GEF-H1 Transduces FcεRI Signaling in Mast Cells to Activate RhoA and Focal Adhesion Formation during Exocytosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and GEF-H1 Knockdown
2.2. Small Molecule Inhibitors and Antibodies
2.3. RNA Isolation and qPCR
2.4. Plasmid Preparation and Transfection of RBL-2H3 Cells
2.5. Degranulation Assay
2.6. Detection of Active Rho Proteins and GEF-H1 by Pulldown Assay
2.7. Microscopy
2.8. Analysis of Focal Adhesions
2.9. Cell Size Measurement by ImageJ
2.10. Statistical Analysis
3. Results
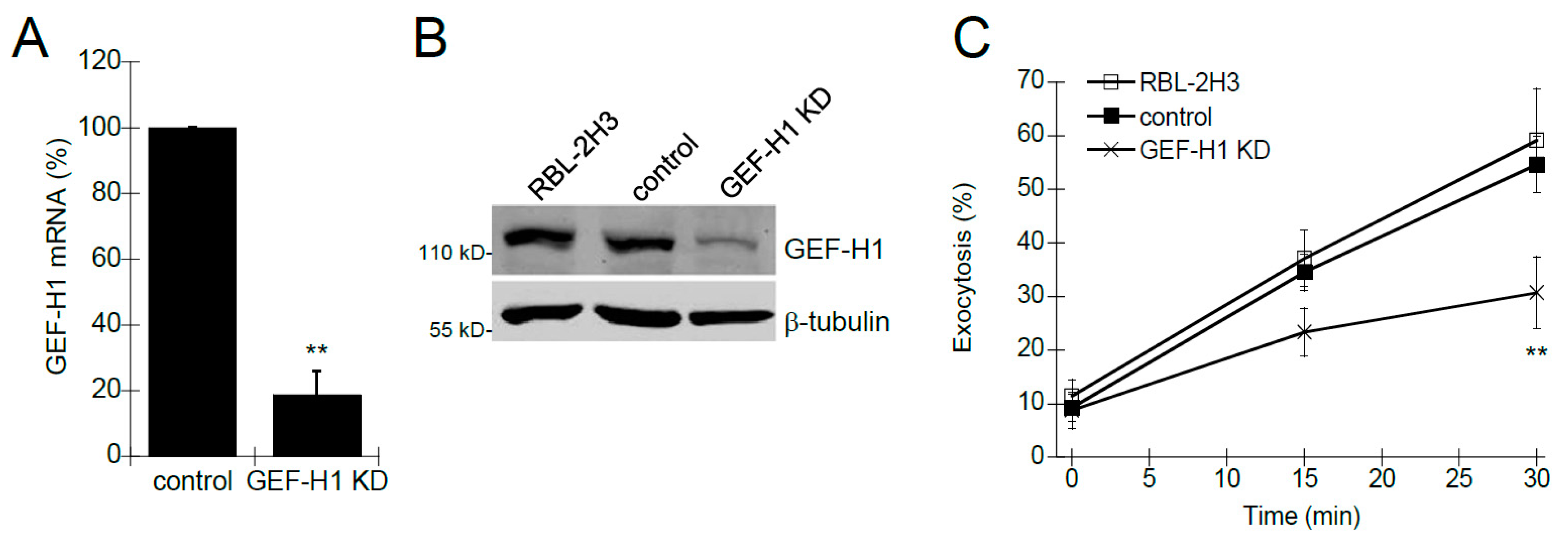
3.1. Establishment of a Role for GEF-H1 (ARHGEF2) in Mast Cell Degranulation
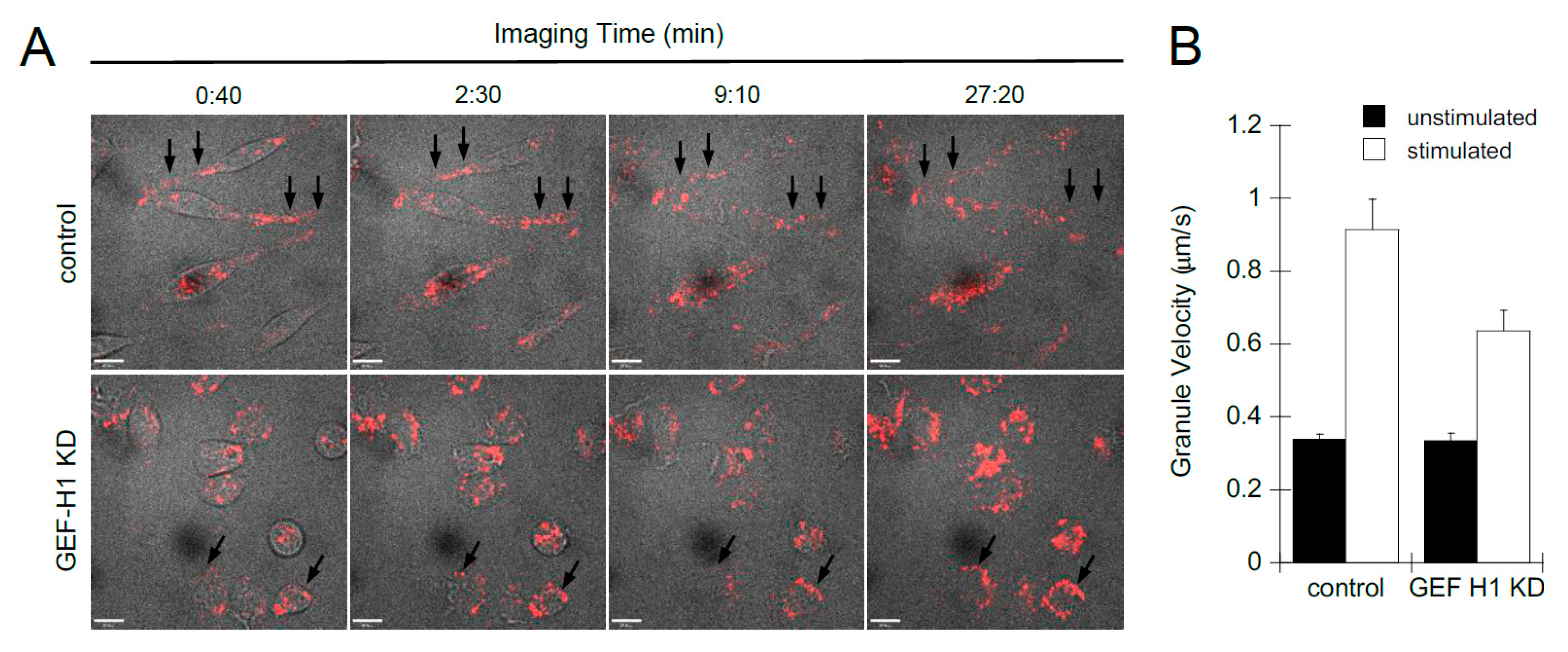
3.2. GEF-H1 Knockdown Results in Reduced Cell Activation and Granule Trafficking
3.3. RhoA, but Not Rac1, Is a Downstream Target of GEF-H1
3.4. Expression of Constitutively Active RhoA Bypasses GEF-H1
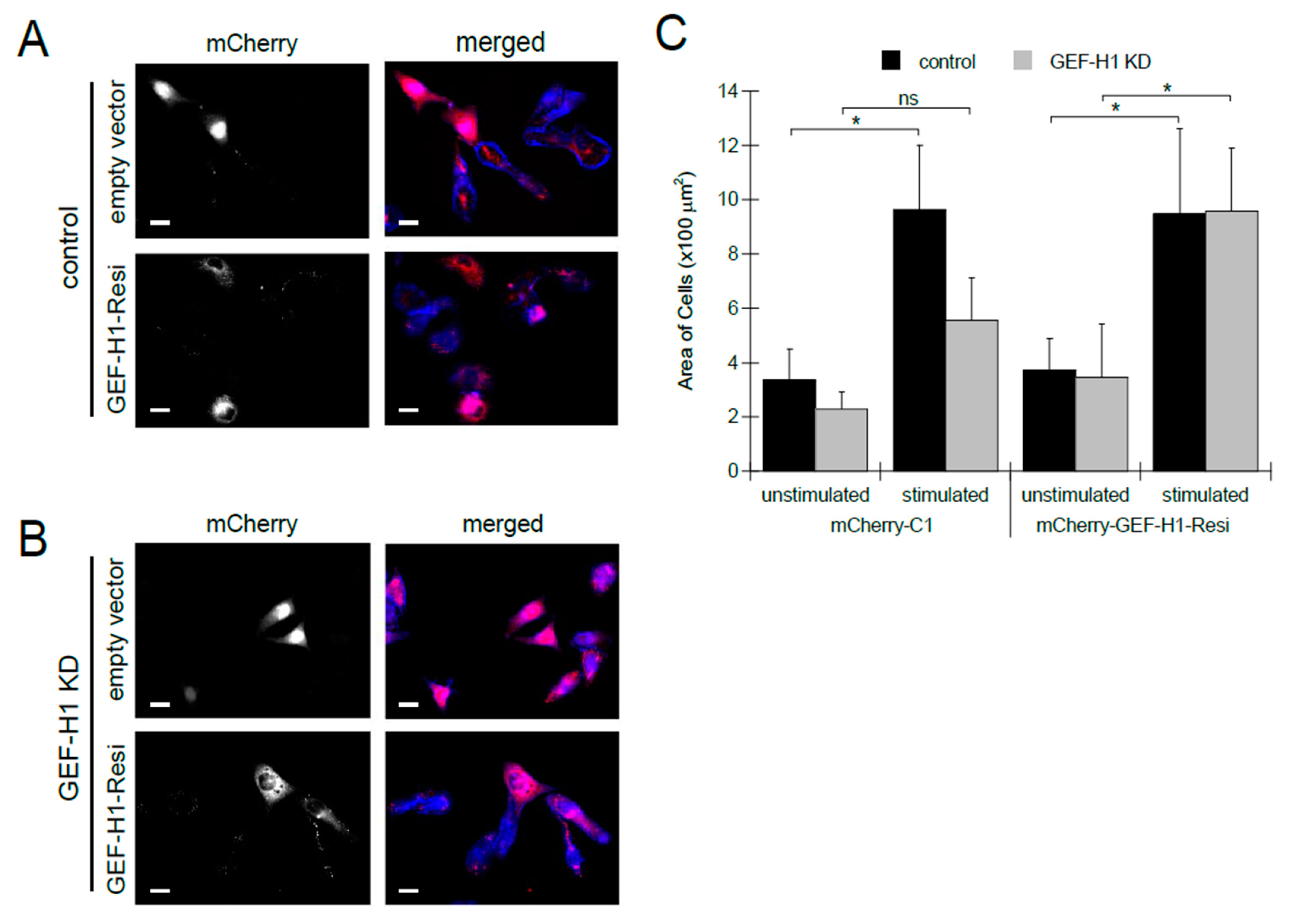
3.5. The GEF-H1-RhoA Signaling Axis Regulates Focal Adhesion (FA) Formation
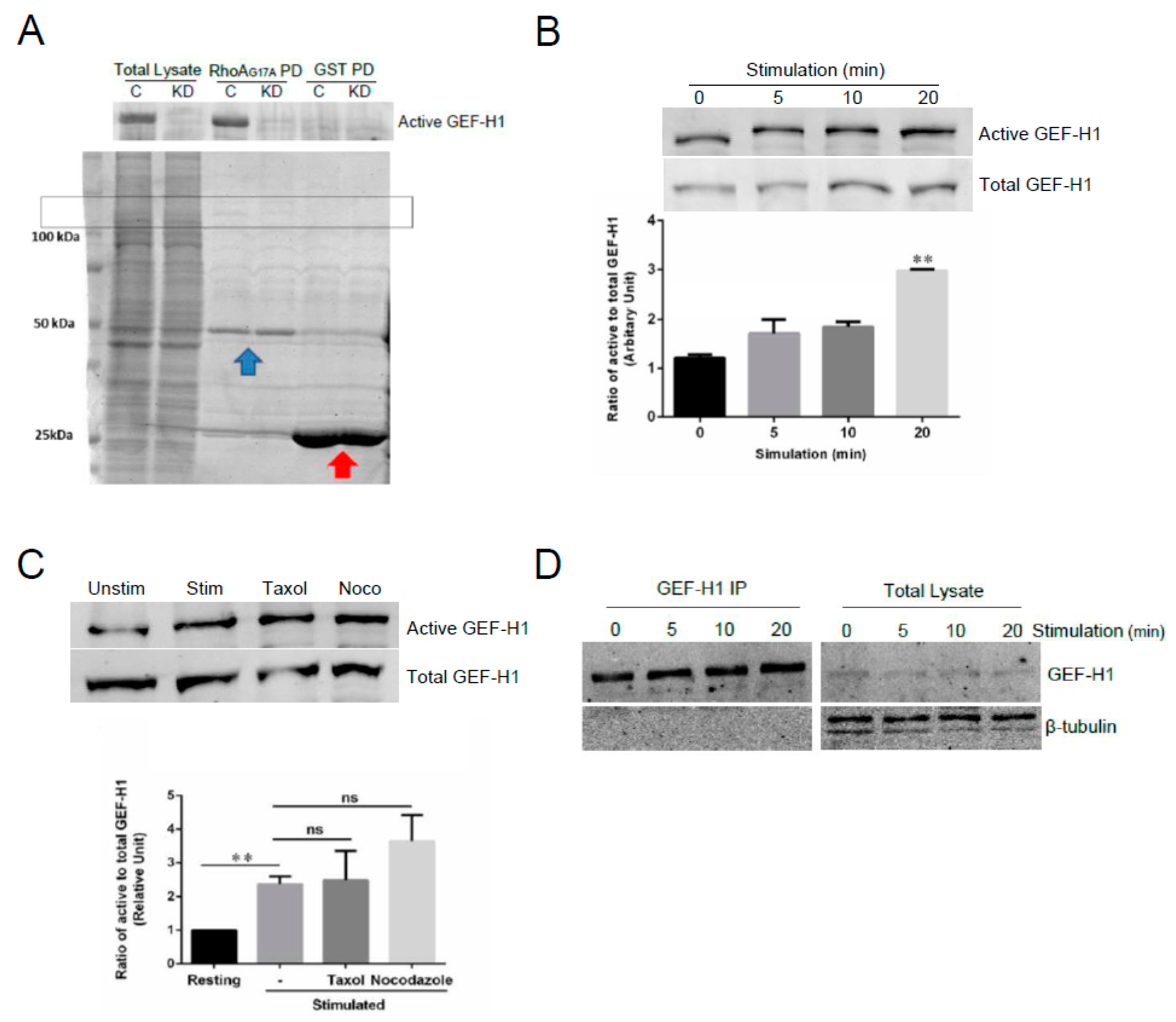
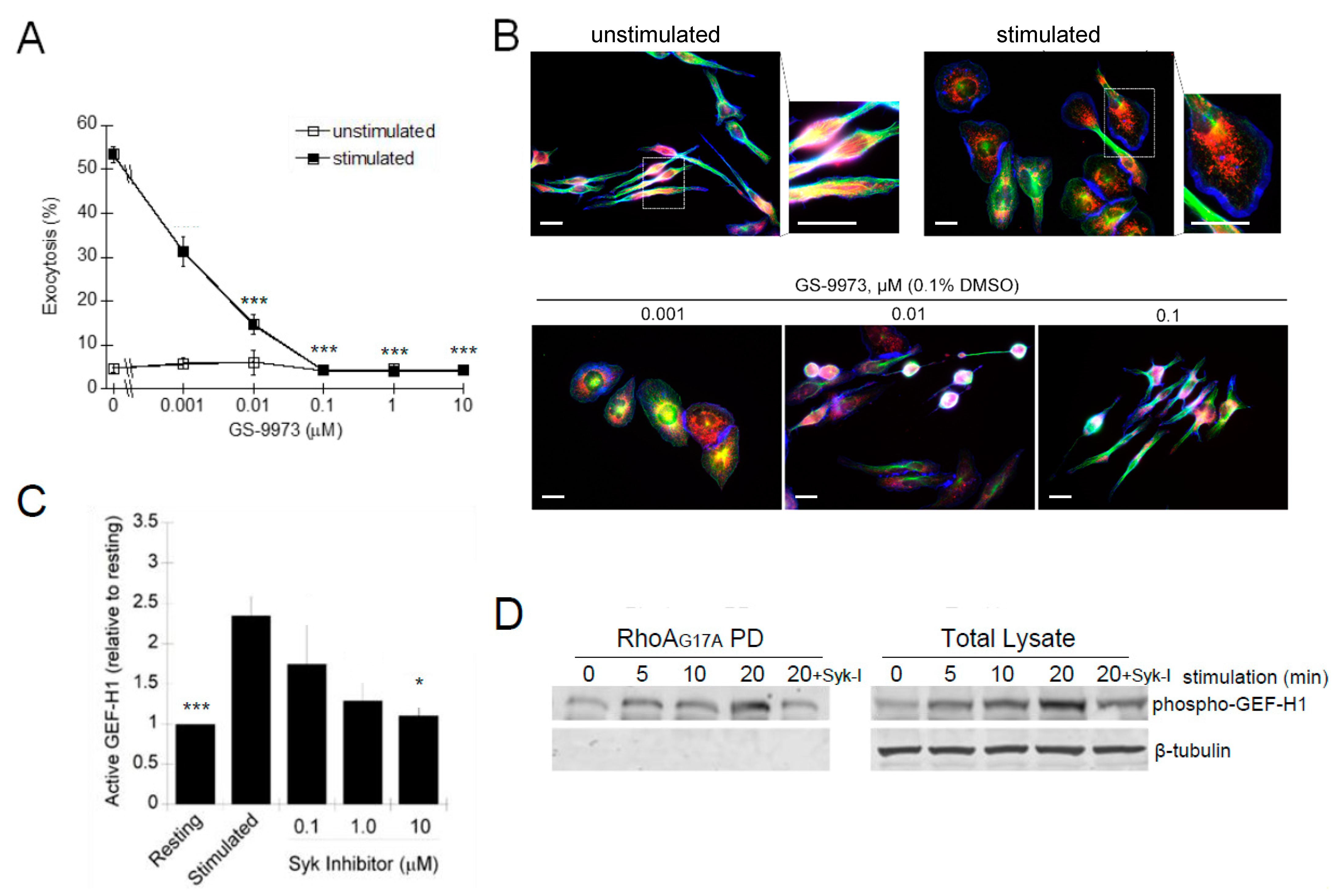
3.6. GEF-H1 Is Activated in Antigen-Stimulated Mast Cells via the FcεRI Signaling Pathway
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Da Silva, E.Z.; Jamur, M.C.; Oliver, C. Mast cell function: A new vision of an old cell. J. Histochem. Cytochem. 2014, 62, 698–738. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Blank, U.; Rivera, J. The ins and outs of IgE-dependent mast-cell exocytosis. Trends Immunol. 2004, 25, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Pribluda, V.S.; Pribluda, C.; Metzger, H. Transphosphorylation as the mechanism by which the high-affinity receptor for IgE is phosphorylated upon aggregation. Proc. Natl. Acad. Sci. USA 1994, 91, 11246–11250. [Google Scholar] [CrossRef] [PubMed]
- Parravicini, V.; Gadina, M.; Kovarova, M.; Odom, S.; González-Espinosa, C.; Furumoto, Y.; Saitoh, S.; Samelson, L.E.; O’Shea, J.J.; Rivera, J. Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat. Immunol. 2002, 3, 741–748. [Google Scholar] [CrossRef]
- Alvarez-Errico, D.; Yamashita, Y.; Suzuki, R.; Odom, S.; Furumoto, Y.; Yamashita, T.; Rivera, J. Functional analysis of Lyn kinase A and B isoforms reveals redundant and distinct roles in Fc epsilon RI-dependent mast cell activation. J. Immunol. 2010, 184, 5000–5008. [Google Scholar] [CrossRef]
- Suzuki, R.; Liu, X.; Olivera, A.; Aguiniga, L.; Yamashita, Y.; Blank, U.; Ambudkar, I.; Rivera, J. Loss of TRPC1-mediated Ca2+ influx contributes to impaired degranulation in Fyn-deficient mouse bone marrow-derived mast cells. J. Leukoc. Biol. 2010, 88, 863–875. [Google Scholar] [CrossRef]
- Brown, A.M.; O’Sullivan, A.J.; Gomperts, B.D. Induction of Exocytosis from Permeabilized Mast Cells by the Guanosine Triphosphatases Rac and Cdc42. Mol. Biol. Cell 1998, 9, 1053–1063. [Google Scholar] [CrossRef]
- Hong-Geller, E.; Cerione, R.A. Cdc42 and Rac stimulate exocytosis of secretory granules by activating the IP(3)/calcium pathway in RBL-2H3 mast cells. J. Cell Biol. 2000, 148, 481–494. [Google Scholar] [CrossRef]
- Baier, A.; Ndoh, V.N.E.; Lacy, P.; Eitzen, G. Rac1 and Rac2 control distinct events during antigen-stimulated mast cell exocytosis. J. Leukoc. Biol. 2014, 95, 763–774. [Google Scholar] [CrossRef]
- Sheshachalam, A.; Baier, A.; Eitzen, G. The effect of Rho drugs on mast cell activation and degranulation. J. Leukoc. Biol. 2017, 102, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Etienne-Manneville, S.; Hall, A. Rho GTPases in cell biology. Nature 2002, 420, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Hall, A. Guanine nucleotide exchange factors for Rho GTPases: Turning on the switch. Genes Dev. 2002, 16, 1587–1609. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.R.; Rossman, K.L.; Der, C.J. Rho guanine nucleotide exchange factors: Regulators of Rho GTPase activity in development and disease. Oncogene 2014, 33, 4021–4035. [Google Scholar] [CrossRef]
- Manetz, T.S.; Gonzalez-Espinosa, C.; Arudchandran, R.; Xirasagar, S.; Tybulewicz, V.; Rivera, J. Vav1 regulates phospholipase cgamma activation and calcium responses in mast cells. Mol. Cell Biol. 2001, 21, 3763–3774. [Google Scholar] [CrossRef]
- Chahdi, A.; Sorokin, A.; Dunn, M.J.; Landry, Y. The Rac/Cdc42 guanine nucleotide exchange factor beta1Pix enhances mastoparan-activated Gi-dependent pathway in mast cells. Biochem. Biophys. Res. Commun. 2004, 317, 384–389. [Google Scholar] [CrossRef]
- Sulimenko, V.; Hájková, Z.; Černohorská, M.; Sulimenko, T.; Sládková, V.; Dráberová, L.; Vinopal, S.; Dráberová, E.; Dráber, P. Microtubule nucleation in mouse bone marrow-derived mast cells is regulated by the concerted action of GIT1/βPIX proteins and calcium. J. Immunol. 2015, 194, 4099–4111. [Google Scholar] [CrossRef]
- Balamatsias, D.; Kong, A.M.; Waters, J.E.; Sriratana, A.; Gurung, R.; Bailey, C.G.; Rasko, J.E.; Tiganis, T.; Macaulay, S.L.; Mitchell, C.A. Identification of P-Rex1 as a Novel Rac1-Guanine Nucleotide Exchange Factor (GEF) That Promotes Actin Remodeling and GLUT4 Protein Trafficking in Adipocytes. J. Biol. Chem. 2011, 286, 43229–43240. [Google Scholar] [CrossRef]
- Qian, F.; Le Breton, G.C.; Chen, J.; Deng, J.; Christman, J.W.; Wu, D.; Ye, R.D. Role for the guanine nucleotide exchange factor phosphatidylinositol-3,4,5-trisphosphate-dependent rac exchanger 1 in platelet secretion and aggregation. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 768–777. [Google Scholar] [CrossRef]
- Ogawa, K.; Tanaka, Y.; Uruno, T.; Duan, X.; Harada, Y.; Sanematsu, F.; Yamamura, K.; Terasawa, M.; Nishikimi, A.; Côté, J.-F.; et al. DOCK5 functions as a key signaling adaptor that links FcεRI signals to microtubule dynamics during mast cell degranulation. J. Exp. Med. 2014, 211, 1407–1419. [Google Scholar] [CrossRef] [Green Version]
- Pathak, R.; Delorme-Walker, V.D.; Howell, M.C.; Anselmo, A.N.; White, M.A.; Bokoch, G.M.; DerMardirossian, C. The Microtubule-Associated Rho Activating Factor GEF-H1 Interacts with Exocyst Complex to Regulate Vesicle Traffic. Dev. Cell 2012, 23, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Sáez, J.J.; Diaz, J.; Ibañez, J.; Bozo, J.P.; Reyes, F.C.; Alamo, M.; Gobert, F.-X.; Obino, D.; Bono, M.R.; Lennon-Duménil, A.-M.; et al. The exocyst controls lysosome secretion and antigen extraction at the immune synapse of B cells. J. Cell Biol. 2019, 218, 2247–2264. [Google Scholar] [CrossRef] [PubMed]
- Passante, E.; Frankish, N. The RBL-2H3 cell line: Its provenance and suitability as a model for the mast cell. Inflamm. Res. 2009, 58, 737–745. [Google Scholar] [CrossRef]
- Kang, M.G.; Guo, Y.; Huganir, R.L. AMPA receptor and GEF-H1/Lfc complex regulates dendritic spine development through RhoA signaling cascade. Proc. Natl. Acad. Sci. USA 2009, 106, 3549–3554. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Riedl, J.; Crevenna, A.; Kessenbrock, K.; Yu, J.H.; Neukirchen, D.; Bista, M.; Bradke, F.; Jenne, D.; Holak, T.A.; Werb, Z.; et al. Lifeact: A versatile marker to visualize F-actin. Nat. Methods 2008, 5, 605–607. [Google Scholar] [CrossRef]
- Zheng, L.; Baumann, U.; Reymond, J.L. An efficient one-step site-directed and site-saturation mutagenesis protocol. Nucleic Acids Res. 2004, 32, e115. [Google Scholar] [CrossRef]
- Cohen, R.; Corwith, K.; Holowka, D.; Baird, B. Spatiotemporal resolution of mast cell granule exocytosis reveals correlation with Ca2+ wave initiation. J. Cell Sci. 2012, 125 Pt 12, 2986–2994. [Google Scholar]
- Naal, R.M.Z.; Tabb, J.; Holowka, D.; Baird, B. In situ measurement of degranulation as a biosensor based on RBL-2H3 mast cells. Biosens. Bioelectron. 2004, 20, 791–796. [Google Scholar] [CrossRef]
- García-Mata, R.; Wennerberg, K.; Arthur, W.T.; Noren, N.K.; Ellerbroek, S.M.; Burridge, K. Analysis of Activated GAPs and GEFs in Cell Lysates. Methods Enzymol. 2006, 406, 425–437. [Google Scholar] [CrossRef]
- Benard, V.; Bokoch, G.M. Assay of Cdc42, Rac, and Rho GTPase Activation by Affinity Methods. Methods Enzymol. 2002, 345, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.-D.; Schwartz, M.A. Determination of GTP loading on Rho. Methods Enzym. 2000, 325, 264–272. [Google Scholar] [CrossRef]
- Kuo, J.C.; Han, X.; Yates, J.R., 3rd; Waterman, C.M. Isolation of focal adhesion proteins for biochemical and proteomic analysis. Methods Mol. Biol. 2012, 757, 297–323. [Google Scholar] [PubMed]
- Colin-York, H.; Li, D.; Korobchevskaya, K.; Chang, V.T.; Betzig, E.; Eggeling, C.; Fritzsche, M. Cytoskeletal actin patterns shape mast cell activation. Commun. Biol. 2019, 2, 93. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.C.; Price, L.S.; Ridley, A.; Koffer, A. The small GTP-binding proteins, Rac and Rho, regulate cytoskeletal organization and exocytosis in mast cells by parallel pathways. Mol. Biol. Cell 1996, 7, 1429–1442. [Google Scholar] [CrossRef]
- Hong-Geller, E.; Holowka, D.; Siraganian, R.P.; Baird, B.; Cerione, R.A. Activated Cdc42/Rac reconstitutes Fcepsilon RI-mediated Ca2+ mobilization and degranulation in mutant RBL mast cells. Proc. Natl. Acad. Sci. USA 2001, 98, 1154–1159. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Li, R.; Zheng, Y.; Busch, H. Cloning and Characterization of GEF-H1, a Microtubule-associated Guanine Nucleotide Exchange Factor for Rac and Rho GTPases. J. Biol. Chem. 1998, 273, 34954–34960. [Google Scholar] [CrossRef] [PubMed]
- Krendel, M.; Zenke, F.T.; Bokoch, G.M. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat. Cell Biol. 2002, 4, 294–301. [Google Scholar] [CrossRef]
- Tonami, K.; Kurihara, Y.; Arima, S.; Nishiyama, K.; Uchijima, Y.; Asano, T.; Sorimachi, H.; Kurihara, H. Calpain-6, a microtubule-stabilizing protein, regulates Rac1 activity and cell motility through interaction with GEF-H1. J. Cell Sci. 2011, 124 Pt 8, 1214–1223. [Google Scholar] [CrossRef]
- Frigeri, L.; Apgar, J.R. The role of actin microfilaments in the down-regulation of the degranulation response in RBL-2H3 mast cells. J. Immunol. 1999, 162, 2243–2250. [Google Scholar] [CrossRef] [PubMed]
- Köberle, M.; Kaesler, S.; Kempf, W.; Wölbing, F.; Biedermann, T. Tetraspanins in Mast Cells. Front. Immunol. 2012, 3, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibanga, J.; Zhang, E.L.; Eitzen, G.; Guo, Y. Mast cell granule motility and exocytosis is driven by dynamic microtubule formation and kinesin-1 motor function. PLoS ONE 2022, 17, e0265122. [Google Scholar] [CrossRef]
- Fujishiro, S.H.; Tanimura, S.; Mure, S.; Kashimoto, Y.; Watanabe, K.; Kohno, M. ERK1/2 phosphorylate GEF-H1 to enhance its guanine nucleotide exchange activity toward RhoA. Biochem. Biophys. Res. Commun. 2008, 368, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Ridley, A.J.; Hall, A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 1992, 70, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Hall, A. Rho GTPases and the Actin Cytoskeleton. Science 1998, 279, 509–514. [Google Scholar] [CrossRef]
- Hamawy, M.M.; Swieter, M.; Mergenhagen, S.E.; Siraganian, R.P. Reconstitution of High Affinity IgE Receptor-mediated Secretion By Transfecting Protein Tyrosine Kinase pp125FAK. J. Biol. Chem. 1997, 272, 30498–30503. [Google Scholar] [CrossRef]
- Smith, A.J.; Pfeiffer, J.R.; Zhang, J.; Martinez, A.M.; Griffiths, G.M.; Wilson, B.S. Microtubule-dependent transport of secretory vesicles in RBL-2H3 cells. Traffic 2003, 4, 302–312. [Google Scholar] [CrossRef]
- Martin-Verdeaux, S.; Pombo, I.; Iannascoli, B.; Roa, M.; Varin-Blank, N.; Rivera, J.; Blank, U. Evidence of a role for Munc18-2 and microtubules in mast cell granule exocytosis. J. Cell Sci. 2003, 116 Pt 2, 325–334. [Google Scholar] [CrossRef]
- Nishida, K.; Yamasaki, S.; Ito, Y.; Kabu, K.; Hattori, K.; Tezuka, T.; Nishizumi, H.; Kitamura, D.; Goitsuka, R.; Geha, R.S.; et al. Fc{epsilon}RI-mediated mast cell degranulation requires calcium-independent microtubule-dependent translocation of granules to the plasma membrane. J. Cell Biol. 2005, 170, 115–126. [Google Scholar] [CrossRef]
- Birkenfeld, J.; Nalbant, P.; Yoon, S.H.; Bokoch, G.M. Cellular functions of GEF-H1, a microtubule-regulated Rho-GEF: Is altered GEF-H1 activity a crucial determinant of disease pathogenesis? Trends Cell Biol. 2008, 18, 210–219. [Google Scholar] [CrossRef]
- Siraganian, R.P.; Zhang, J.; Suzuki, K.; Sada, K. Protein tyrosine kinase Syk in mast cell signaling. Mol. Immunol. 2002, 38, 1229–1233. [Google Scholar] [CrossRef] [PubMed]
- Sandí, M.-J.; Marshall, C.B.; Balan, M.; Coyaud, É.; Zhou, M.; Monson, D.M.; Ishiyama, N.; Chandrakumar, A.A.; La Rose, J.; Couzens, A.L.; et al. MARK3-mediated phosphorylation of ARHGEF2 couples microtubules to the actin cytoskeleton to establish cell polarity. Sci. Signal. 2017, 10, eaan3286. [Google Scholar] [CrossRef]
- Azoitei, M.L.; Noh, J.; Marston, D.J.; Roudot, P.; Marshall, C.B.; Daugird, T.A.; Lisanza, S.L.; Sandí, M.-J.; Ikura, M.; Sondek, J.; et al. Spatiotemporal dynamics of GEF-H1 activation controlled by microtubule- and Src-mediated pathways. J. Cell Biol. 2019, 218, 3077–3097. [Google Scholar] [CrossRef] [PubMed]
- Birkenfeld, J.; Nalbant, P.; Bohl, B.P.; Pertz, O.; Hahn, K.M.; Bokoch, G.M. GEF-H1 modulates localized RhoA activation during cytokinesis under the control of mitotic kinases. Dev. Cell. 2007, 12, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Momboisse, F.; Ory, S.; Ceridono, M.; Calco, V.; Vitale, N.; Bader, M.-F.; Gasman, S. The Rho Guanine Nucleotide Exchange Factors Intersectin 1L and β-Pix Control Calcium-Regulated Exocytosis in Neuroendocrine PC12 Cells. Cell. Mol. Neurobiol. 2010, 30, 1327–1333. [Google Scholar] [CrossRef]
- Sun, Y.; Bamji, S.X. β-Pix modulates actin-mediated recruitment of synaptic vesicles to synapses. J. Neurosci. 2011, 31, 17123–17133. [Google Scholar] [CrossRef] [PubMed]
- Machin, P.A.; Tsonou, E.; Hornigold, D.C.; Welch, H.C.E. Rho Family GTPases and Rho GEFs in Glucose Homeostasis. Cells 2021, 10, 915. [Google Scholar] [CrossRef]
- Pathak, R.; Dermardirossian, C. GEF-H1: Orchestrating the interplay between cytoskeleton and vesicle trafficking. Small GTPases 2013, 4, 174–179. [Google Scholar] [CrossRef]
- Porat-Shliom, N.; Milberg, O.; Masedunskas, A.; Weigert, R. Multiple roles for the actin cytoskeleton during regulated exocytosis. Cell. Mol. Life Sci. 2013, 70, 2099–2121. [Google Scholar] [CrossRef]
- Synek, L.; Sekeres, J.; Žárský, V. The exocyst at the interface between cytoskeleton and membranes in eukaryotic cells. Front. Plant Sci. 2014, 4, 543. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Nishida-Fukuda, H.; Li, Y.; McDonald, W.H.; Gradinaru, C.C.; Macara, I.G. Exocyst dynamics during vesicle tethering and fusion. Nat. Commun. 2018, 9, 5140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, I.-H.; Hsiao, C.-T.; Wu, J.-C.; Shen, R.-F.; Liu, C.-Y.; Wang, Y.-K.; Chen, Y.-C.; Huang, C.-M.; del Alamo, J.C.; Chang, Z.-F.; et al. GEF-H1 controls focal adhesion signaling that regulates mesenchymal stem cell lineage commitment. J. Cell Sci. 2014, 127 Pt 19, 4186–4200. [Google Scholar] [CrossRef] [PubMed]
- Gonon, E.M.; Skalski, M.; Kean, M.; Coppolino, M.G. SNARE-mediated membrane traffic modulates RhoA-regulated focal adhesion formation. FEBS Lett. 2005, 579, 6169–6178. [Google Scholar] [CrossRef] [PubMed]
- Eisler, S.A.; Curado, F.; Link, G.; Schulz, S.; Noack, M.; Steinke, M.; Olayioye, M.A.; Hausser, A. A Rho signaling network links microtubules to PKD controlled carrier transport to focal adhesions. Elife 2018, 7, e35907. [Google Scholar] [CrossRef]
- Yu, B.; Martins, I.R.; Li, P.; Amarasinghe, G.K.; Umetani, J.; Fernandez-Zapico, M.E.; Billadeau, D.D.; Machius, M.; Tomchick, D.R.; Rosen, M.K. Structural and Energetic Mechanisms of Cooperative Autoinhibition and Activation of Vav1. Cell 2010, 140, 246–256. [Google Scholar] [CrossRef]
- Kosoff, R.; Chow, H.Y.; Radu, M.; Chernoff, J. Pak2 kinase restrains mast cell FcεRI receptor signaling through modulation of Rho protein guanine nucleotide exchange factor (GEF) activity. J. Biol. Chem. 2013, 288, 974–983. [Google Scholar] [CrossRef]
- Joo, E.; Olson, M.F. Regulation and functions of the RhoA regulatory guanine nucleotide exchange factor GEF-H1. Small GTPases 2020, 12, 358–371. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Nalbant, P.; Birkenfeld, J.; Chang, Z.-F.; Bokoch, G.M. GEF-H1 Couples Nocodazole-induced Microtubule Disassembly to Cell Contractility via RhoA. Mol. Biol. Cell 2008, 19, 2147–2153. [Google Scholar] [CrossRef]
- Kashyap, A.S.; Fernandez-Rodriguez, L.; Zhao, Y.; Monaco, G.; Trefny, M.P.; Yoshida, N.; Martin, K.; Sharma, A.; Olieric, N.; Shah, P.; et al. GEF-H1 Signaling upon Microtubule Destabilization Is Required for Dendritic Cell Activation and Specific Anti-tumor Responses. Cell Rep. 2019, 28, 3367–3380.e8. [Google Scholar] [CrossRef]
- Zenke, F.T.; Krendel, M.; DerMardirossian, C.; King, C.C.; Bohl, B.P.; Bokoch, G.M. p21-activated Kinase 1 Phosphorylates and Regulates 14-3-3 Binding to GEF-H1, a Microtubule-localized Rho Exchange Factor. J. Biol. Chem. 2004, 279, 18392–18400. [Google Scholar] [CrossRef] [Green Version]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Negre, J.; Eitzen, G. GEF-H1 Transduces FcεRI Signaling in Mast Cells to Activate RhoA and Focal Adhesion Formation during Exocytosis. Cells 2023, 12, 537. https://doi.org/10.3390/cells12040537
Guo Y, Negre J, Eitzen G. GEF-H1 Transduces FcεRI Signaling in Mast Cells to Activate RhoA and Focal Adhesion Formation during Exocytosis. Cells. 2023; 12(4):537. https://doi.org/10.3390/cells12040537
Chicago/Turabian StyleGuo, Yitian, Judeah Negre, and Gary Eitzen. 2023. "GEF-H1 Transduces FcεRI Signaling in Mast Cells to Activate RhoA and Focal Adhesion Formation during Exocytosis" Cells 12, no. 4: 537. https://doi.org/10.3390/cells12040537



