cAMP Compartmentalisation in Human Myometrial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Myometrial Tissue Collection
2.2. Primary Myometrial Cell Isolation
2.3. Protein Extraction
2.4. Western Blotting
2.5. RNA Extraction
2.6. Quantitative RT-PCR
2.7. hTERT-HM Cell Seeding and Infection
2.8. HPMC Seeding and Adenoviral Infection
2.9. Passage Experiments
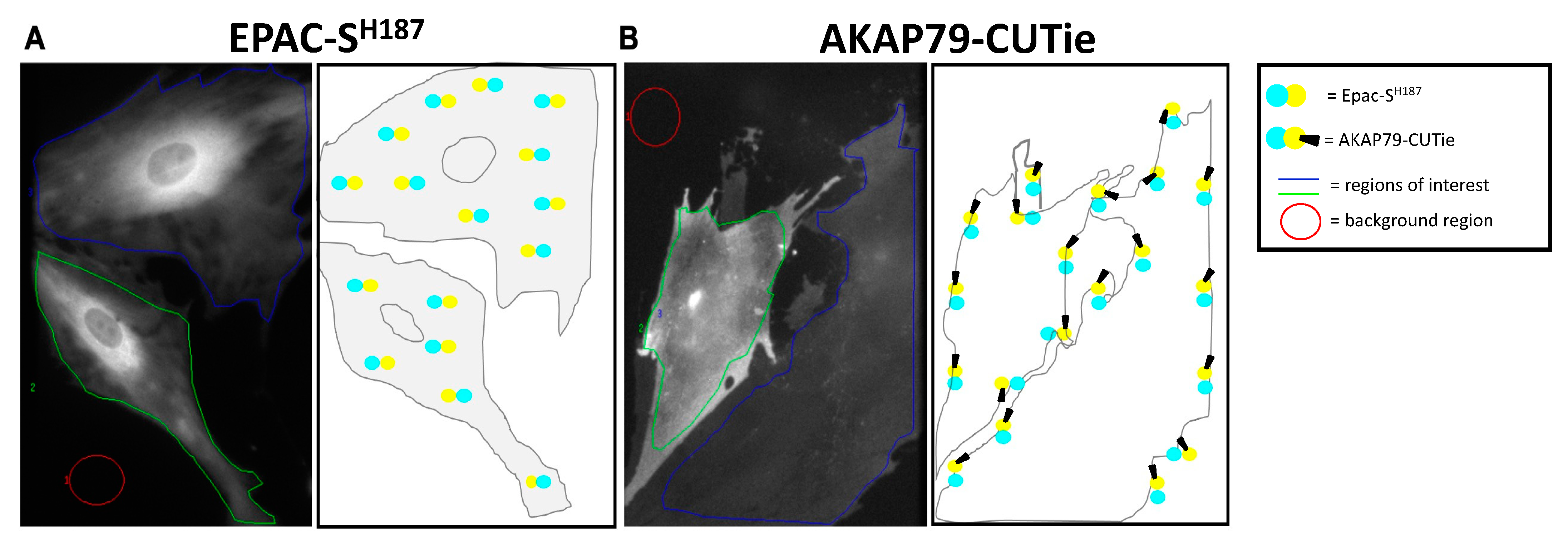
2.10. FRET Imaging
2.11. Statistical Analysis
3. Results
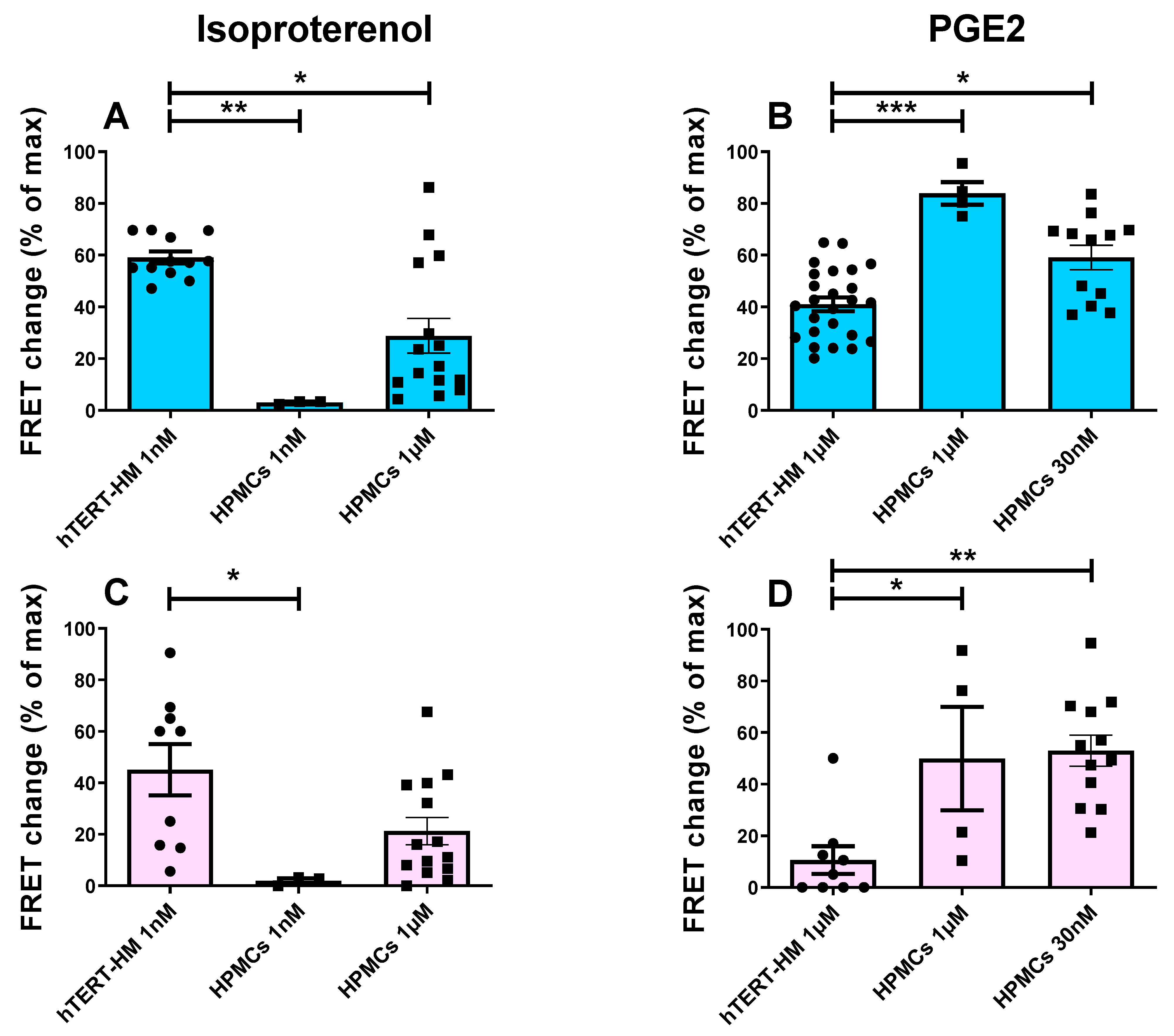
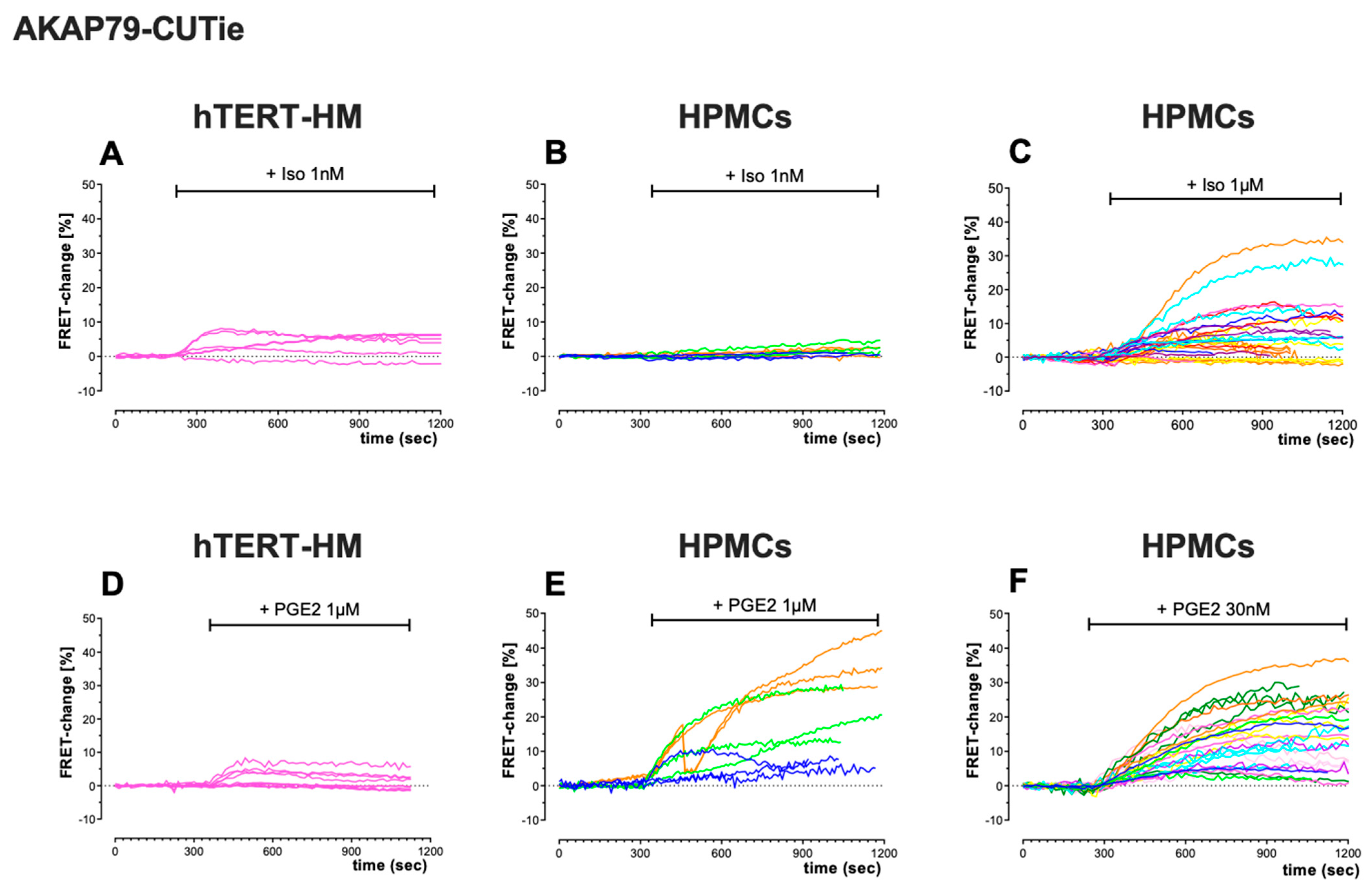
3.1. Comparison of cAMP Responses in hTERT-HM Cells and HPMCs
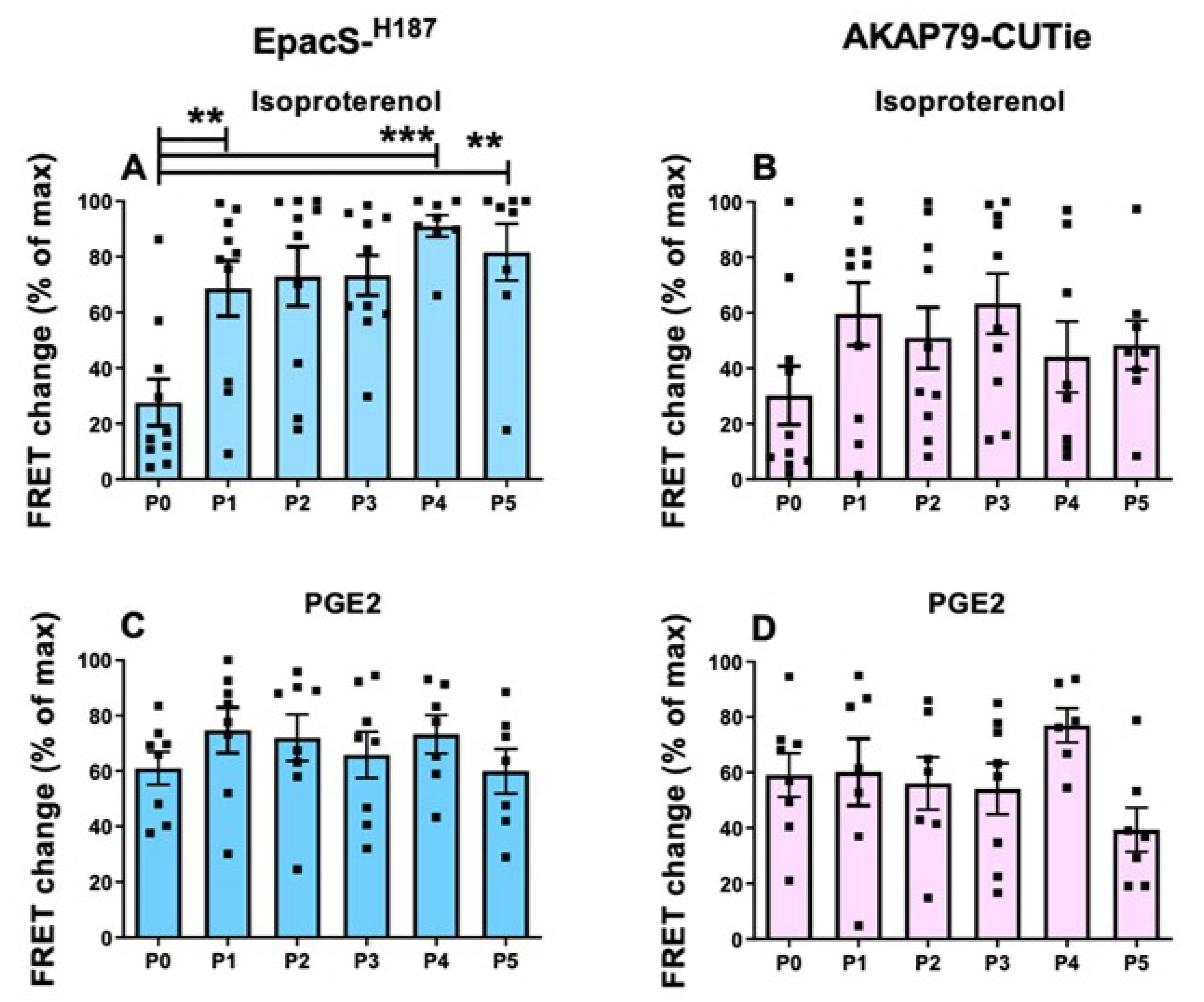
3.2. Effects of Sub-Culture on HPMC Phenotype
3.3. Variability of the cAMP Response in HPMCs from Different Donors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blencowe, H.; Lee, A.C.; Cousens, S.; Bahalim, A.; Narwal, R.; Zhong, N.; Chou, D.; Say, L.; Modi, N.; Katz, J.; et al. Preterm birth–associated neurodevelopmental impairment estimates at regional and global levels for 2010. Pediatr. Res. 2013, 74, 17–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saigal, S.; Doyle, L.W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008, 371, 261–269. [Google Scholar] [CrossRef] [PubMed]
- WHO. Preterm Birth. 2018. Available online: https://www.who.int/en/news-room/fact-sheets/detail/preterm-birth (accessed on 15 December 2021).
- Liu, L.; Oza, S.; Hogan, D.; Chu, Y.; Perin, J.; Zhu, J.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, regional, and national causes of under-5 mortality in 2000-15: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016, 388, 3027–3035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walani, S.R. Global burden of preterm birth. Int. J. Gynecol. Obstet. 2020, 150, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Yulia, A.; Singh, N.; Lei, K.; Sooranna, S.R.; Johnson, M.R. Cyclic AMP Effectors Regulate Myometrial Oxytocin Receptor Expression. Endocrinology 2016, 157, 4411–4422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodge, K.L.; Carr, D.W.; Yue, C.; Sanborn, B.M. A Role for AKAP (A Kinase Anchoring Protein) Scaffolding in the Loss of a Cyclic Adenosine 3′,5′-Monophosphate Inhibitory Response in Late Pregnant Rat Myometrium. Mol. Endocrinol. 1999, 13, 1977–1987. [Google Scholar]
- MacDougall, M.W.; Europe-Finner, G.N.; Robson, S.C. Human myometrial quiescence and activation during gestation and parturition involve dramatic changes in expression and activity of particulate type II (RII alpha) protein kinase A holoenzyme. J. Clin. Endocrinol. Metab. 2003, 88, 2194–2205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chun-Ying, K.; Word, R.A.; Barbara, M.S. Differential Expression of Protein Kinase A, AKAP 79, and PP2B in Pregnant Human Myometrial Membranes Prior to and During Labor. J. Soc. Gynecol. Investig. 2005, 12, 421–427. [Google Scholar]
- Kofinas, A.D.; Rose, J.C.; Meis, P.J. Changes in cyclic adenosine monophosphate-phosphodiesterase activity in nonpregnant and pregnant human myometrium. Am. J. Obstet. Gynecol. 1987, 157, 733–738. [Google Scholar] [CrossRef]
- Méhats, C.; Tanguy, G.; Paris, B.; Robert, B.; Pernin, N.; Ferré, F.; Leroy, M.J. Pregnancy induces a modulation of the cAMP phosphodiesterase 4-conformers ratio in human myometrium: Consequences for the utero-relaxant effect of PDE4-selective inhibitors. J. Pharmacol. Exp. Ther. 2000, 292, 817–823. [Google Scholar]
- Li, J.K.; Lai, P.F.; Tribe, R.M.; Johnson, M.R. Transcription factors regulated by cAMP in smooth muscle of the myometrium at human parturition. Biochem. Soc. Trans. 2021, 49, 997–1011. [Google Scholar] [CrossRef] [PubMed]
- Yulia, A.; Varley, A.J.; Singh, N.; Lei, K.; Tribe, R.; Johnson, M.R. Changes in cAMP effector predominance are associated with increased oxytocin receptor expression in twin but not infection-associated or idiopathic preterm labour. PLoS ONE 2020, 15, e0240325. [Google Scholar] [CrossRef] [PubMed]
- Zaccolo, M.; Zerio, A.; Lobo, M.J. Subcellular Organization of the cAMP Signaling Pathway. Pharmacol. Rev. 2021, 73, 278–309. [Google Scholar] [CrossRef] [PubMed]
- DiPilato, L.M.; Cheng, X.; Zhang, J. Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc. Natl. Acad. Sci. USA 2004, 101, 16513–16518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefkimmiatis, K.; Zaccolo, M. cAMP signaling in subcellular compartments. Pharmacol. Ther. 2014, 143, 295–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koschinski, A.; Zaccolo, M. Activation of PKA in cell requires higher concentration of cAMP than in vitro: Implications for compartmentalization of cAMP signalling. Sci. Rep. 2017, 7, 14090. [Google Scholar] [CrossRef] [Green Version]
- Anton, S.E.; Kayser, C.; Maiellaro, I.; Nemec, K.; Möller, J.; Koschinski, A.; Zaccolo, M.; Annibale, P.; Falcke, M.; Lohse, M.J.; et al. Receptor-associated independent cAMP nanodomains mediate spatiotemporal specificity of GPCR signaling. Cell 2022, 185, 1130–1142.e11. [Google Scholar] [CrossRef]
- Lobo, M.J.; Amaral, M.D.; Zaccolo, M.; Farinha, C.M. EPAC1 activation by cAMP stabilizes CFTR at the membrane by promoting its interaction with NHERF1. J. Cell Sci. 2016, 129, 2599–2612. [Google Scholar] [CrossRef] [Green Version]
- Zaccolo, M. Spatial control of cAMP signalling in health and disease. Curr. Opin. Pharmacol. 2011, 11, 649–655. [Google Scholar] [CrossRef] [Green Version]
- Nikolaev, V.O.; Moshkov, A.; Lyon, A.R.; Miragoli, M.; Novak, P.; Paur, H.; Lohse, M.J.; Korchev, Y.E.; Harding, S.E.; Gorelik, J. Beta2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science 2010, 327, 1653–1657. [Google Scholar] [CrossRef]
- Aye, T.-T.; Soni, S.; van Veen, T.A.; van der Heyden, M.A.; Cappadona, S.; Varro, A.; de Weger, R.A.; de Jonge, N.; Vos, M.A.; Heck, A.J.; et al. Reorganized PKA-AKAP associations in the failing human heart. J. Mol. Cell. Cardiol. 2012, 52, 511–518. [Google Scholar] [CrossRef]
- Omar, M.H.; Byrne, D.P.; Jones, K.N.; Lakey, T.M.; Collins, K.B.; Lee, K.-S.; Daly, L.A.; Forbush, K.A.; Lau, H.-T.; Golkowski, M.; et al. Mislocalization of protein kinase A drives pathology in Cushing’s syndrome. Cell Rep. 2022, 40, 111073. [Google Scholar] [CrossRef]
- Brescia, M.; Zaccolo, M. Modulation of Compartmentalised Cyclic Nucleotide Signalling via Local Inhibition of Phosphodiesterase Activity. Int. J. Mol. Sci. 2016, 17, 1672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baillie, G.S.; Tejeda, G.S.; Kelly, M.P. Therapeutic targeting of 3′,5′-cyclic nucleotide phosphodiesterases: Inhibition and beyond. Nat. Rev. Drug Discov. 2019, 18, 770–796. [Google Scholar] [CrossRef]
- Canadian Preterm Labor Investigators Group. Treatment of Preterm Labor with the Beta-Adrenergic Agonist Ritodrine. New Engl. J. Med. 1992, 327, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Coler, B.; Shynlova, O.; Boros-Rausch, A.; Lye, S.; McCartney, S.; Leimert, K.; Xu, W.; Chemtob, S.; Olson, D.; Li, M.; et al. Landscape of Preterm Birth Therapeutics and a Path Forward. J. Clin. Med. 2021, 10, 2912. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, L.; Shynlova, O.; Matysiak-Zablocki, E.; Mesiano, S.; Dong, X.; Lye, S. Molecular evidence of functional progesterone withdrawal in human myometrium. Nat. Commun. 2016, 7, 11565. [Google Scholar] [CrossRef] [Green Version]
- Peters, G.A.; Yi, L.; Skomorovska-Prokvolit, Y.; Patel, B.; Amini, P.; Tan, H.; Mesiano, S. Inflammatory Stimuli Increase Progesterone Receptor-A Stability and Transrepressive Activity in Myometrial Cells. Endocrinology 2017, 158, 158–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadeem, L.; Shynlova, O.; Mesiano, S.; Lye, S. Progesterone Via its Type-A Receptor Promotes Myometrial Gap Junction Coupling. Sci. Rep. 2017, 7, 13357. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.; Yi, L.; Rote, N.S.; Hurd, W.W.; Mesiano, S. Progesterone Receptor-A and -B Have Opposite Effects on Proinflammatory Gene Expression in Human Myometrial Cells: Implications for Progesterone Actions in Human Pregnancy and Parturition. J. Clin. Endocrinol. Metab. 2012, 97, E719–E730. [Google Scholar] [CrossRef] [Green Version]
- Klarenbeek, J.; Goedhart, J.; van Batenburg, A.; Groenewald, D.; Jalink, K. Fourth-Generation Epac-Based FRET Sensors for cAMP Feature Exceptional Brightness, Photostability and Dynamic Range: Characterization of Dedicated Sensors for FLIM, for Ratiometry and with High Affinity. PLoS ONE 2015, 10, e0122513. [Google Scholar] [CrossRef] [Green Version]
- Surdo, N.C.; Berrera, M.; Koschinski, A.; Brescia, M.; Machado, M.R.; Carr, C.; Wright, P.; Gorelik, J.; Morotti, S.; Grandi, E.; et al. FRET biosensor uncovers cAMP nano-domains at β-adrenergic targets that dictate precise tuning of cardiac contractility. Nat. Commun. 2017, 8, 15031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stangherlin, A.; Gesellchen, F.; Zoccarato, A.; Terrin, A.; Fields, L.A.; Berrera, M.; Surdo, N.C.; Craig, M.A.; Smith, G.; Hamilton, G.; et al. cGMP Signals Modulate cAMP Levels in a Compartment-Specific Manner to Regulate Catecholamine-Dependent Signaling in Cardiac Myocytes. Circ. Res. 2011, 108, 929–939. [Google Scholar] [CrossRef] [Green Version]
- Rouget, C.; Bardou, M.; Breuiller-Fouché, M.; Loustalot, C.; Qi, H.; Naline, E.; Croci, T.; Cabrol, D.; Advenier, C.; Leroy, M.J. β3-Adrenoceptor Is the Predominant β-Adrenoceptor Subtype in Human Myometrium and Its Expression Is Up-Regulated in Pregnancy. J. Clin. Endocrinol. Metab. 2005, 90, 1644–1650. [Google Scholar] [CrossRef] [Green Version]
- Slater, D.M.; Astle, S.; Woodcock, N.; Chivers, J.E.; de Wit, N.C.; Thornton, S.; Vatish, M.; Newton, R. Anti-inflammatory and relaxatory effects of prostaglandin E2 in myometrial smooth muscle. Mol. Hum. Reprod. 2006, 12, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Sooranna, S.; Engineer, N.; Liang, Z.; Bennett, P.; Johnson, M. Stretch and interleukin 1 beta: Pro-labour factors with similar mitogen-activated protein kinase effects but differential patterns of transcription factor activation and gene expression. J. Cell. Physiol. 2007, 212, 195–206. [Google Scholar] [CrossRef]
- Chin-Smith, E.C.; Willey, F.R.; Slater, D.M.; Taggart, M.J.; Tribe, R.M. Nuclear factor of activated T-cell isoform expression and regulation in human myometrium. Reprod. Biol. Endocrinol. 2015, 13, 83. [Google Scholar] [CrossRef] [Green Version]
- Wakle-Prabagaran, M.; Lorca, R.A.; Ma, X.; Stamnes, S.J.; Amazu, C.; Hsiao, J.J.; Karch, C.M.; Hyrc, K.L.; Wright, M.E.; England, S.K. BKCa channel regulates calcium oscillations induced by alpha-2-macroglobulin in human myometrial smooth muscle cells. Proc. Natl. Acad. Sci. USA 2016, 113, E2335–E2344. [Google Scholar] [CrossRef] [Green Version]
- Mosher, A.A.; Rainey, K.J.; Bolstad, S.S.; Lye, S.J.; Mitchell, B.F.; Olson, D.M.; Wood, S.L.; Slater, D.M. Development and validation of primary human myometrial cell culture models to study pregnancy and labour. BMC Pregnancy Childbirth 2013, 13, S7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaitseva, M.; Vollenhoven, B.J.; Rogers, P.A. In vitro culture significantly alters gene expression profiles and reduces differences between myometrial and fibroid smooth muscle cells. Mol. Hum. Reprod. 2006, 12, 187–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Europe-Finner, G.N.; Phaneuf, S.; Watson, S.P.; López Bernal, A. Identification and expression of G-proteins in human myometrium: Up-regulation of G alpha s in pregnancy. Endocrinology 1993, 132, 2484–2490. [Google Scholar] [CrossRef] [PubMed]
- Tyson, E.K.; MacIntyre, D.A.; Smith, R.; Chan, E.-C.; Read, M. Evidence that a Protein Kinase A Substrate, Small Heat-Shock Protein 20, Modulates Myometrial Relaxation in Human Pregnancy. Endocrinology 2008, 149, 6157–6165. [Google Scholar] [CrossRef] [Green Version]
- Guerra, D.D.; Bok, R.; Hurt, K.J. Cyclic nucleotides and myometrial contractility. Curr. Opin. Physiol. 2020, 13, 102–107. [Google Scholar] [CrossRef]
- Europe-Finner, G.; Phaneuf, S.; Tolkovsky, A.M.; Watson, S.P.; López Bernal, A. Down-regulation of Gαs in human myometrium in term and preterm labor: A mechanism for parturition. J. Clin. Endocrinol. Metab. 1995, 79, 1835–1839. [Google Scholar]
- Sanborn, B.M.; Yue, C.; Wang, W.; Dodge, K.L. G protein signalling pathways in myometrium: Affecting the balance between contraction and relaxation. Rev. Reprod. 1998, 3, 196–205. [Google Scholar] [CrossRef]
- Zaccolo, M.; De Giorgi, F.; Cho, C.Y.; Feng, L.; Knapp, T.; Negulescu, P.A.; Taylor, S.S.; Tsien, R.Y.; Pozzan, T. A genetically encoded, fluorescent indicator for cyclic AMP in living cells. Nature 2000, 2, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Regan, J.W. EP2 and EP4 prostanoid receptor signaling. Life Sci. 2003, 74, 143–153. [Google Scholar] [CrossRef]
- Bastien, L.; Sawyer, N.; Grygorczyk, R.; Metters, K.M.; Adam, M. Cloning, functional expression, and characterization of the human prostaglandin E2 receptor EP2 subtype. J. Biol. Chem. 1994, 269, 11873–11877. [Google Scholar] [CrossRef]
- Funk, C.D.; Furci, L.; A Fitzgerald, G.; Grygorczyk, R.; Rochette, C.; A Bayne, M.; Abramovitz, M.; Adam, M.; Metters, K.M. Cloning and expression of a cDNA for the human prostaglandin E receptor EP1 subtype. J. Biol. Chem. 1994, 268, 26767–26772. [Google Scholar] [CrossRef]
- Kotani, M.; Tanaka, I.; Ogawa, Y.; Suganami, T.; Matsumoto, T.; Muro, S.; Yamamoto, Y.; Sugawara, A.; Yoshimasa, Y.; Sagawa, N.; et al. Multiple signal transduction pathways through two prostaglandin E receptor EP3 subtype isoforms expressed in human uterus. J. Clin. Endocrinol. Metab. 2000, 85, 4315–4322. [Google Scholar] [CrossRef]
- Asbóth, G.; Phaneuf, S.; López Bernal, A.L. Prostaglandin E receptors in myometrial cells. Acta Physiol. Hung. 1997, 85, 39–50. [Google Scholar] [PubMed]
- Condon, J.; Yin, S.; Mayhew, B.; Word, R.A.; Wright, W.E.; Shay, J.W.; Rainey, W.E. Telomerase Immortalization of Human Myometrial Cells. Biol. Reprod. 2002, 67, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Duckworth, N.; Marshall, K.; Clayton, J.K. An investigation of the effect of the prostaglandin EP2 receptor agonist, butaprost, on the human isolated myometrium from pregnant and non-pregnant women. J. Endocrinol. 2002, 172, 263–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astle, S.; Thornton, S.; Slater, D.M. Identification and localization of prostaglandin E2 receptors in upper and lower segment human myometrium during pregnancy. Mol. Hum. Reprod. 2005, 11, 279–287. [Google Scholar] [CrossRef]
- Popat, A.; Crankshaw, D.J. Variable responses to prostaglandin E2 in human non-pregnant myometrium. Eur. J. Pharmacol. 2001, 416, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Chanrachakul, B.; Matharoo-Ball, B.; Turner, A.; Robinson, G.; Broughton-Pipkin, F.; Arulkumaran, S.; Khan, R.N. Reduced Expression of Immunoreactive β2-Adrenergic Receptor Protein in Human Myometrium with Labor. J. Clin. Endocrinol. Metab. 2003, 88, 4997–5001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakakibara, T.; Inoue, Y.; Uzue, S.; Tsukamoto, T.; Kobayashi, M.; Kojima, M.; Akahane, M.; Kitamura, K.; Kawarabayashi, T. Diversity of inhibitorsy responses to beta2-stimulants shown by term-pregnant human myometria in vitro is partly due to differences in receptor density. Am. J. Obstet. Gynecol. 2002, 186, 997–1004. [Google Scholar] [CrossRef]
- Story, M.E.; Hall, S.; Ziccone, S.P.; Paull, J.D. Effects of adrenaline, isoprenaline and forskolin on pregnant human myometrial preparations. Clin. Exp. Pharmacol. Physiol. 1988, 15, 703–713. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Nwosu, U.C.; Rice, P.J. Relaxation of isolated human myometrial muscle by β2-adrenergic receptors but not β1-adrenergic receptors. Am. J. Obstet. Gynecol. 1998, 179, 895–898. [Google Scholar] [CrossRef]
- MacKenzie, S.J.; Baillie, G.S.; McPhee, I.; MacKenzie, C.; Seamons, R.; McSorley, T.; Millen, J.; Beard, M.B.; van Heeke, G.; Houslay, M.D. Long PDE4 cAMP specific phosphodiesterases are activated by protein kinase A-mediated phosphorylation of a single serine residue in Upstream Conserved Region 1 (UCR1). Br. J. Pharmacol. 2002, 136, 421–433. [Google Scholar] [CrossRef] [Green Version]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varley, A.; Koschinski, A.; Johnson, M.R.; Zaccolo, M. cAMP Compartmentalisation in Human Myometrial Cells. Cells 2023, 12, 718. https://doi.org/10.3390/cells12050718
Varley A, Koschinski A, Johnson MR, Zaccolo M. cAMP Compartmentalisation in Human Myometrial Cells. Cells. 2023; 12(5):718. https://doi.org/10.3390/cells12050718
Chicago/Turabian StyleVarley, Alice, Andreas Koschinski, Mark R. Johnson, and Manuela Zaccolo. 2023. "cAMP Compartmentalisation in Human Myometrial Cells" Cells 12, no. 5: 718. https://doi.org/10.3390/cells12050718




