Composite Fibrin and Carbon Microfibre Implant to Modulate Postraumatic Inflammation after Spinal Cord Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Preparation and Bio-Functionalization of Carbon MFs
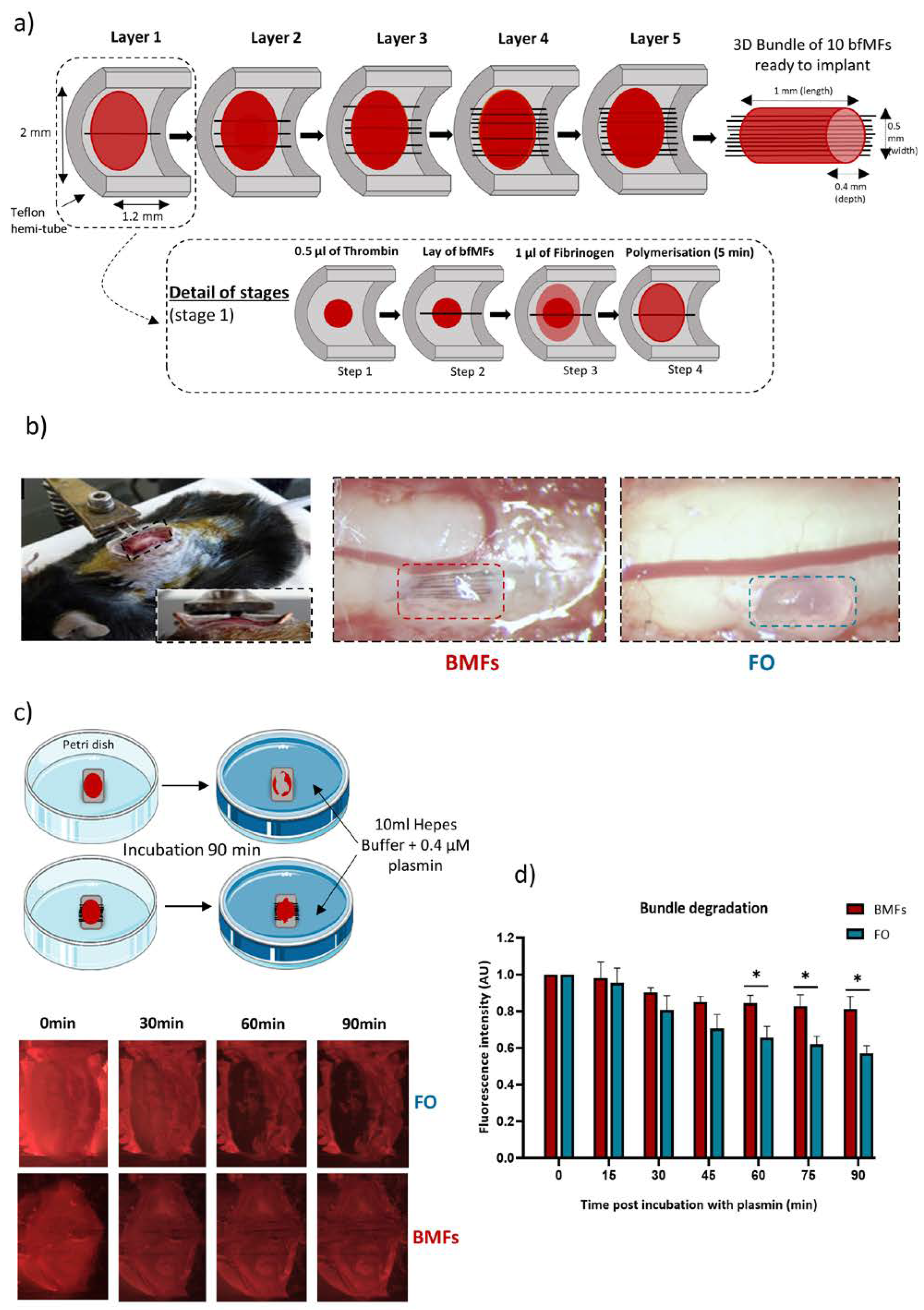
2.3. Preparation of the MF Bundle in a Fibrin/Thrombin Gel
2.4. Partial Unilateral Dorsal Quadrant Lesion (PUDQL) and Glass Window Implantation
2.5. Intravital Imaging
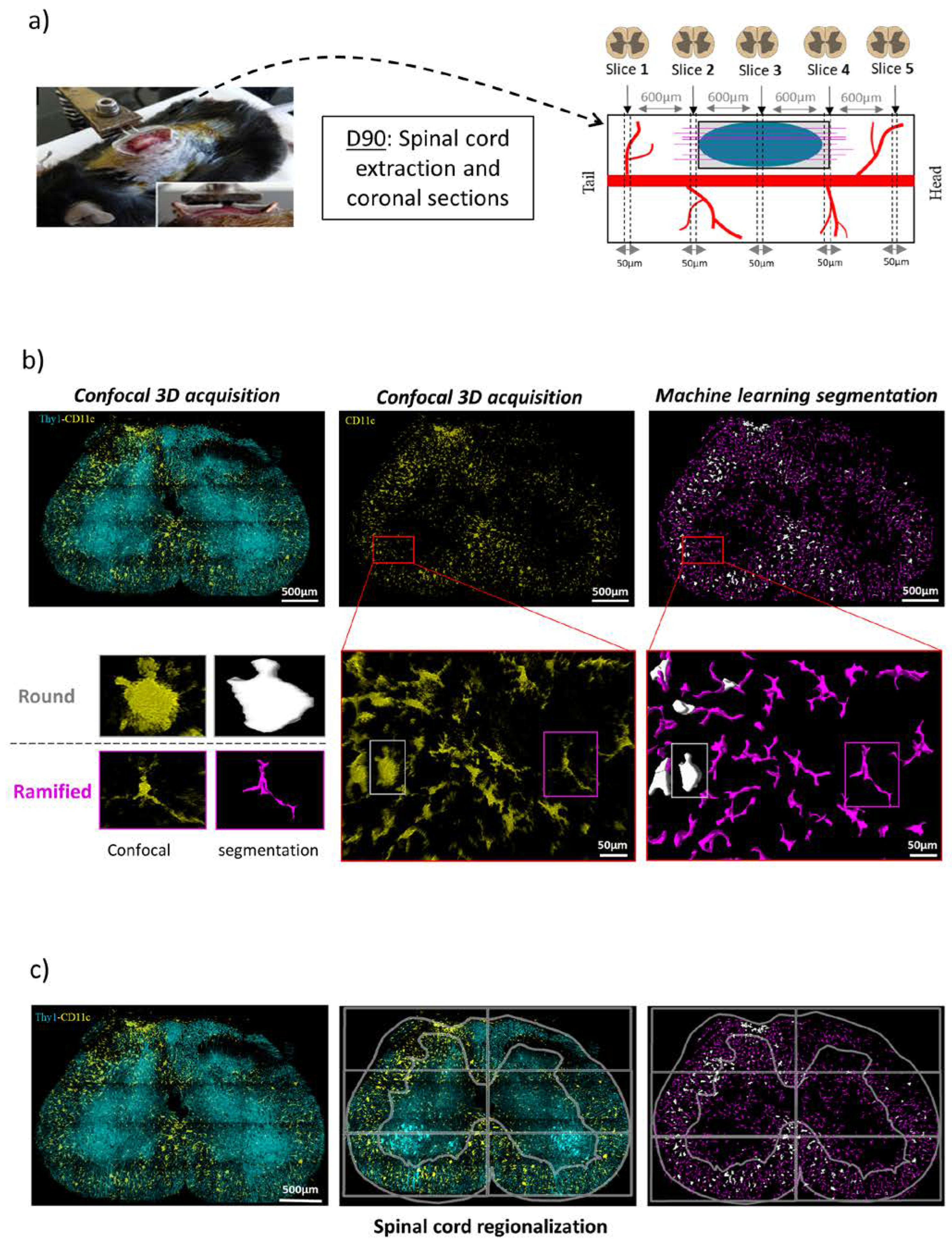
2.6. Histological Study
2.7. Image Analysis
2.8. Statistics
3. Results
3.1. Tailoring a Fibrin/Carbon Based Implant
3.2. Plasmin Mediated Resorption of the Implant
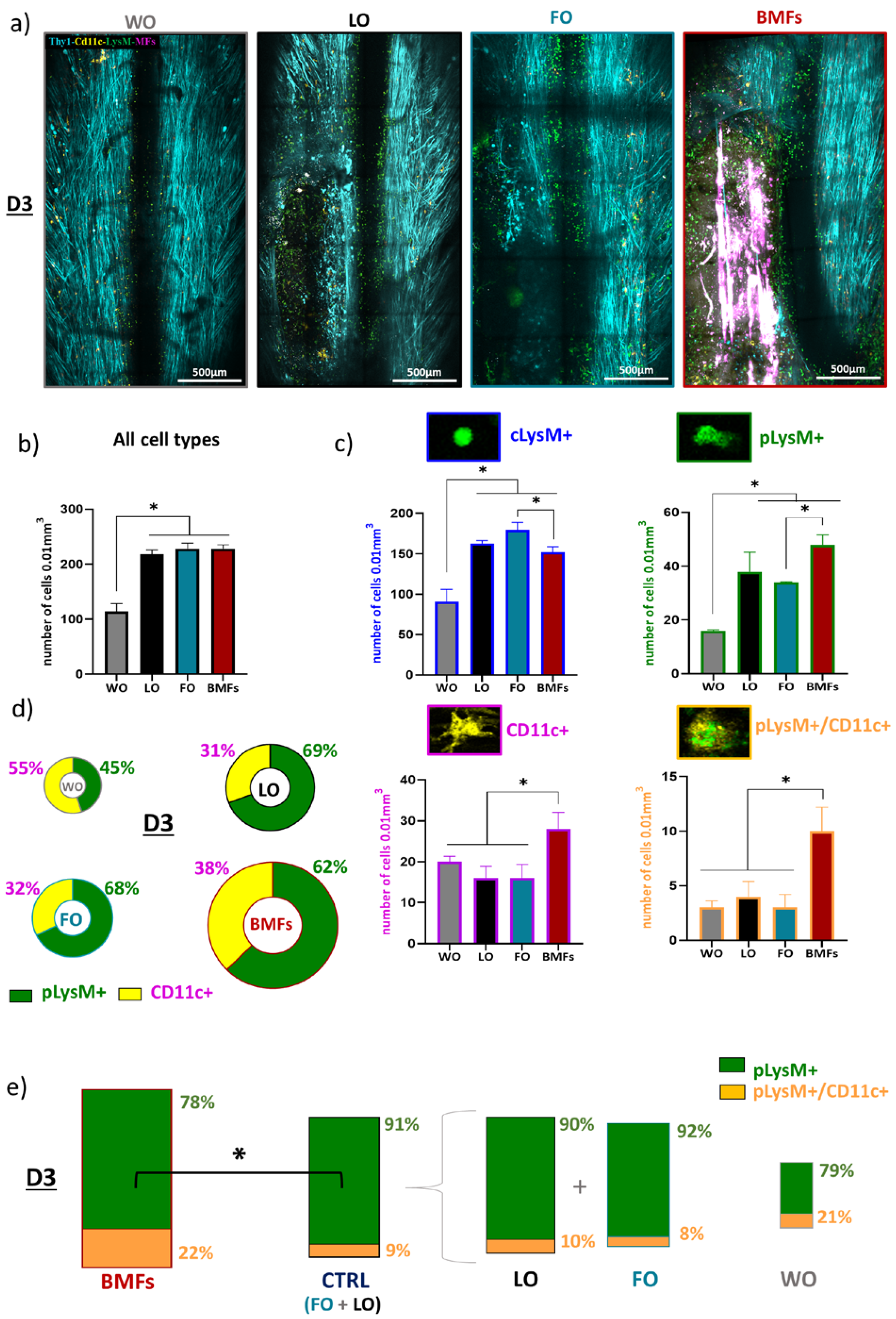
3.3. Immunomodulatory Effects of the Composite Implant after Spinal Cord Injury
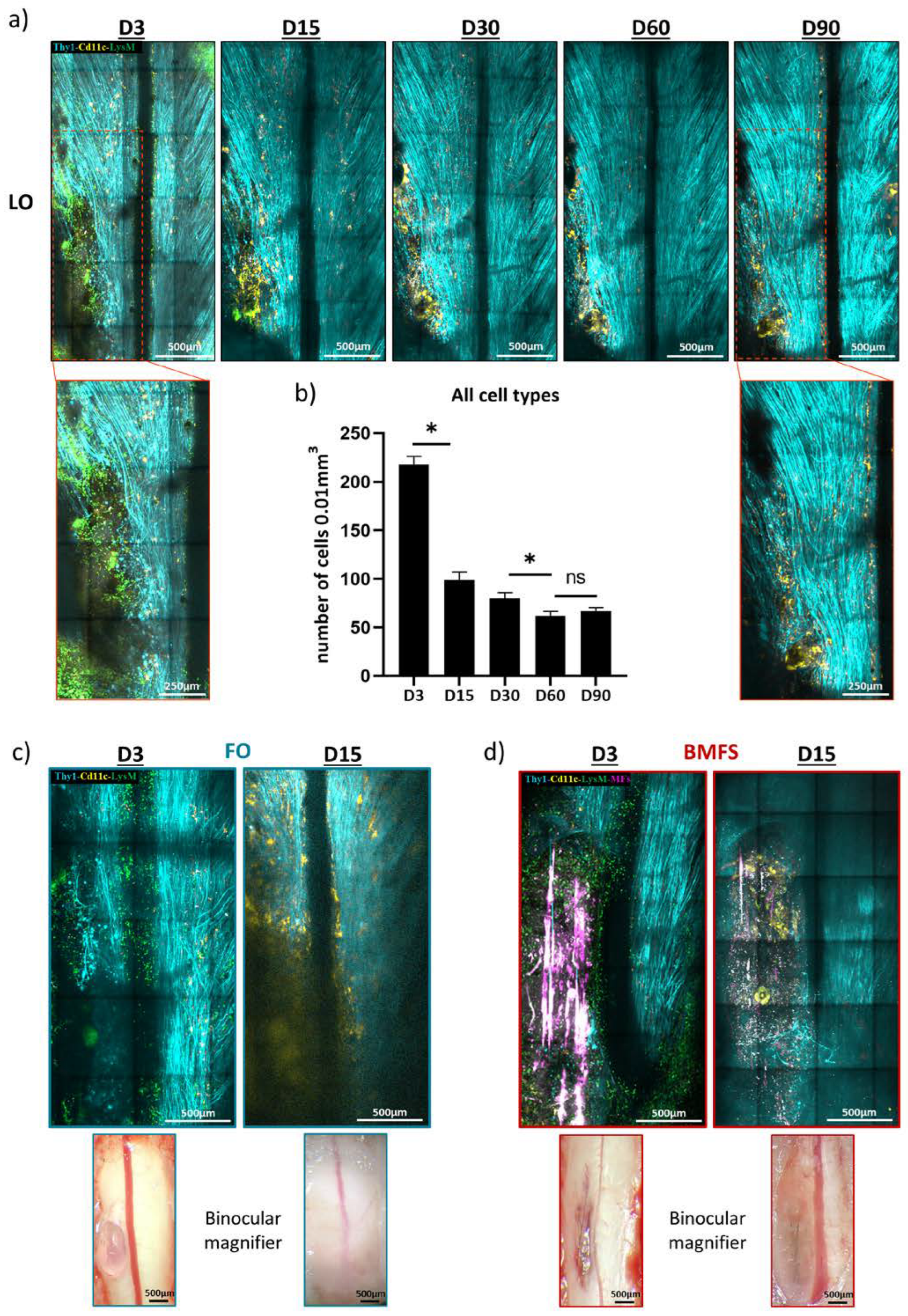
3.4. Fibrin Degradation Precludes Longitudinal Intravital Imaging
3.5. Chronic Inflammatory Responses to the Different Types of Implants
3.6. The Composit Fibrin-MF Implant Improves the Axonal Regeneration
4. Discussion
4.1. Implantation Strategy
4.2. Interest of Biofunctionalized Carbon MFs
4.3. Fibrin, an Ideal Matrix?
4.4. Imaging to Study Biocompatibility and Cell Responses to Implants in the CNS
4.5. Limitation and Prospects
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, H.; Imajo, Y.; Funaba, M.; Ikeda, H.; Nishida, N.; Sakai, T. Current Concepts of Biomaterial Scaffolds and Regenerative Therapy for Spinal Cord Injury. Int. J. Mol. Sci. 2023, 24, 2528. [Google Scholar] [CrossRef] [PubMed]
- Saremi, J.; Mahmoodi, N.; Rasouli, M.; Ranjbar, F.E.; Mazaheri, E.L.; Akbari, M.; Hasanzadeh, E.; Azami, M. Advanced Approaches to Regenerate Spinal Cord Injury: The Development of Cell and Tissue Engineering Therapy and Combinational Treatments. Biomed. Pharm. 2022, 146, 112529. [Google Scholar] [CrossRef] [PubMed]
- Alves-Sampaio, A.; García-Rama, C.; Collazos-Castro, J.E. Biofunctionalized PEDOT-Coated Microfibers for the Treatment of Spinal Cord Injury. Biomaterials 2016, 89, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Collazos-Castro, J.E.; Hernández-Labrado, G.R.; Polo, J.L.; García-Rama, C. N-Cadherin- and L1-Functionalised Conducting Polymers for Synergistic Stimulation and Guidance of Neural Cell Growth. Biomaterials 2013, 34, 3603–3617. [Google Scholar] [CrossRef]
- Collazos-Castro, J.E.; García-Rama, C.; Alves-Sampaio, A. Glial Progenitor Cell Migration Promotes CNS Axon Growth on Functionalized Electroconducting Microfibers. Acta Biomater. 2016, 35, 42–56. [Google Scholar] [CrossRef]
- El Waly, B.; Escarrat, V.; Perez-Sanchez, J.; Kaur, J.; Pelletier, F.; Collazos-Castro, J.E.; Debarbieux, F. Intravital Assessment of Cells Responses to Conducting Polymer-Coated Carbon Microfibres for Bridging Spinal Cord Injury. Cells 2021, 10, 73. [Google Scholar] [CrossRef]
- Nakkala, J.R.; Li, Z.; Ahmad, W.; Wang, K.; Gao, C. Immunomodulatory Biomaterials and Their Application in Therapies for Chronic Inflammation-Related Diseases. Acta Biomater 2021, 123, 1–30. [Google Scholar] [CrossRef]
- Silva, D.; Sousa, R.A.; Salgado, A.J. Hydrogels as Delivery Systems for Spinal Cord Injury Regeneration. Mater. Today Bio. 2021, 9, 100093. [Google Scholar] [CrossRef]
- Yu, Z.; Li, H.; Xia, P.; Kong, W.; Chang, Y.; Fu, C.; Wang, K.; Yang, X.; Qi, Z. Application of Fibrin-Based Hydrogels for Nerve Protection and Regeneration after Spinal Cord Injury. J. Biol. Eng. 2020, 14, 22. [Google Scholar] [CrossRef]
- Luyendyk, J.P.; Schoenecker, J.G.; Flick, M.J. The Multifaceted Role of Fibrinogen in Tissue Injury and Inflammation. Blood 2019, 133, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C.; Barker, T.H. Fibrin-Based Biomaterials: Modulation of Macroscopic Properties through Rational Design at the Molecular Level. Acta Biomater. 2014, 10, 1502–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakovlev, S.; Strickland, D.K.; Medved, L. Current View on the Molecular Mechanisms Underlying Fibrin(Ogen)-Dependent Inflammation. Thromb. Haemost. 2022, 122, 1858–1868. [Google Scholar] [CrossRef]
- Yao, S.; Yu, S.; Cao, Z.; Yang, Y.; Yu, X.; Mao, H.-Q.; Wang, L.-N.; Sun, X.; Zhao, L.; Wang, X. Hierarchically Aligned Fibrin Nanofiber Hydrogel Accelerated Axonal Regrowth and Locomotor Function Recovery in Rat Spinal Cord Injury. Int. J. Nanomed. 2018, 13, 2883–2895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Z.; Man, W.; Xiong, Y.; Guo, Y.; Yang, S.; Liu, D.; Zhao, H.; Yang, Y.; Yao, S.; Li, C.; et al. White Matter Regeneration Induced by Aligned Fibrin Nanofiber Hydrogel Contributes to Motor Functional Recovery in Canine T12 Spinal Cord Injury. Regen. Biomater. 2022, 9, rbab069. [Google Scholar] [CrossRef] [PubMed]
- Brennan, F.H.; Li, Y.; Wang, C.; Ma, A.; Guo, Q.; Li, Y.; Pukos, N.; Campbell, W.A.; Witcher, K.G.; Guan, Z.; et al. Microglia Coordinate Cellular Interactions during Spinal Cord Repair in Mice. Nat. Commun. 2022, 13, 4096. [Google Scholar] [CrossRef]
- Caravagna, C.; Jaouën, A.; Desplat-Jégo, S.; Fenrich, K.K.; Bergot, E.; Luche, H.; Grenot, P.; Rougon, G.; Malissen, M.; Debarbieux, F. Diversity of Innate Immune Cell Subsets across Spatial and Temporal Scales in an EAE Mouse Model. Sci. Rep. 2018, 8, 5146. [Google Scholar] [CrossRef]
- Vara, H.; Collazos-Castro, J.E. Biofunctionalized Conducting Polymer/Carbon Microfiber Electrodes for Ultrasensitive Neural Recordings. ACS Appl. Mater. Interfaces 2015, 7, 27016–27026. [Google Scholar] [CrossRef]
- Vara, H.; Collazos-Castro, J.E. Enhanced Spinal Cord Microstimulation Using Conducting Polymer-Coated Carbon Microfibers. Acta Biomater. 2019, 90, 71–86. [Google Scholar] [CrossRef]
- Janmey, P.A.; Winer, J.P.; Weisel, J.W. Fibrin Gels and Their Clinical and Bioengineering Applications. J. R. Soc. Interface 2009, 6, 1–10. [Google Scholar] [CrossRef]
- Feller, T.; Hársfalvi, J.; Csányi, C.; Kiss, B.; Kellermayer, M. Plasmin-Driven Fibrinolysis in a Quasi-Two-Dimensional Nanoscale Fibrin Matrix. J. Struct. Biol. 2018, 203, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Ricard, C.; Tchoghandjian, A.; Luche, H.; Grenot, P.; Figarella-Branger, D.; Rougon, G.; Malissen, M.; Debarbieux, F. Phenotypic Dynamics of Microglial and Monocyte-Derived Cells in Glioblastoma-Bearing Mice. Sci. Rep. 2016, 6, 26381. [Google Scholar] [CrossRef] [Green Version]
- Patel, P.R.; Zhang, H.; Robbins, M.T.; Nofar, J.B.; Marshall, S.P.; Kobylarek, M.J.; Kozai, T.D.Y.; Kotov, N.A.; Chestek, C.A. Chronic in vivo Stability Assessment of Carbon Fiber Microelectrode Arrays. J. Neural. Eng. 2016, 13, 066002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Zhu, J.; Wu, Q.; An, Y.; Wang, K.; Xuan, T.; Zhang, J.; Song, W.; He, H.; Song, L.; et al. Mussel-Inspired Surface Immobilization of Heparin on Magnetic Nanoparticles for Enhanced Wound Repair via Sustained Release of a Growth Factor and M2 Macrophage Polarization. ACS Appl. Mater. Interfaces 2021, 13, 2230–2244. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, J.K.; Mano, F.; Iezzi, R.; LoBue, S.A.; Holman, B.H.; Fautsch, M.P.; Olsen, T.W.; Pulido, J.S.; Marmorstein, A.D. Fibrin Hydrogels Are Safe, Degradable Scaffolds for Sub-Retinal Implantation. PLoS ONE 2020, 15, e0227641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolbank, S.; Pichler, V.; Ferguson, J.C.; Meinl, A.; van Griensven, M.; Goppelt, A.; Redl, H. Non-Invasive in vivo Tracking of Fibrin Degradation by Fluorescence Imaging. J. Tissue Eng. Regen. Med. 2015, 9, 973–976. [Google Scholar] [CrossRef]
- Gersh, K.C.; Edmondson, K.E.; Weisel, J.W. Flow Rate and Fibrin Fiber Alignment. J. Thromb. Haemost. 2010, 8, 2826–2828. [Google Scholar] [CrossRef] [Green Version]
- Lynch, S.R.; Laverty, S.M.; Bannish, B.E.; Hudson, N.E. Microscale Structural Changes of Individual Fibrin Fibers during Fibrinolysis. Acta Biomater. 2022, 141, 114–122. [Google Scholar] [CrossRef]
- Wolberg, A.S. Thrombin Generation and Fibrin Clot Structure. Blood Rev. 2007, 21, 131–142. [Google Scholar] [CrossRef]
- Jara, C.P.; Wang, O.; Paulino do Prado, T.; Ismail, A.; Fabian, F.M.; Li, H.; Velloso, L.A.; Carlson, M.A.; Burgess, W.; Lei, Y.; et al. Novel Fibrin-Fibronectin Matrix Accelerates Mice Skin Wound Healing. Bioact. Mater. 2020, 5, 949–962. [Google Scholar] [CrossRef]
- Tognatta, R.; Merlini, M.; Yan, Z.; Schuck, R.; Davalos, D.; Akassoglou, K. In Vivo Two-Photon Microscopy Protocol for Imaging Microglial Responses and Spine Elimination at Sites of Fibrinogen Deposition in Mouse Brain. STAR Protoc. 2021, 2, 100638. [Google Scholar] [CrossRef]
- Davalos, D.; Ryu, J.K.; Merlini, M.; Baeten, K.M.; Le Moan, N.; Petersen, M.A.; Deerinck, T.J.; Smirnoff, D.S.; Bedard, C.; Hakozaki, H.; et al. Fibrinogen-Induced Perivascular Microglial Clustering Is Required for the Development of Axonal Damage in Neuroinflammation. Nat. Commun. 2012, 3, 1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, T.J.; Leavell, K.J.; Peterson, M.W. CD11b/CD18 Mediates the Neutrophil Chemotactic Activity of Fibrin Degradation Product D Domain. Thromb. Haemost. 1997, 77, 894–900. [Google Scholar]
- Fenrich, K.K.; Weber, P.; Hocine, M.; Zalc, M.; Rougon, G.; Debarbieux, F. Long-Term in vivo Imaging of Normal and Pathological Mouse Spinal Cord with Subcellular Resolution Using Implanted Glass Windows. J. Physiol. 2012, 590, 3665–3675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eles, J.R.; Vazquez, A.L.; Kozai, T.D.Y.; Cui, X.T. Meningeal Inflammatory Response and Fibrous Tissue Remodeling around Intracortical Implants: An in Vivo Two-Photon Imaging Study. Biomaterials 2019, 195, 111–123. [Google Scholar] [CrossRef]
- Eles, J.R.; Vazquez, A.L.; Snyder, N.R.; Lagenaur, C.; Murphy, M.C.; Kozai, T.D.Y.; Cui, X.T. Neuroadhesive L1 Coating Attenuates Acute Microglial Attachment to Neural Electrodes as Revealed by Live Two-Photon Microscopy. Biomaterials 2017, 113, 279–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vereyken, E.J.F.; Heijnen, P.D.A.M.; Baron, W.; de Vries, E.H.E.; Dijkstra, C.D.; Teunissen, C.E. Classically and Alternatively Activated Bone Marrow Derived Macrophages Differ in Cytoskeletal Functions and Migration towards Specific CNS Cell Types. J. Neuroinflamm. 2011, 8, 58. [Google Scholar] [CrossRef] [Green Version]
- Sikkema, A.H.; Stoffels, J.M.J.; Wang, P.; Basedow, F.J.; Bulsink, R.; Bajramovic, J.J.; Baron, W. Fibronectin Aggregates Promote Features of a Classically and Alternatively Activated Phenotype in Macrophages. J. Neuroinflamm. 2018, 15, 218. [Google Scholar] [CrossRef] [PubMed]
- Yim, A.; Smith, C.; Brown, A.M. Osteopontin/Secreted Phosphoprotein-1 Harnesses Glial-, Immune-, and Neuronal Cell Ligand-Receptor Interactions to Sense and Regulate Acute and Chronic Neuroinflammation. Immunol. Rev. 2022, 311, 224–233. [Google Scholar] [CrossRef]
- Jin, X.; Liu, M.-Y.; Zhang, D.-F.; Zhong, X.; Du, K.; Qian, P.; Gao, H.; Wei, M.-J. Natural Products as a Potential Modulator of Microglial Polarization in Neurodegenerative Diseases. Pharmacol. Res. 2019, 145, 104253. [Google Scholar] [CrossRef]
- Schaefer, C.J.; Lawrence, W.D.; Wooley, P.H. Influence of Long Term Silicone Implantation on Type II Collagen Induced Arthritis in Mice. Ann. Rheum. Dis. 1999, 58, 503–509. [Google Scholar] [CrossRef]
- Zilionis, R.; Engblom, C.; Pfirschke, C.; Savova, V.; Zemmour, D.; Saatcioglu, H.D.; Krishnan, I.; Maroni, G.; Meyerovitz, C.V.; Kerwin, C.M.; et al. Single-Cell Transcriptomics of Human and Mouse Lung Cancers Reveals Conserved Myeloid Populations across Individuals and Species. Immunity 2019, 50, 1317–1334.e10. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Dissecting Spinal Cord Regeneration. Nature 2018, 557, 343–350. [Google Scholar] [CrossRef]
- Cregg, J.M.; DePaul, M.A.; Filous, A.R.; Lang, B.T.; Tran, A.; Silver, J. Functional Regeneration beyond the Glial Scar. Exp. Neurol. 2014, 253, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; He, S.; Wu, J.; Chen, C.; Li, X.; Liu, K.; Qu, J.Y. Long-Term in Vivo Imaging of Mouse Spinal Cord through an Optically Cleared Intervertebral Window. Nat. Commun. 2022, 13, 1959. [Google Scholar] [CrossRef]
- El Waly, B.; Buttigieg, E.; Karakus, C.; Brustlein, S.; Debarbieux, F. Longitudinal Intravital Microscopy Reveals Axon Degeneration Concomitant With Inflammatory Cell Infiltration in an LPC Model of Demyelination. Front. Cell Neurosci. 2020, 14, 165. [Google Scholar] [CrossRef]
- Dray, C.; Rougon, G.; Debarbieux, F. Quantitative Analysis by in Vivo Imaging of the Dynamics of Vascular and Axonal Networks in Injured Mouse Spinal Cord. Proc. Natl. Acad. Sci. USA 2009, 106, 9459–9464. [Google Scholar] [CrossRef] [Green Version]
- Park, S.-S.; Lee, Y.J.; Lee, S.H.; Lee, D.; Choi, K.; Kim, W.-H.; Kweon, O.-K.; Han, H.J. Functional Recovery after Spinal Cord Injury in Dogs Treated with a Combination of Matrigel and Neural-Induced Adipose-Derived Mesenchymal Stem Cells. Cytotherapy 2012, 14, 584–597. [Google Scholar] [CrossRef]
- Dias, D.O.; Kim, H.; Holl, D.; Werne Solnestam, B.; Lundeberg, J.; Carlén, M.; Göritz, C.; Frisén, J. Reducing Pericyte-Derived Scarring Promotes Recovery after Spinal Cord Injury. Cell 2018, 173, 153–165.e22. [Google Scholar] [CrossRef] [Green Version]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A Sensitive and Reliable Locomotor Rating Scale for Open Field Testing in Rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef]
- Arlotti, M.; Colombo, M.; Bonfanti, A.; Mandat, T.; Lanotte, M.M.; Pirola, E.; Borellini, L.; Rampini, P.; Eleopra, R.; Rinaldo, S.; et al. A New Implantable Closed-Loop Clinical Neural Interface: First Application in Parkinson’s Disease. Front. Neurosci. 2021, 15, 763235. [Google Scholar] [CrossRef]
- Gilron, R.; Little, S.; Perrone, R.; Wilt, R.; de Hemptinne, C.; Yaroshinsky, M.S.; Racine, C.A.; Wang, S.S.; Ostrem, J.L.; Larson, P.S.; et al. Long-Term Wireless Streaming of Neural Recordings for Circuit Discovery and Adaptive Stimulation in Individuals with Parkinson’s Disease. Nat. Biotechnol. 2021, 39, 1078–1085. [Google Scholar] [CrossRef]
- Bonizzato, M.; Martinez, M. An Intracortical Neuroprosthesis Immediately Alleviates Walking Deficits and Improves Recovery of Leg Control after Spinal Cord Injury. Sci. Transl. Med. 2021, 13, eabb4422. [Google Scholar] [CrossRef]
- Ngan, C.G.Y.; Kapsa, R.M.I.; Choong, P.F.M. Strategies for Neural Control of Prosthetic Limbs: From Electrode Interfacing to 3D Printing. Materials 2019, 12, 1927. [Google Scholar] [CrossRef] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escarrat, V.; Perez-Sanchez, J.; El-Waly, B.; Collazos-Castro, J.E.; Debarbieux, F. Composite Fibrin and Carbon Microfibre Implant to Modulate Postraumatic Inflammation after Spinal Cord Injury. Cells 2023, 12, 839. https://doi.org/10.3390/cells12060839
Escarrat V, Perez-Sanchez J, El-Waly B, Collazos-Castro JE, Debarbieux F. Composite Fibrin and Carbon Microfibre Implant to Modulate Postraumatic Inflammation after Spinal Cord Injury. Cells. 2023; 12(6):839. https://doi.org/10.3390/cells12060839
Chicago/Turabian StyleEscarrat, Vincent, Jimena Perez-Sanchez, Bilal El-Waly, Jorge E. Collazos-Castro, and Franck Debarbieux. 2023. "Composite Fibrin and Carbon Microfibre Implant to Modulate Postraumatic Inflammation after Spinal Cord Injury" Cells 12, no. 6: 839. https://doi.org/10.3390/cells12060839






