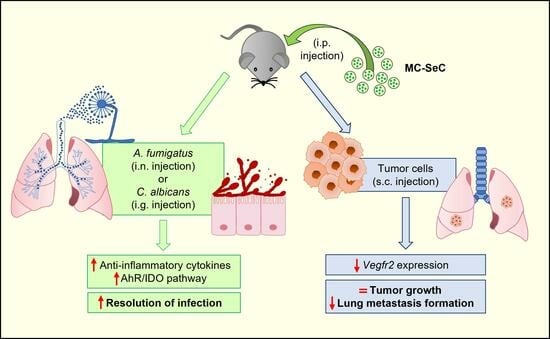
Grafted Sertoli Cells Exert Immunomodulatory Non-Immunosuppressive Effects in Preclinical Models of Infection and Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Animal Models of Tumor Growth and Fungal Infection
2.3. SeC Purification and Characterization
2.4. Preparation of the MC-SeCs
2.5. Histology and Immunohistochemistry
2.6. Morphological Quantification
2.7. Bronchoalveolar Lavage Fluid (BALF) Morphometry
2.8. Real-Time PCR
2.9. Statistical Analysis
2.10. Ethics Approval
3. Results
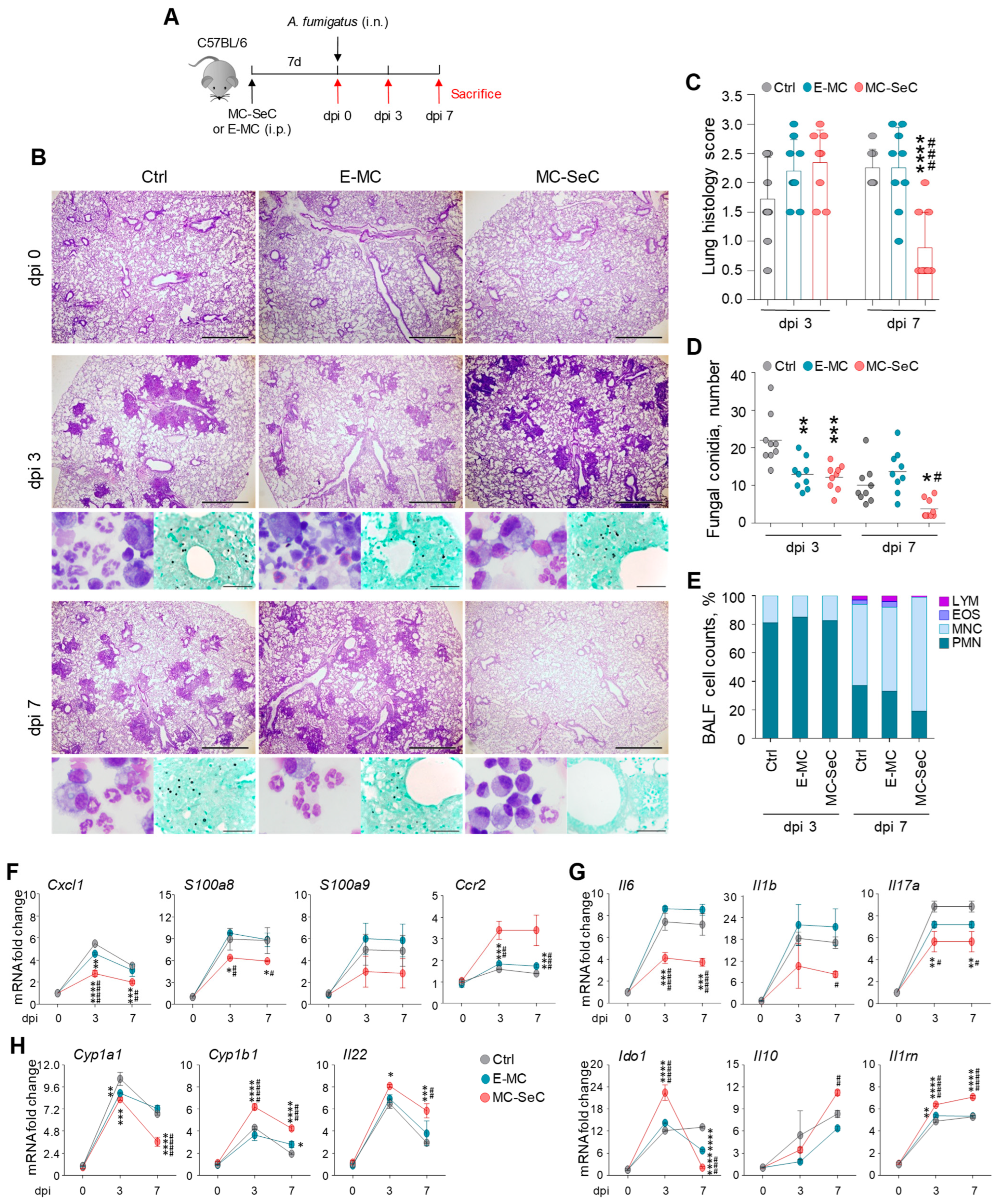
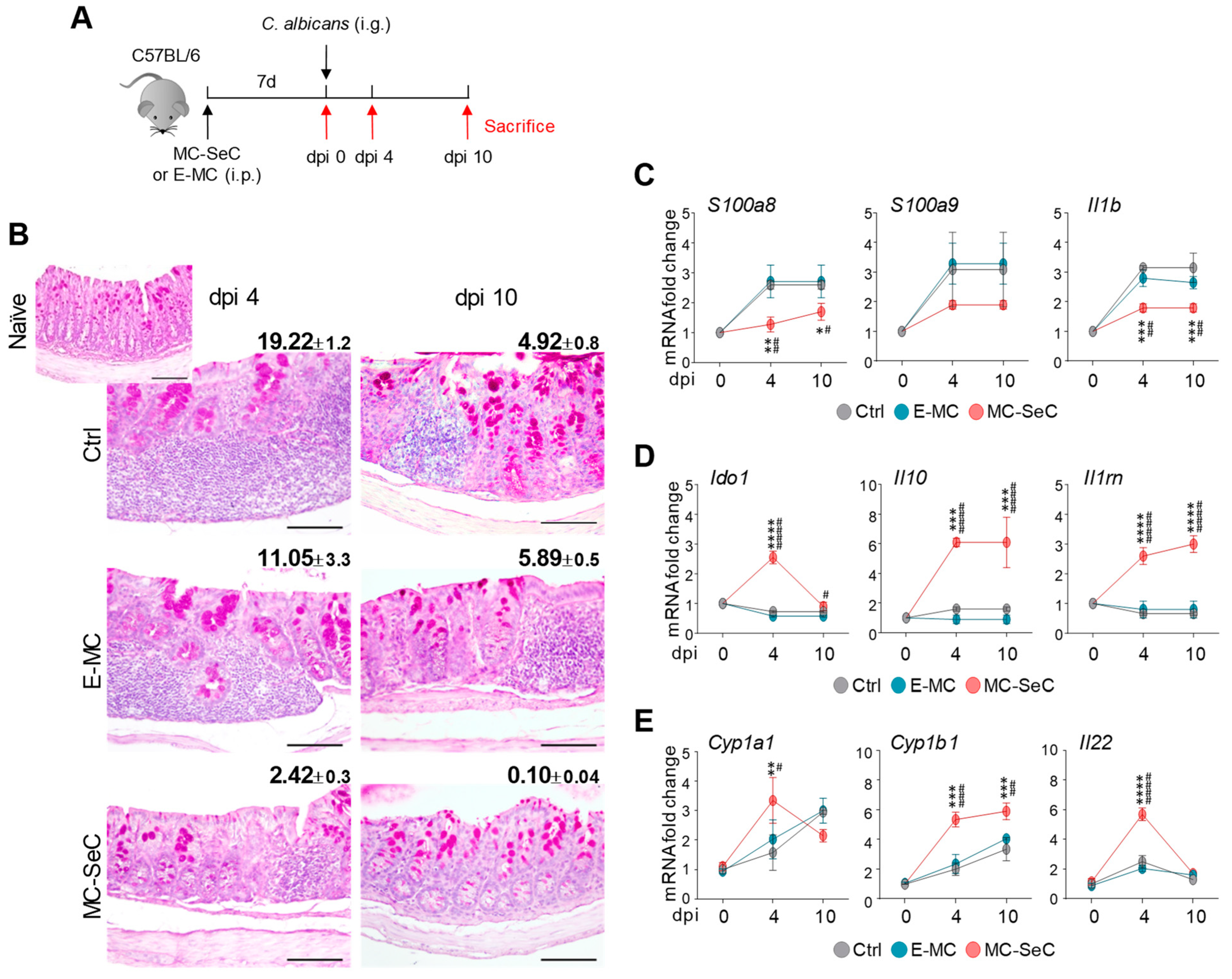
3.1. Grafted MC-SeCs Restrain Immunopathology in Infection
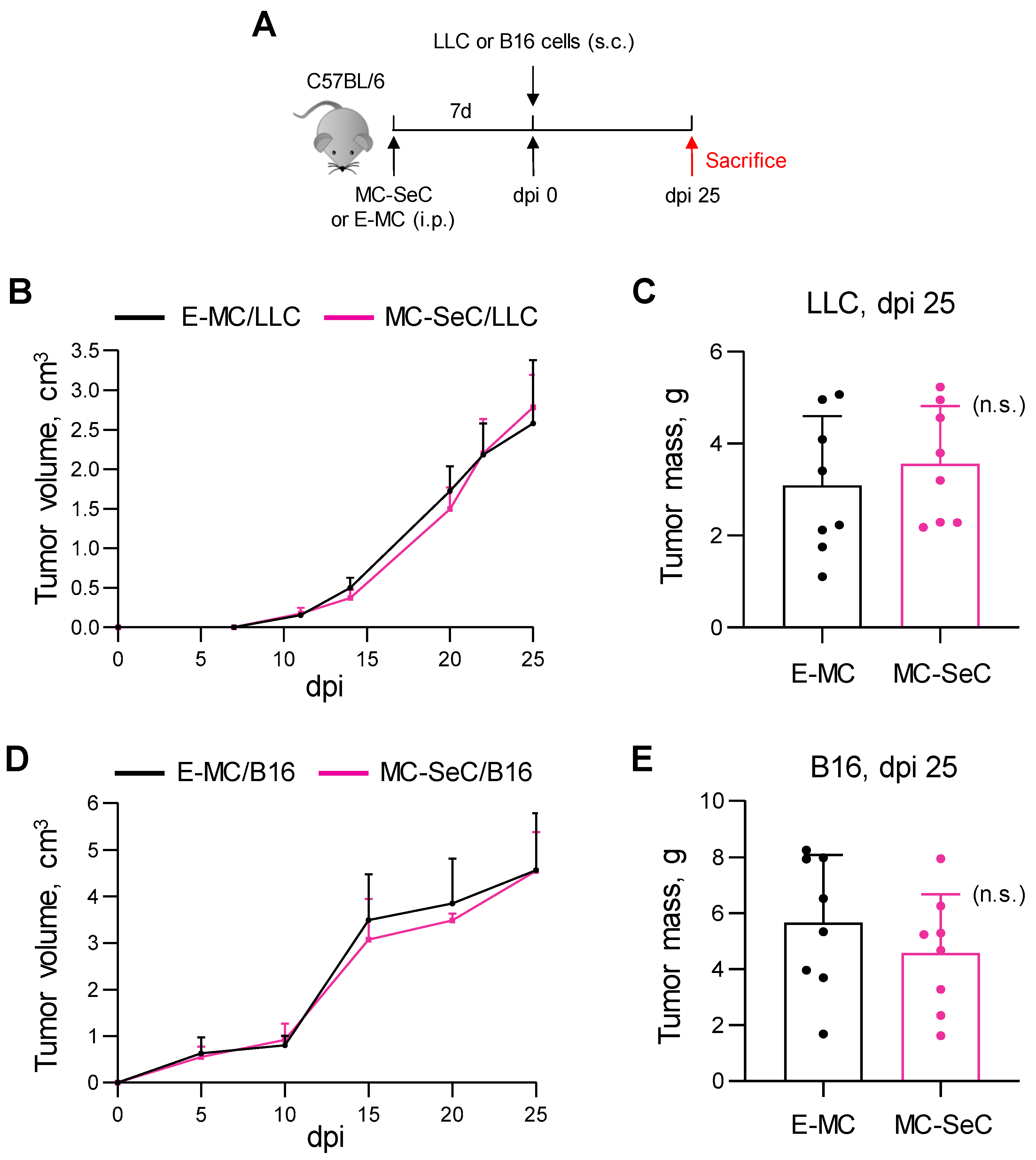
3.2. Grafted MC-SeCs Reduce Metastatic Cancer Spread
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mital, P.; Kaur, G.; Dufour, J.M. Immunoprotective Sertoli cells: Making allogeneic and xenogeneic trasplatantion feasible. Reproduction 2010, 139, 495–504. [Google Scholar] [CrossRef]
- Kaur, G.; Thompson, L.A.; Dufour, J.M. Sertoli cells-immunological sentinels of spermatogenesis. Semin. Cell Dev. Biol. 2014, 30, 36–44. [Google Scholar] [CrossRef]
- Selawry, H.P.; Kotb, M.; Herrod, H.G.; Lu, Z.N. Production of a factor, or factors, suppressing IL-2 production and T cell proliferation by Sertoli cell-enriched preparations. A potential role for islet transplantation in an immunologically privileged site. Transplantation 1991, 52, 846–850. [Google Scholar] [CrossRef]
- De Cesaris, P.; Filippini, A.; Cervelli, C.; Riccioli, A.; Muci, S.; Starace, G.; Stefanini, M.; Ziparo, E. Immunosuppressive molecules produced by Sertoli cells cultured in vitro: Biological effects on lymphocytes. Biochem. Biophys. Res. Commun. 1992, 186, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Pinzon, W.; Korbutt, G.S.; Power, R.; Hooton, J.; Rajotte, R.V.; Rabinovitch, A. Testicular sertoli cells protect islet beta-cells from autoimmune destruction in NOD mice by a transforming growth factor-beta1-dependent mechanism. Diabetes 2000, 49, 1810–1818. [Google Scholar] [CrossRef]
- Campese, A.F.; Grazioli, P.; de Cesaris, P.; Riccioli, A.; Bellavia, D.; Pelullo, M.; Padula, F.; Noce, C.; Verkhovskaia, S.; Filippini, A.; et al. Mouse Sertoli cells sustain de novo generation of regulatory T cells by triggering the notch pathway through soluble JAGGED1. Biol. Reprod. 2014, 90, 53. [Google Scholar] [CrossRef]
- Chiappalupi, S.; Salvadori, L.; Luca, G.; Riuzzi, F.; Calafiore, R.; Donato, R.; Sorci, G. Do porcine Sertoli cells represent an opportunity for Duchenne muscular dystrophy? Cell. Prolif. 2019, 52, e12599. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Gotoh, M.; Dono, K.; Nishihara, M.; Grochowiecki, T.; Kimura, F.; Yoshida, T.; Ohta, Y.; Ota, H.; Ohzato, H.; et al. Protection of islet allografts transplanted together with Fas ligand expressing testicular allografts. Diabetologia 1998, 41, 315–321. [Google Scholar] [CrossRef]
- Wright, K.; Dziuk, R.; Mital, P.; Kaur, G.; Dufour, J.M. Xenotransplanted Pig Sertoli Cells Inhibit Both the Alternative and Classical Pathways of Complement-Mediated Cell Lysis While Pig Islets Are Killed. Cell Transplant. 2016, 25, 2027–2040. [Google Scholar] [CrossRef]
- Tokuda, N.; Kasahara, M.; Levy, R.B. Differential regulation and expression of major histocompatibility complex (MHC) and Ly-6 gene products on mouse testicular Leydig and Sertoli cell lines. J. Autoimmun. 1990, 3, 457–471. [Google Scholar] [CrossRef]
- Kaur, G.; Wright, K.; Mital, P.; Hibler, T.; Miranda, J.M.; Thompson, L.A.; Halley, K.; Dufour, J.M. Neonatal Pig Sertoli Cells Survive Xenotransplantation by Creating an Immune Modulatory Environment Involving CD4 and CD8 Regulatory T Cells. Cell Transplant. 2020, 29, 963689720947102. [Google Scholar] [CrossRef]
- Qu, N.; Ogawa, Y.; Kuramasu, M.; Nagahori, K.; Sakabe, K.; Itoh, M. Immunological microenvironment in the testis. Reprod. Med. Biol. 2019, 19, 24–31. [Google Scholar] [CrossRef]
- Luca, G.; Arato, I.; Sorci, G.; Cameron, D.F.; Hansen, B.C.; Baroni, T.; Donato, R.; White, D.G.J.; Calafiore, R. Sertoli cells for cell transplantation: Pre-clinical studies and future perspectives. Andrology 2018, 6, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Washburn, R.L.; Hibler, T.; Thompson, L.A.; Kaur, G.; Dufour, J.M. Therapeutic application of Sertoli cells for treatment of various diseases. Semin. Cell Dev. Biol. 2022, 121, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Chiappalupi, S.; Luca, G.; Mancuso, F.; Madaro, L.; Fallarino, F.; Nicoletti, C.; Calvitti, M.; Arato, I.; Falabella, G.; Salvadori, L.; et al. Intraperitoneal injection of microencapsulated Sertoli cells restores muscle morphology and performance in dystrophic mice. Biomaterials 2016, 75, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Chiappalupi, S.; Salvadori, L.; Mancuso, F.; Arato, I.; Calvitti, M.; Riuzzi, F.; Calafiore, R.; Luca, G.; Sorci, G. Microencapsulated Sertoli cells sustain myoblast proliferation without affecting the myogenic potential. In vitro data. Data Brief 2021, 40, 107744. [Google Scholar] [CrossRef] [PubMed]
- Borghi, M.; Puccetti, M.; Pariano, M.; Renga, G.; Stincardini, C.; Ricci, M.; Giovagnoli, S.; Costantini, C.; Romani, L. Tryptophan as a Central Hub for Host/Microbial Symbiosis. Int. J. Tryptophan Res. 2020, 13, 1178646920919755. [Google Scholar] [CrossRef]
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef]
- Stockinger, B.; Di Meglio, P.; Gialitakis, M.; Duarte, J.H. The aryl hydrocarbon receptor: Multitasking in the immune system. Annu. Rev. Immunol. 2014, 32, 403–432. [Google Scholar] [CrossRef]
- Swann, J.B.; Smyth, M.J. Immune surveillance of tumors. J. Clin. Investig. 2007, 117, 1137–1146. [Google Scholar] [CrossRef]
- Ballarò, R.; Costelli, P.; Penna, F. Animal models for cancer cachexia. Curr. Opin. Support. Palliat. Care. 2016, 10, 281–287. [Google Scholar] [CrossRef]
- Giavazzi, R.; Decio, A. Syngeneic murine metastasis models: B16 melanoma. Methods Mol. Biol. 2014, 1070, 131–140. [Google Scholar] [CrossRef]
- Ya, Z.; Hailemichael, Y.; Overwijk, W.; Restifo, N.P. Mouse model for pre-clinical study of human cancer immunotherapy. Curr. Protoc. Immunol. 2015, 108, 20.1.1–20.1.43. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell. Biol. 2020, 21, 341–352, published correction appears in Nat. Rev. Mol. Cell. Biol. 2021, 22, 834. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, W.; Wei, X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef]
- Lian, L.; Li, X.L.; Xu, M.D.; Li, X.M.; Wu, M.Y.; Zhang, Y.; Tao, M.; Li, W.; Shen, X.M.; Zhou, C.; et al. VEGFR2 promotes tumorigenesis and metastasis in a pro-angiogenic-independent way in gastric cancer. BMC Cancer 2019, 19, 183. [Google Scholar] [CrossRef]
- Ezekian, B.; Schroder, P.M.; Freischlag, K.; Yoon, J.; Kwun, J.; Knechtle, S.J. Contemporary Strategies and Barriers to Transplantation Tolerance. Transplantation 2018, 102, 1213–1222. [Google Scholar] [CrossRef]
- Marcen, R. Immunosuppressive drugs in kidney transplantation: Impact on patient survival, and incidence of cardiovascular disease, malignancy and infection. Drugs 2009, 69, 2227–2243. [Google Scholar] [CrossRef]
- Riccioli, A.; Starace, D.; Galli, R.; Fuso, A.; Scarpa, S.; Palombi, F.; De Cesaris, P.; Ziparo, E.; Filippini, A. Sertoli cells initiate testicular innate immune responses through TLR activation. J. Immunol. 2006, 177, 7122–7130. [Google Scholar] [CrossRef]
- Lakpour, M.R.; Koruji, M.; Shahverdi, A.; Aghajanpour, S.; Rajabian Naghandar, M.; Sadighi Gilani, M.A.; Sabbaghian, M.; Aflatoonian, R. The Expression of TLR2 and TLR3 in Sertoli Cells of Azoospermic Patients. Cell J. 2017, 19, 375–385. [Google Scholar] [CrossRef]
- Renga, G.; D’Onofrio, F.; Pariano, M.; Galarini, R.; Barola, C.; Stincardini, C.; Bellet, M.M.; Ellemunter, H.; Lass-Flörl, C.; Costantini, C.; et al. Bridging of host-microbiota tryptophan partitioning by the serotonin pathway in fungal pneumonia. Nat. Commun. 2023, 14, 5753. [Google Scholar] [CrossRef] [PubMed]
- Shiratsuchi, A.; Osada, Y.; Nakanishi, Y. Differences in the mode of phagocytosis of bacteria between macrophages and testicular Sertoli cells. Drug Discov. Ther. 2013, 7, 73–77. [Google Scholar]
- Washburn, R.L.; Hibler, T.; Kaur, G.; Dufour, J.M. Sertoli Cell Immune Regulation: A Double-Edged Sword. Front. Immunol. 2022, 13, 913502. [Google Scholar] [CrossRef]
- Engels, E.A.; Pfeiffer, R.M.; Fraumeni, J.F.; Kasiske, B.L., Jr.; Israni, A.K.; Snyder, J.J.; Wolfe, R.A.; Goodrich, N.P.; Bayakly, A.R.; Clarke, C.A.; et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA 2011, 306, 1891–1901. [Google Scholar] [CrossRef] [PubMed]
- Grimberg, A. Mechanisms by which IGF-I may promote cancer. Cancer Biol. Ther. 2003, 2, 630–635. [Google Scholar] [CrossRef]
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625. [Google Scholar] [CrossRef]
- Qvist, G. John Hunter 1728–1793; William Heinemann Medical Books Ltd.: London, UK, 1981. [Google Scholar]
- Doyle, T.J.; Kaur, G.; Putrevu, S.M.; Dyson, E.L.; Dyson, M.; McCunniff, W.T.; Pasham, M.R.; Kim, K.H.; Dufour, J.M. Immunoprotective properties of primary Sertoli cells in mice: Potential functional pathways that confer immune privilege. Biol. Reprod. 2012, 86, 1–14. [Google Scholar] [CrossRef]
- Chamberlain, J.S.; Metzger, J.; Reyes, M.; Townsend, D.; Faulkner, J.A. Dystrophin-deficient mdx mice display a reduced life span and are susceptible to spontaneous rhabdomyosarcoma. FASEB J. 2007, 21, 2195–2204. [Google Scholar] [CrossRef]
- Valdés-González, R.A.; Dorantes, L.M.; Garibay, G.N.; Bracho-Blanchet, E.; Mendez, A.J.; Dávila-Pérez, R.; Elliott, R.B.; Terán, L.; White, D.J. Xenotransplantation of porcine neonatal islets of Langerhans and Sertoli cells: A 4-year study. Eur. J. Endocrinol. 2005, 153, 419–427. [Google Scholar] [CrossRef]
- Esquivel-Pérez, R.; Rodriguez-Ventura, A.L.; Dorantes, L.M.; Ramírez-González, B.; López-Santos, M.G.; Valdes-Gonzalez, R. Correlation between insulin requirements and anti-galactose antibodies in patients with type 1 diabetes transplanted with neonatal pig islets. Clin. Exp. Immunol. 2011, 165, 104–109. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiappalupi, S.; Salvadori, L.; Borghi, M.; Mancuso, F.; Pariano, M.; Riuzzi, F.; Luca, G.; Romani, L.; Arato, I.; Sorci, G. Grafted Sertoli Cells Exert Immunomodulatory Non-Immunosuppressive Effects in Preclinical Models of Infection and Cancer. Cells 2024, 13, 544. https://doi.org/10.3390/cells13060544
Chiappalupi S, Salvadori L, Borghi M, Mancuso F, Pariano M, Riuzzi F, Luca G, Romani L, Arato I, Sorci G. Grafted Sertoli Cells Exert Immunomodulatory Non-Immunosuppressive Effects in Preclinical Models of Infection and Cancer. Cells. 2024; 13(6):544. https://doi.org/10.3390/cells13060544
Chicago/Turabian StyleChiappalupi, Sara, Laura Salvadori, Monica Borghi, Francesca Mancuso, Marilena Pariano, Francesca Riuzzi, Giovanni Luca, Luigina Romani, Iva Arato, and Guglielmo Sorci. 2024. "Grafted Sertoli Cells Exert Immunomodulatory Non-Immunosuppressive Effects in Preclinical Models of Infection and Cancer" Cells 13, no. 6: 544. https://doi.org/10.3390/cells13060544