vis. Exp. 2023, 198, e65682. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.A.; Lutz, D.; Shen, J.; Yuan, X.; Shen, H.; Cunningham, J.; Rivers, H.M. Topical Drug Delivery to the Posterior Segment of the Eye: Addressing the Challenge of Preclinical to Clinical Translation. Pharm. Res. 2018, 35, 245. [Google Scholar] [CrossRef]
- Wu, K.Y.; Joly-Chevrier, M.; Akbar, D.; Tran, S.D. Overcoming Treatment Challenges in Posterior Segment Diseases with Biodegradable Nano-Based Drug Delivery Systems. Pharmaceutics 2023, 15, 1094. [Google Scholar] [CrossRef]
- Thareja, A.; Hughes, H.; Alvarez-Lorenzo, C.; Hakkarainen, J.J.; Ahmed, Z. Penetration Enhancers for Topical Drug Delivery to the Ocular Posterior Segment-A Systematic Review. Pharmaceutics 2021, 13, 276. [Google Scholar] [CrossRef]
- Evans, J. Antioxidant supplements to prevent or slow down the progression of AMD: A systematic review and meta-analysis. Eye 2008, 22, 751–760. [Google Scholar] [CrossRef]
- Hobbs, S.D.; Pierce, K. Wet age-related macular degeneration (Wet AMD). In StatPearls [Internet]; StatPearls Publishing: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Jurkunas, U.V.; Bitar, M.S.; Funaki, T.; Azizi, B. Evidence of oxidative stress in the pathogenesis of fuchs endothelial corneal dystrophy. Am. J. Pathol. 2010, 177, 2278–2289. [Google Scholar] [CrossRef]
- Toma, C.; De Cilla, S.; Palumbo, A.; Garhwal, D.P.; Grossini, E. Oxidative and Nitrosative Stress in Age-Related Macular Degeneration: A Review of Their Role in Different Stages of Disease. Antioxidants 2021, 10, 653. [Google Scholar] [CrossRef] [PubMed]
- Haddad, W.M.; Coscas, G.; Soubrane, G. Age-related macular degeneration and apoptosis. J. Fr. Ophtalmol. 2003, 26, 307–311. [Google Scholar]
- Dunaief, J.L.; Dentchev, T.; Ying, G.S.; Milam, A.H. The role of apoptosis in age-related macular degeneration. Arch. Ophthalmol. 2002, 120, 1435–1442. [Google Scholar] [CrossRef]
- Zeng, H.Y.; Tso, M.O.; Lai, S.; Lai, H. Activation of nuclear factor-kappaB during retinal degeneration in rd mice. Mol. Vis. 2008, 14, 1075–1080. [Google Scholar]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Bardhan, K.; Yang, D.; Thangaraju, M.; Ganapathy, V.; Waller, J.L.; Liles, G.B.; Lee, J.R.; Liu, K. NF-kappaB directly regulates Fas transcription to modulate Fas-mediated apoptosis and tumor suppression. J. Biol. Chem. 2012, 287, 25530–25540. [Google Scholar] [CrossRef]
- Jiang, N.; Chen, X.L.; Yang, H.W.; Ma, Y.R. Effects of nuclear factor kappaB expression on retinal neovascularization and apoptosis in a diabetic retinopathy rat model. Int. J. Ophthalmol. 2015, 8, 448–452. [Google Scholar] [CrossRef]
- Hikage, F.; Lennikov, A.; Mukwaya, A.; Lachota, M.; Ida, Y.; Utheim, T.P.; Chen, D.F.; Huang, H.; Ohguro, H. NF-κB activation in retinal microglia is involved in the inflammatory and neovascularization signaling in laser-induced choroidal neovascularization in mice. Exp. Cell Res. 2021, 403, 112581. [Google Scholar] [CrossRef] [PubMed]
- Crescenzi, E.; Pacifico, F.; Lavorgna, A.; De Palma, R.; D’Aiuto, E.; Palumbo, G.; Formisano, S.; Leonardi, A. NF-kappaB-dependent cytokine secretion controls Fas expression on chemotherapy-induced premature senescent tumor cells. Oncogene 2011, 30, 2707–2717. [Google Scholar] [CrossRef] [PubMed]
- Jurisic, V.; Bogdanovic, G.; Kojic, V.; Jakimov, D.; Srdic, T. Effect of TNF-α on Raji cells at different cellular levels estimated by various methods. Ann. Hematol. 2006, 85, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Merlin, J.P.J.; Rupasinghe, H.P.V.; Dellaire, G.; Murphy, K. Role of Dietary Antioxidants in p53-Mediated Cancer Chemoprevention and Tumor Suppression. Oxid. Med. Cell. Longev. 2021, 2021, 9924328. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-X.; Li, Y.; Sun, C.; Jiang, D.; Lin, Y.-J.; Jin, F.-X.; Lee, S.-K.; Jin, Y.-H. p53-dependent Fas expression is critical for Ginsenoside Rh2 triggered caspase-8 activation in HeLa cells. Protein Cell 2014, 5, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Munsch, D.; Watanabe-Fukunaga, R.; Bourdon, J.-C.; Nagata, S.; May, E.; Yonish-Rouach, E.; Reisdorf, P. Human and mouse Fas (APO-1/CD95) death receptor genes each contain a p53-responsive element that is activated by p53 mutants unable to induce apoptosis. J. Biol. Chem. 2000, 275, 3867–3872. [Google Scholar] [CrossRef]
- Semont, A.; Nowak, E.B.; Silva Lages, C.; Mathieu, C.; Mouthon, M.A.; May, E.; Allemand, I.; Millet, P.; Boussin, F.D. Involvement of p53 and Fas/CD95 in murine neural progenitor cell response to ionizing irradiation. Oncogene 2004, 23, 8497–8508. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, D.; Wang, V.M.; Yu, C.R.; Wang, R.X.; Tuo, J.; Chan, C.C. Enhanced apoptosis in retinal pigment epithelium under inflammatory stimuli and oxidative stress. Apoptosis 2012, 17, 1144–1155. [Google Scholar] [CrossRef]
- Zhao, H.; Roychoudhury, J.; Doggett, T.A.; Apte, R.S.; Ferguson, T.A. Age-dependent changes in FasL (CD95L) modulate macrophage function in a model of age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5321–5331. [Google Scholar] [CrossRef]
- Zacks, D.N.; Kocab, A.J.; Choi, J.J.; Gregory-Ksander, M.S.; Cano, M.; Handa, J.T. Cell Death in AMD: The Rationale for Targeting Fas. J. Clin. Med. 2022, 11, 592. [Google Scholar] [CrossRef]
- Besirli, C.G.; Chinskey, N.D.; Zheng, Q.D.; Zacks, D.N. Inhibition of retinal detachment-induced apoptosis in photoreceptors by a small peptide inhibitor of the fas receptor. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2177–2184. [Google Scholar] [CrossRef]
- Jurisic, V.; Bogdanovic, G.; Srdic, T.; Jakimov, D.; Mrdjanovic, J.; Baltic, M.; Baltic, V.V. Modulation of TNF-alpha activity in tumor PC cells using anti-CD45 and anti-CD95 monoclonal antibodies. Cancer Lett. 2004, 214, 55–61. [Google Scholar] [CrossRef]
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old, new and emerging functions of caspases. Cell Death Differ. 2015, 22, 526–539. [Google Scholar] [CrossRef]
- Li, H.; Zhu, H.; Xu, C.J.; Yuan, J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 1998, 94, 491–501. [Google Scholar] [CrossRef]
- Sui, A.; Chen, X.; Demetriades, A.M.; Shen, J.; Cai, Y.; Yao, Y.; Yao, Y.; Zhu, Y.; Shen, X.; Xie, B. Inhibiting NF-kappaB Signaling Activation Reduces Retinal Neovascularization by Promoting a Polarization Shift in Macrophages. Investig. Ophthalmol. Vis. Sci. 2020, 61, 4. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Cai, B.; Feng, Y.; Chen, J.; Wu, Y.; Zhuang, J.; Liu, Z.; Wu, Y. Activation of JNK signaling promotes all-trans-retinal-induced photoreceptor apoptosis in mice. J. Biol. Chem. 2020, 295, 6958–6971. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, R.; Satoh, R.; Takasaki, T. ERK: A double-edged sword in cancer. ERK-dependent apoptosis as a potential therapeutic strategy for cancer. Cells 2021, 10, 2509. [Google Scholar] [CrossRef]
- Du, H.; Sun, X.; Guma, M.; Luo, J.; Ouyang, H.; Zhang, X.; Zeng, J.; Quach, J.; Nguyen, D.H.; Shaw, P.X.; et al. JNK inhibition reduces apoptosis and neovascularization in a murine model of age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 2377–2382. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, A.; Cheng, X.; Kajino, K.; Berezov, A.; Murata, K.; Nakayama, T.; Yagita, H.; Murali, R.; Greene, M.I. Fas-disabling small exocyclic peptide mimetics limit apoptosis by an unexpected mechanism. Proc. Natl. Acad. Sci. USA 2004, 101, 6599–6604. [Google Scholar] [CrossRef]
- Glotin, A.L.; Calipel, A.; Brossas, J.Y.; Faussat, A.M.; Treton, J.; Mascarelli, F. Sustained versus transient ERK1/2 signaling underlies the anti- and proapoptotic effects of oxidative stress in human RPE cells. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4614–4623. [Google Scholar] [CrossRef]
- Cagnol, S.; Chambard, J.C. ERK and cell death: Mechanisms of ERK-induced cell death-apoptosis, autophagy and senescence. FEBS J. 2010, 277, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.C.; Chien, Y.; Yarmishyn, A.A.; Lim, L.Y.; Tsai, H.Y.; Kuo, W.C.; Tsai, P.H.; Yang, S.H.; Hong, S.I.; Chen, S.J.; et al. Inhibition of oxidative stress-induced epithelial-mesenchymal transition in retinal pigment epithelial cells of age-related macular degeneration model by suppressing ERK activation. J. Adv. Res. 2023. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Piva, R.; Belardo, G.; Santoro, M.G. NF-kappaB: A stress-regulated switch for cell survival. Antioxid. Redox Signal. 2006, 8, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Papa, S.; Bubici, C.; Zazzeroni, F.; Pham, C.G.; Kuntzen, C.; Knabb, J.R.; Dean, K.; Franzoso, G. The NF-kappaB-mediated control of the JNK cascade in the antagonism of programmed cell death in health and disease. Cell Death Differ. 2006, 13, 712–729. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, Z.; Zhang, J.; Robinson, A.G.; Lyu, B.; Chen, Z.; Wang, C.; Wei, B.; Xia, X.; Zhang, Q. Apoptotic caspase inhibits innate immune signaling by cleaving NF-κBs in both Mammals and Flies. Cell Death Dis. 2022, 13, 731. [Google Scholar] [CrossRef]
- Ahmed, S.; Amin, M.M.; Sayed, S. Ocular Drug Delivery: A Comprehensive Review. AAPS PharmSciTech 2023, 24, 66. [Google Scholar] [CrossRef]
- Cholkar, K.; Dasari, S.R.; Pal, D.; Mitra, A.K. Eye: Anatomy, physiology and barriers to drug delivery. In Ocular Transporters and Receptors; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–36. [Google Scholar]
- Diwan, P.; Jangde, R.; Khunte, S.; Bhardwaj, H.; Suresh, P.K. Ocular Drug Delivery System: Barrier for Drug Permeation, Method to Overcome Barrier. In Drug Development Life Cycle; IntechOpen: London, UK, 2022. [Google Scholar]
- Nhan, N.T.T.; Maidana, D.E.; Yamada, K.H. Ocular Delivery of Therapeutic Agents by Cell-Penetrating Peptides. Cells 2023, 12, 1071. [Google Scholar] [CrossRef]
- Zivanovic, M.; Gazdic Jankovic, M.; Ramovic Hamzagic, A.; Virijevic, K.; Milivojevic, N.; Pecic, K.; Seklic, D.; Jovanovic, M.; Kastratovic, N.; Miric, A.; et al. Combined Biological and Numerical Modeling Approach for Better Understanding of the Cancer Viability and Apoptosis. Pharmaceutics 2023, 15, 1628. [Google Scholar] [CrossRef]
- Ullah, I.; Chung, K.; Bae, S.; Li, Y.; Kim, C.; Choi, B.; Nam, H.Y.; Kim, S.H.; Yun, C.O.; Lee, K.Y.; et al. Nose-to-Brain Delivery of Cancer-Targeting Paclitaxel-Loaded Nanoparticles Potentiates Antitumor Effects in Malignant Glioblastoma. Mol. Pharm. 2020, 17, 1193–1204. [Google Scholar] [CrossRef]
- Ullah, I.; Chung, K.; Oh, J.; Beloor, J.; Bae, S.; Lee, S.C.; Lee, M.; Kumar, P.; Lee, S.K. Intranasal delivery of a Fas-blocking peptide attenuates Fas-mediated apoptosis in brain ischemia. Sci. Rep. 2018, 8, 15041. [Google Scholar] [CrossRef]
- Chung, S.; Yi, Y.; Ullah, I.; Chung, K.; Park, S.; Lim, J.; Kim, C.; Pyun, S.H.; Kim, M.; Kim, D.; et al. Systemic Treatment with Fas-Blocking Peptide Attenuates Apoptosis in Brain Ischemia. Int. J. Mol. Sci. 2024, 25, 661. [Google Scholar] [CrossRef] [PubMed]
- Lukinova, N.; Iacovelli, J.; Dentchev, T.; Wolkow, N.; Hunter, A.; Amado, D.; Ying, G.S.; Sparrow, J.R.; Dunaief, J.L. Iron chelation protects the retinal pigment epithelial cell line ARPE-19 against cell death triggered by diverse stimuli. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Horita, S.; Watanabe, M.; Katagiri, M.; Nakamura, H.; Haniuda, H.; Nakazato, T.; Kagawa, Y. Species differences in ocular pharmacokinetics and pharmacological activities of regorafenib and pazopanib eye-drops among rats, rabbits and monkeys. Pharmacol. Res. Perspect. 2019, 7, e00545. [Google Scholar] [CrossRef] [PubMed]
- Beloor, J.; Maes, N.; Ullah, I.; Uchil, P.; Jackson, A.; Fikrig, E.; Lee, S.K.; Kumar, P. Small Interfering RNA-Mediated Control of Virus Replication in the CNS Is Therapeutic and Enables Natural Immunity to West Nile Virus. Cell Host Microbe 2018, 23, 549–556.e543. [Google Scholar] [CrossRef]
- Zeller, S.; Choi, C.S.; Uchil, P.D.; Ban, H.S.; Siefert, A.; Fahmy, T.M.; Mothes, W.; Lee, S.K.; Kumar, P. Attachment of cell-binding ligands to arginine-rich cell-penetrating peptides enables cytosolic translocation of complexed siRNA. Chem. Biol. 2015, 22, 50–62. [Google Scholar] [CrossRef]
- Iwase, T.; Oveson, B.C.; Hashida, N.; Lima e Silva, R.; Shen, J.; Krauss, A.H.; Gale, D.C.; Adamson, P.; Campochiaro, P.A. Topical pazopanib blocks VEGF-induced vascular leakage and neovascularization in the mouse retina but is ineffective in the rabbit. Investig. Ophthalmol. Vis. Sci. 2013, 54, 503–511. [Google Scholar] [CrossRef]
- Del Amo, E.M.; Rimpela, A.K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic aspects of retinal drug delivery. Prog. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef]
- Cheng, S.-C.; Wu, Y.-H.; Huang, W.-C.; Pang, J.-H.S.; Huang, T.-H.; Cheng, C.-Y. Anti-inflammatory property of quercetin through downregulation of ICAM-1 and MMP-9 in TNF-α-activated retinal pigment epithelial cells. Cytokine 2019, 116, 48–60. [Google Scholar] [CrossRef]
- Lambuk, L.; Ahmad, S.; Sadikan, M.Z.; Nordin, N.A.; Kadir, R.; Nasir, N.A.A.; Chen, X.; Boer, J.; Plebanski, M.; Mohamud, R. Targeting Differential Roles of Tumor Necrosis Factor Receptors as a Therapeutic Strategy for Glaucoma. Front. Immunol. 2022, 13, 857812. [Google Scholar] [CrossRef]
- Huang, X.; Lu, Z.; Lv, Z.; Yu, T.; Yang, P.; Shen, Y.; Ding, Y.; Fu, D.; Zhang, X.; Fu, Q.; et al. The Fas/Fas ligand death receptor pathway contributes to phenylalanine-induced apoptosis in cortical neurons. PLoS ONE 2013, 8, e71553. [Google Scholar] [CrossRef]
- Chien, M.H.; Chang, W.M.; Lee, W.J.; Chang, Y.C.; Lai, T.C.; Chan, D.V.; Sharma, R.; Lin, Y.F.; Hsiao, M. A Fas Ligand (FasL)-Fused Humanized Antibody Against Tumor-Associated Glycoprotein 72 Selectively Exhibits the Cytotoxic Effect Against Oral Cancer Cells with a Low FasL/Fas Ratio. Mol. Cancer Ther. 2017, 16, 1102–1113. [Google Scholar] [CrossRef]
- Simunovic, M.P.; Puscas, L.; Mallick, S.; Kocab, A.J.; Kleinman, D.M.; Zacks, D.N. Safety and tolerability of intravitreal ONL1204 in patients with macula-off rhegmatogenous retinal detachment. Investig. Ophthalmol. Vis. Sci. 2023, 64, 2491. [Google Scholar]
- Yao, J.; Yang, M.; Jia, L.; Kocab, A.J.; Zacks, D.N. Protective effect of pharmacological Fas inhibition in two mouse models of inherited retinal degeneration. Investig. Ophthalmol. Vis. Sci. 2023, 64, 1540. [Google Scholar]
- Yaylaci, S.; Dinç, E.; Aydın, B.; Tekinay, A.B.; Guler, M.O. Peptide Nanofiber System for Sustained Delivery of Anti-VEGF Proteins to the Eye Vitreous. Pharmaceutics 2023, 15, 1264. [Google Scholar] [CrossRef] [PubMed]
- Parsons, D.E.; Lee, S.H.; Sun, Y.J.; Velez, G.; Bassuk, A.G.; Smith, M.; Mahajan, V.B. Peptidomimetics Therapeutics for Retinal Disease. Biomolecules 2021, 11, 339. [Google Scholar] [CrossRef] [PubMed]
- Sidman, R.L.; Li, J.; Lawrence, M.; Hu, W.; Musso, G.F.; Giordano, R.J.; Cardo-Vila, M.; Pasqualini, R.; Arap, W. The peptidomimetic Vasotide targets two retinal VEGF receptors and reduces pathological angiogenesis in murine and nonhuman primate models of retinal disease. Sci. Transl. Med. 2015, 7, 309ra165. [Google Scholar] [CrossRef] [PubMed]


| Peptide Name | Peptide Sequence | Molecular Weight (Da) |
|---|---|---|
| FBP | YCDEHFCY | 1079.1 |
| 4-mer | DEHF | 546.5 |
| 5-mer | CDEHF | 649.7 |
| 4R-FBP | RRRRGGGGYCDEHFCY | 1932.1 |
| 5R-FBP | RRRRRGGGGYCDEHFCY | 2088.3 |
| 6R-FBP | RRRRRRGGGGYCDEHFCY | 2244.5 |
| 9R-FBP | RRRRRRRRRGGGGYCDEHFCY | 2713.1 |
| Ctrl | GGGRRGGG | 672.7 |
| Target Gene | Primer Sequence |
|---|---|
| Fas(CD95) | Forward: 5′-GGAGGTGGTGATAGCCGGTAT-3′ |
| Reverse: 5′-TGGGTAATCCATAGAGCCCAG-3′ | |
| TNF-α | Forward: 5′-GGTGCCTATGTCTCAGCCTCTT-3′ |
| Reverse: 5′-GCCATAGAACTGATGAGAGGGAG-3′ | |
| IL-6 | Forward: 5′-TACCACTTCACAAGTCGGAGGC-3′ |
| Reverse: 5′-CTGCAAGTGCATCATCGTTGTTC-3′ | |
| CD11c | Forward: 5′-TTCTTCTGCTGTTGGGGTTTG-3′ |
| Reverse: 5′-CAACCACCACCCAGGAACTAT-3′ | |
| CD206 | Forward: 5′-GGCAGGATCTTGGCAACCTAGTA-3′ |
| Reverse: 5′-CCTTTCTTCCGACTCTTCACCC-3′ | |
| IL-1β | Forward: 5′-TTCAGGCAGGCAGTATCACTC-3′ |
| Reverse: 5′-GAAGGTCCACGGGAAAGACAC-3′ | |
| MCP-1 | Forward: 5′-TAAAAACCTGGATCGGAACCAAA-3′ |
| Reverse: 5′-GCATTAGCTTCAGATTTACGGGT-3′ | |
| F4/80 | Forward: 5′-CTGAGGATGAATTCCCGTGT-3′ |
| Reverse: 5′-GTCTCGGATGCTTCCACAAT-3′ | |
| GAPDH | Forward: 5′-AGGTCGGTGTGAACGGATTTG-3′ |
| Reverse: 5′-TGTAGACCATGTAGTTGAGGTCA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, Y.; Pyun, S.-H.; Kim, C.-Y.; Yun, G.; Kang, E.; Heo, S.; Ullah, I.; Lee, S.-K. Eye Drop with Fas-Blocking Peptide Attenuates Age-Related Macular Degeneration. Cells 2024, 13, 548. https://doi.org/10.3390/cells13060548
Yi Y, Pyun S-H, Kim C-Y, Yun G, Kang E, Heo S, Ullah I, Lee S-K. Eye Drop with Fas-Blocking Peptide Attenuates Age-Related Macular Degeneration. Cells. 2024; 13(6):548. https://doi.org/10.3390/cells13060548
Chicago/Turabian StyleYi, Yujong, Seon-Hong Pyun, Chae-Yeon Kim, Gyeongju Yun, Eunhwa Kang, Seoyoun Heo, Irfan Ullah, and Sang-Kyung Lee. 2024. "Eye Drop with Fas-Blocking Peptide Attenuates Age-Related Macular Degeneration" Cells 13, no. 6: 548. https://doi.org/10.3390/cells13060548