MiR-630 Promotes Radioresistance by Induction of Anti-Apoptotic Effect via Nrf2–GPX2 Molecular Axis in Head–Neck Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Plasma miR-630 Detection
2.2. Cell Line and Cell Culture
2.3. MiR-630 Mimic Oligonucleotides and Transfection
2.4. Determination of miRNA or mRNA Expressions by RT-qPCR Method
2.5. Determination of Protein Expressions by Western Blot Analysis
2.6. Assessment of Cell Proliferation and Radiosensitivity
2.7. Determination of Apoptotic Cells by Annexin V-Labeled Flow Cytometry
2.8. Determination of Double-Strand DNA Breaking Status by Fluorescent Microscopy
2.9. Determination of Intrinsic Apoptosis Associated with Mitochondrial Permeabilization
2.10. Determination of Cellular Reactive Oxygen Species (ROS) Level
2.11. Luciferase Report Assay for the Transcriptional Activity of Nrf2
3. Results
3.1. MiR-630 Is Overexpressed in Patients with HNC and Associated with Poor Prognosis
3.2. MiR-630 Has a Minimal Effect on Cell Growth but Promotes Radioresistance in HNC Cells
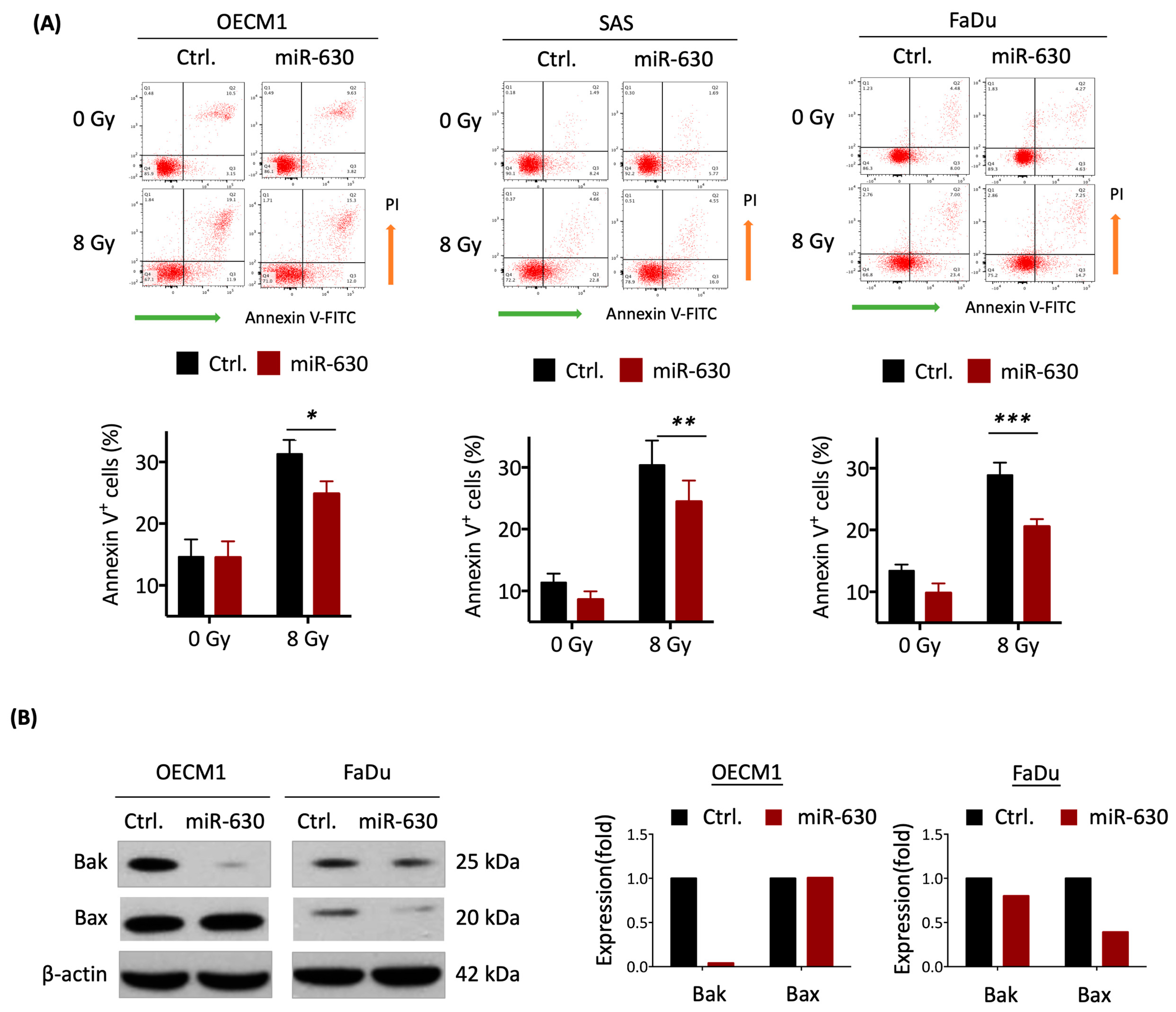
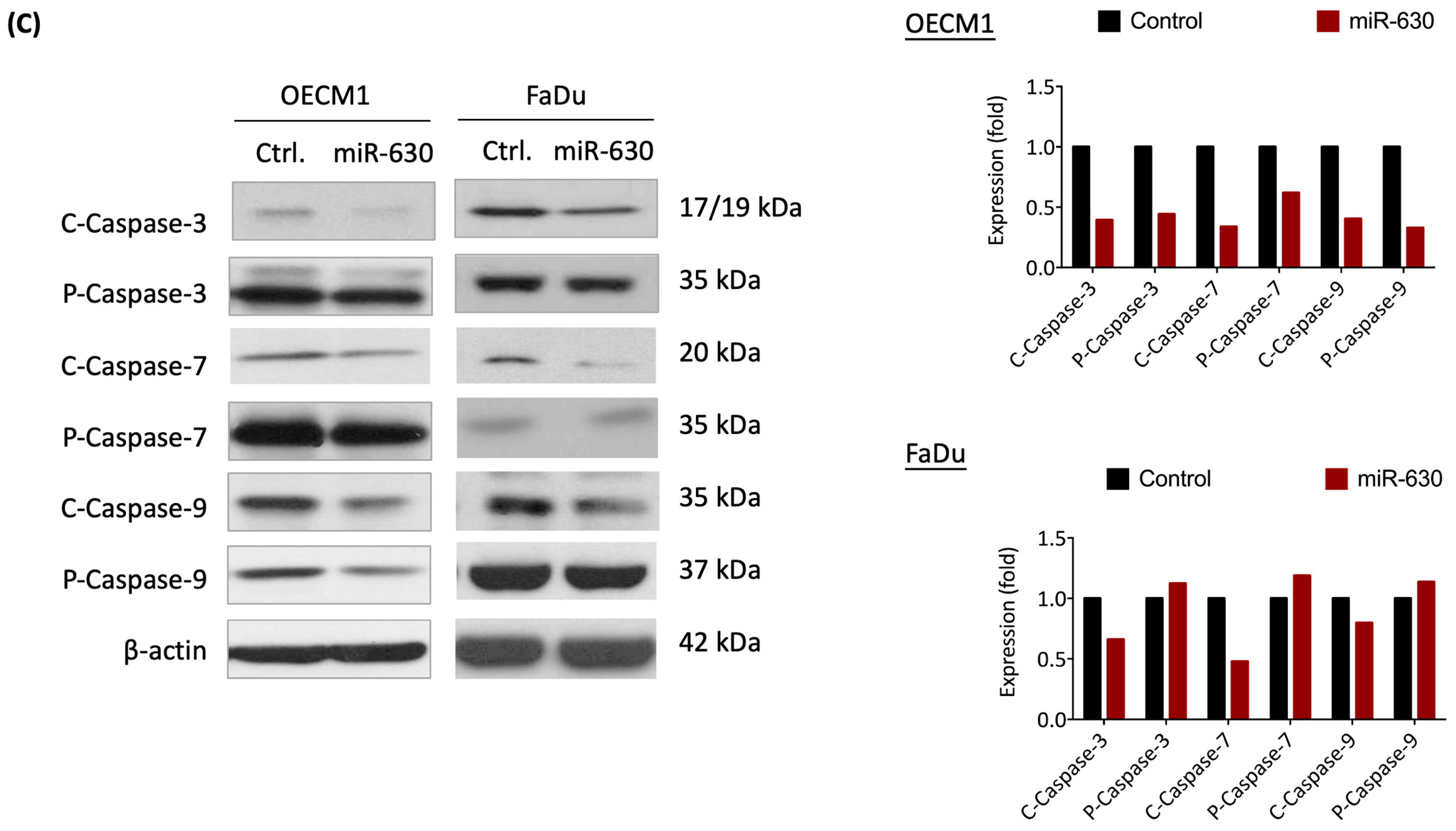
3.3. MiR-630 Suppresses Cellular Intrinsic Apoptotic Pathways
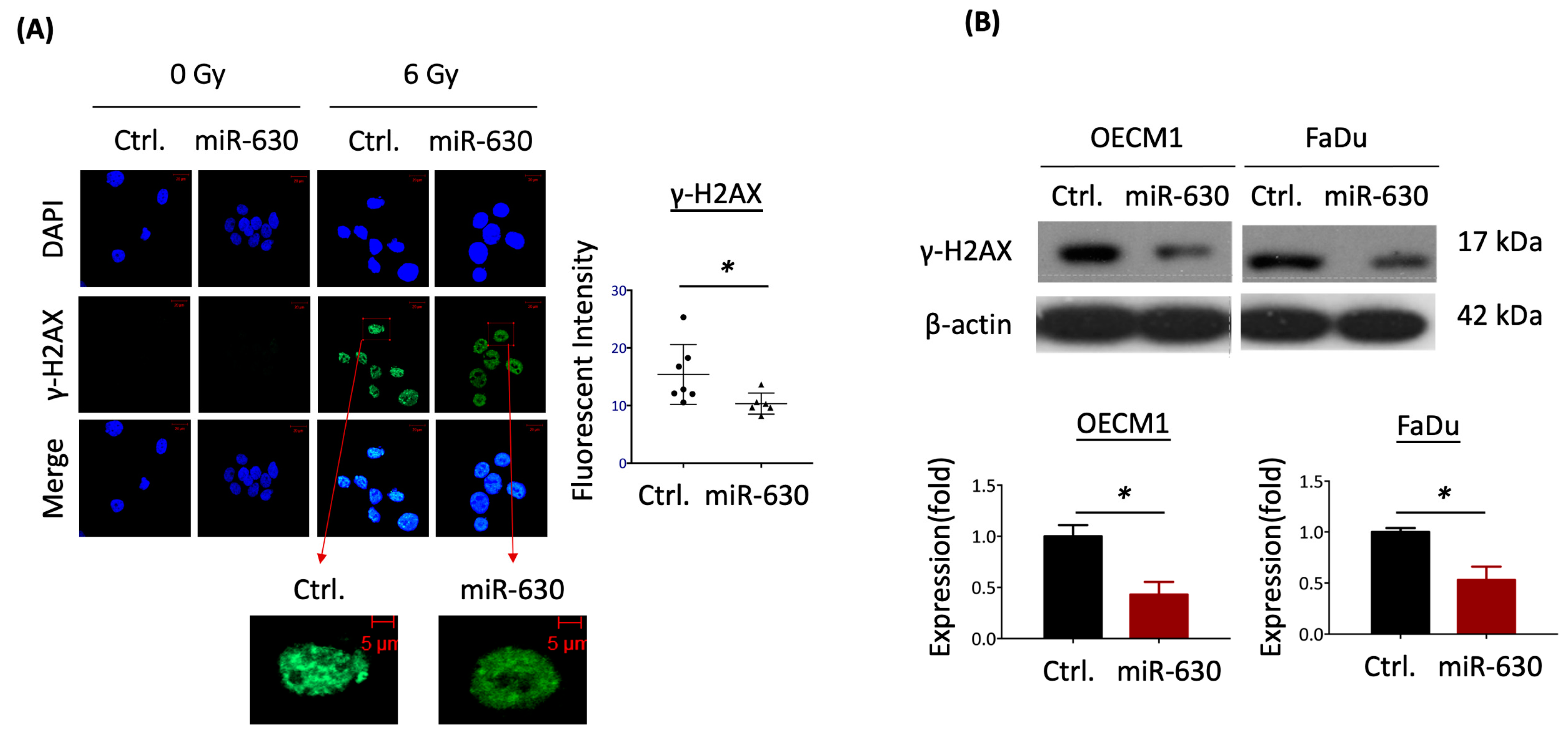
3.4. MiR-630 Mitigates IR-Induced DNA Damage in HNC Cells
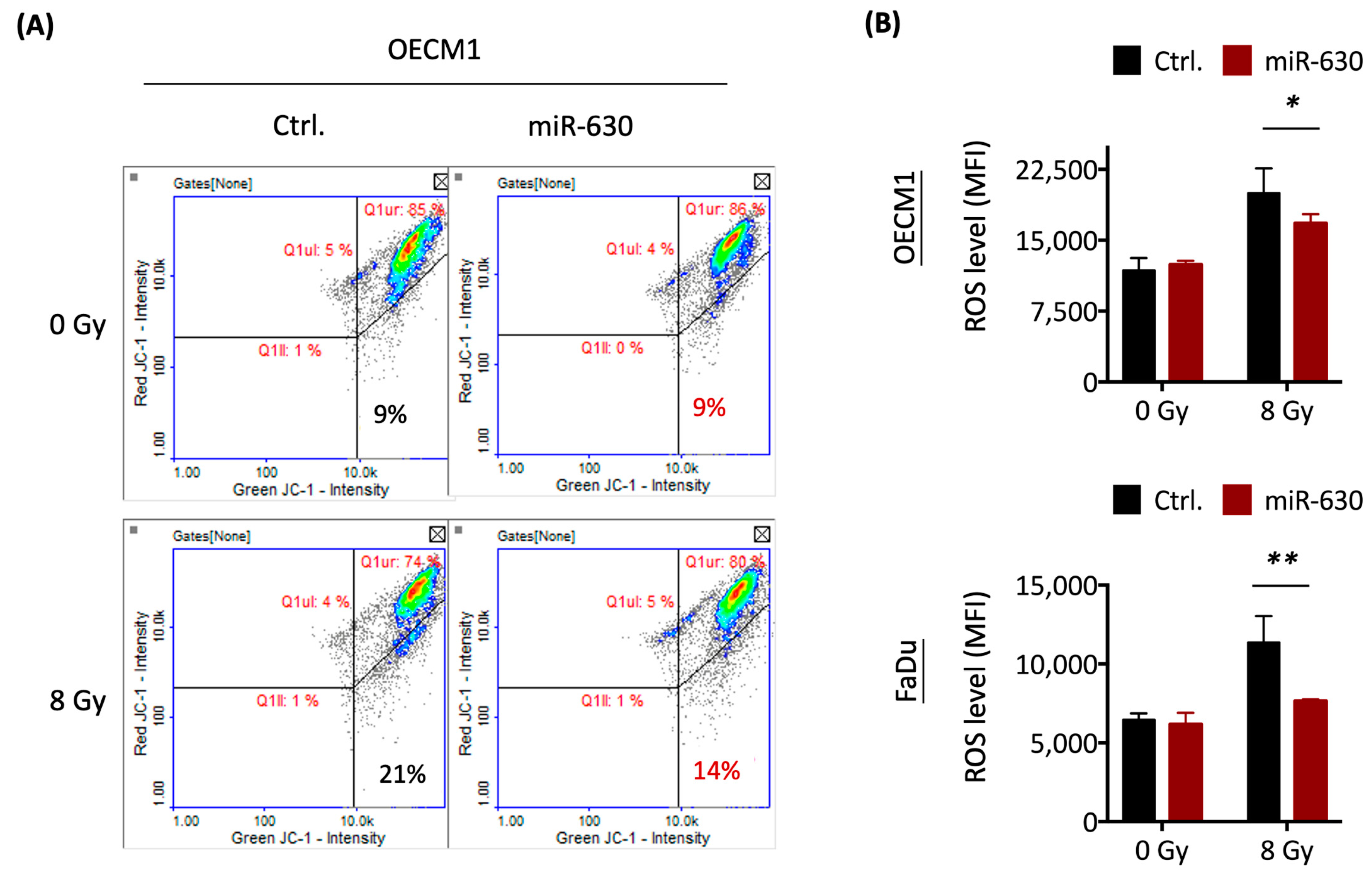
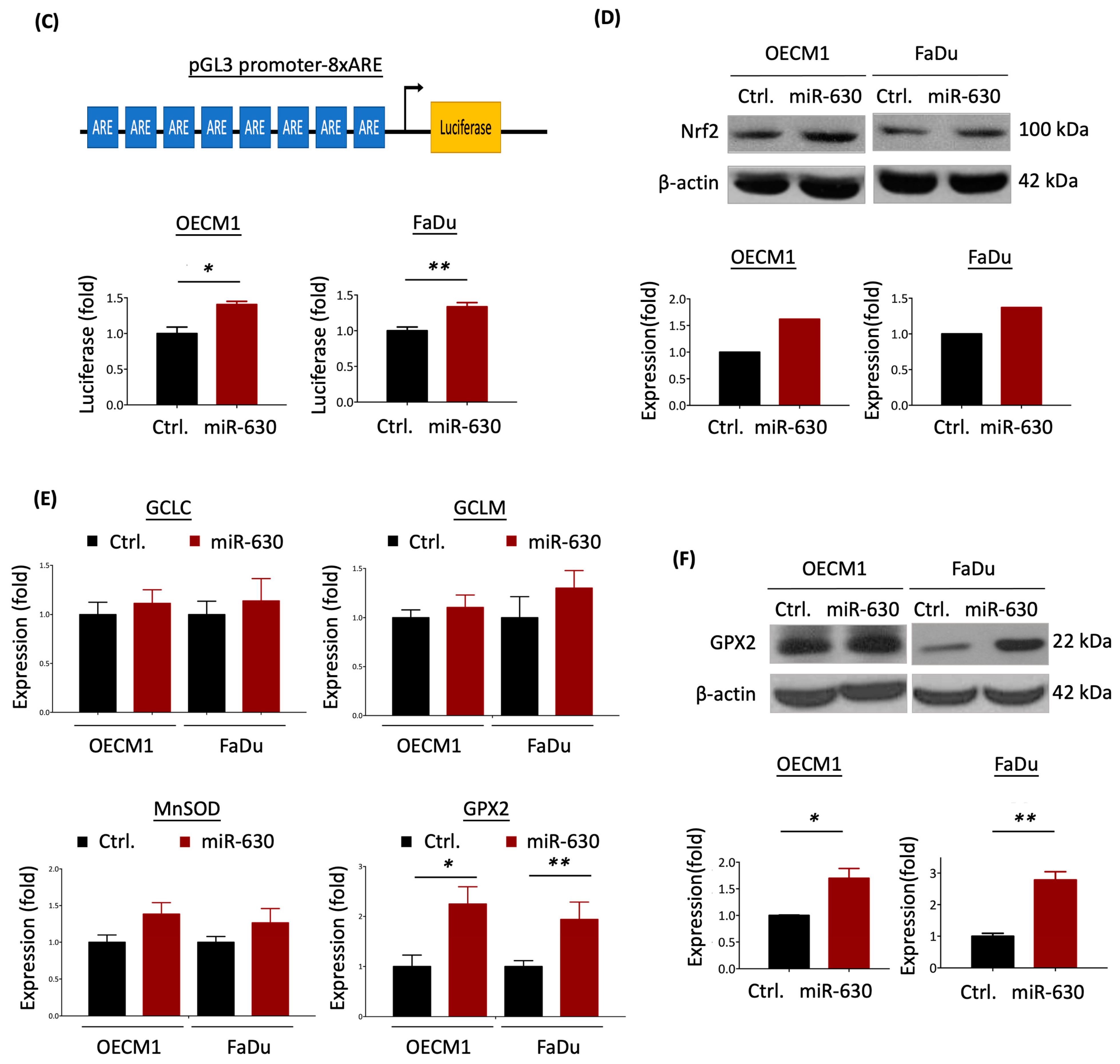
3.5. MiR-630 Attenuates Mitochondrial-Mediated ROS Level via Nrf2–GPX2 Molecular Axis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Johansen, S.; Norman, M.H.; Dale, E.; Amdal, C.D.; Furre, T.; Malinen, E.; Evensen, J.F. Patterns of local-regional recurrence after conformal and intensity-modulated radiotherapy for head and neck cancer. Radiat. Oncol. 2017, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Han, Z.; Luo, Q.; Wang, Y.; Li, Q.; Zhou, L.; Zuo, H. Radiotherapy modulates tumor cell fate decisions: A review. Radiat. Oncol. 2022, 17, 196. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.C.; Lee, W.C.; Wang, C.C.; Yeh, S.A.; Chen, W.H.; Chen, P.J. Targeting PI3K/AKT/mTOR Signaling Pathway as a Radiosensitization in Head and Neck Squamous Cell Carcinomas. Int. J. Mol. Sci. 2022, 23, 15749. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, H.; De Ridder, M. Targeting antioxidant enzymes as a radiosensitizing strategy. Cancer Lett. 2018, 438, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Drusco, A.; Croce, C.M. MicroRNAs and Cancer: A Long Story for Short RNAs. Adv. Cancer Res. 2017, 135, 1–24. [Google Scholar] [CrossRef]
- Vienberg, S.; Geiger, J.; Madsen, S.; Dalgaard, L.T. MicroRNAs in metabolism. Acta Physiol. 2017, 219, 346–361. [Google Scholar] [CrossRef]
- Lu, Y.C.; Chen, Y.J.; Wang, H.M.; Tsai, C.Y.; Chen, W.H.; Huang, Y.C.; Fan, K.H.; Tsai, C.N.; Huang, S.F.; Kang, C.J.; et al. Oncogenic function and early detection potential of miRNA-10b in oral cancer as identified by microRNA profiling. Cancer Prev. Res. 2012, 5, 665–674. [Google Scholar] [CrossRef]
- Mazumder, S.; Datta, S.; Ray, J.G.; Chaudhuri, K.; Chatterjee, R. Liquid biopsy: miRNA as a potential biomarker in oral cancer. Cancer Epidemiol. 2019, 58, 137–145. [Google Scholar] [CrossRef]
- Rashed, M.H.; Bayraktar, E.; Helal, G.K.; Abd-Ellah, M.F.; Amero, P.; Chavez-Reyes, A.; Rodriguez-Aguayo, C. Exosomes: From Garbage Bins to Promising Therapeutic Targets. Int. J. Mol. Sci. 2017, 18, 538. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Wen, Y.; Zhang, S.; Cheng, X. Circular RNA MTO1 intercorrelates with microRNA-630, both associate with Enneking stage and/or pathological fracture as well as prognosis in osteosarcoma patients. J. Clin. Lab. Anal. 2021, 35, e23987. [Google Scholar] [CrossRef] [PubMed]
- Nam, E.J.; Kim, S.; Lee, T.S.; Kim, H.J.; Lee, J.Y.; Kim, S.W.; Kim, J.H.; Kim, Y.T. Primary and recurrent ovarian high-grade serous carcinomas display similar microRNA expression patterns relative to those of normal ovarian tissue. Oncotarget 2016, 7, 70524–70534. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Zheng, J.; Li, J.; Li, Y.; Zhang, J.; Zhao, Q.; Wang, W.; Ji, G. MicroRNA-630 is a prognostic marker for patients with colorectal cancer. Tumour Biol. 2014, 35, 9787–9792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Feng, G.; Zhang, X.; Ding, Y.; Wang, X. microRNA-630 promotes cell proliferation and inhibits apoptosis in the HCT116 human colorectal cancer cell line. Mol. Med. Rep. 2017, 16, 4843–4848. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Zhang, W.; Yang, J.J.; Song, D.K.; Wei, J.X. Expression of miRNA-630 in bladder urothelial carcinoma and its clinical significance. J. Huazhong Univ. Sci. Technol. Med. Sci. 2016, 36, 705–709. [Google Scholar] [CrossRef]
- Zhang, J.W.; Li, Y.; Zeng, X.C.; Zhang, T.; Fu, B.S.; Yi, H.M.; Zhang, Q.; Jiang, N. miR-630 overexpression in hepatocellular carcinoma tissues is positively correlated with alpha-fetoprotein. Med. Sci. Monit. 2015, 21, 667–673. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H.; Li, W.; Yu, J.; Li, J.; Shen, Z.; Ye, G.; Qi, X.; Li, G. CircRNA_100269 is downregulated in gastric cancer and suppresses tumor cell growth by targeting miR-630. Aging 2017, 9, 1585–1594. [Google Scholar] [CrossRef]
- Zhao, J.J.; Chen, P.J.; Duan, R.Q.; Li, K.J.; Wang, Y.Z.; Li, Y. Up-regulation of miR-630 in clear cell renal cell carcinoma is associated with lower overall survival. Int. J. Clin. Exp. Pathol. 2014, 7, 3318–3323. [Google Scholar]
- Cui, Y.; Wang, D.; Xie, M. Tumor-Derived Extracellular Vesicles Promote Activation of Carcinoma-Associated Fibroblasts and Facilitate Invasion and Metastasis of Ovarian Cancer by Carrying miR-630. Front. Cell Dev. Biol. 2021, 9, 652322. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, J.Y.; Lu, L.J.; Wang, C.H.; Wang, L.H. MiR-630 promotes epithelial ovarian cancer proliferation and invasion via targeting KLF6. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4542–4547. [Google Scholar] [PubMed]
- Chen, L.; Chen, L.; Qin, Z.; Lei, J.; Ye, S.; Zeng, K.; Wang, H.; Ying, M.; Gao, J.; Zeng, S.; et al. Upregulation of miR-489-3p and miR-630 inhibits oxaliplatin uptake in renal cell carcinoma by targeting OCT2. Acta Pharm. Sin. B 2019, 9, 1008–1020. [Google Scholar] [CrossRef]
- Eoh, K.J.; Lee, S.H.; Kim, H.J.; Lee, J.Y.; Kim, S.; Kim, S.W.; Kim, Y.T.; Nam, E.J. MicroRNA-630 inhibitor sensitizes chemoresistant ovarian cancer to chemotherapy by enhancing apoptosis. Biochem. Biophys. Res. Commun. 2018, 497, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Millino, C.; Maretto, I.; Pacchioni, B.; Digito, M.; De Paoli, A.; Canzonieri, V.; D’Angelo, E.; Agostini, M.; Rizzolio, F.; Giordano, A.; et al. Gene and MicroRNA Expression Are Predictive of Tumor Response in Rectal Adenocarcinoma Patients Treated with Preoperative Chemoradiotherapy. J. Cell Physiol. 2017, 232, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chen, J.; Ren, Z.; Chen, Y.; Li, J.; Miao, X.; Song, Y.; Zhao, T.; Li, Y.; Shi, Y.; et al. A specific miRNA signature promotes radioresistance of human cervical cancer cells. Cancer Cell Int. 2013, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Ren, Z.; Jiao, S.; Guo, J.; Miao, X.; Wang, J.; Liu, J. HIF-3alpha-Induced miR-630 Expression Promotes Cancer Hallmarks in Cervical Cancer Cells by Forming a Positive Feedback Loop. J. Immunol. Res. 2022, 2022, 5262963. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, W.; Zhang, S.; Tan, W.; Qiu, Y.; Liao, K.; Yang, K. Effect of miR-630 expression on esophageal cancer cell invasion and migration. J. Clin. Lab. Anal. 2021, 35, e23815. [Google Scholar] [CrossRef]
- Gong, X.F.; Yu, A.L.; Tang, J.; Wang, C.L.; He, J.R.; Chen, G.Q.; Zhao, Q.; He, M.; Zhou, C.X. MicroRNA-630 inhibits breast cancer progression by directly targeting BMI1. Exp. Cell Res. 2018, 362, 378–385. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, C.; Xue, Z.X. Inhibition of miR-630 enhances the cell resistance to radiation by directly targeting CDC14A in human glioma. Am. J. Transl. Res. 2017, 9, 1255–1265. [Google Scholar]
- Li, Y.H.; Xu, C.L.; He, C.J.; Pu, H.H.; Liu, J.L.; Wang, Y. circMTDH.4/miR-630/AEG-1 axis participates in the regulation of proliferation, migration, invasion, chemoresistance, and radioresistance of NSCLC. Mol. Carcinog. 2020, 59, 141–153. [Google Scholar] [CrossRef]
- Lu, Y.C.; Chang, J.T.; Huang, Y.C.; Huang, C.C.; Chen, W.H.; Lee, L.Y.; Huang, B.S.; Chen, Y.J.; Li, H.F.; Cheng, A.J. Combined determination of circulating miR-196a and miR-196b levels produces high sensitivity and specificity for early detection of oral cancer. Clin. Biochem. 2015, 48, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.H.; You, G.R.; Tang, S.J.; Chang, J.T.; Cheng, A.J. Molecular Signature of Long Non-Coding RNA Associated with Areca Nut-Induced Head and Neck Cancer. Cells 2023, 12, 873. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.J.; Fan, K.H.; You, G.R.; Huang, S.F.; Kang, C.J.; Huang, Y.F.; Huang, Y.C.; Chang, J.T.; Cheng, A.J. Tumor Suppressor miRNA-503 Inhibits Cell Invasion in Head and Neck Cancer through the Wnt Signaling Pathway via the WNT3A/MMP Molecular Axis. Int. J. Mol. Sci. 2022, 23, 15900. [Google Scholar] [CrossRef] [PubMed]
- You, G.R.; Chang, J.T.; Li, H.F.; Cheng, A.J. Multifaceted and Intricate Oncogenic Mechanisms of NDRG1 in Head and Neck Cancer Depend on Its C-Terminal 3R-Motif. Cells 2022, 11, 1581. [Google Scholar] [CrossRef] [PubMed]
- You, G.R.; Chang, J.T.; Li, Y.L.; Chen, Y.J.; Huang, Y.C.; Fan, K.H.; Chen, Y.C.; Kang, C.J.; Cheng, A.J. Molecular Interplays Between Cell Invasion and Radioresistance That Lead to Poor Prognosis in Head-Neck Cancer. Front. Oncol. 2021, 11, 681717. [Google Scholar] [CrossRef]
- Li, Y.L.; Chang, J.T.; Lee, L.Y.; Fan, K.H.; Lu, Y.C.; Li, Y.C.; Chiang, C.H.; You, G.R.; Chen, H.Y.; Cheng, A.J. GDF15 contributes to radioresistance and cancer stemness of head and neck cancer by regulating cellular reactive oxygen species via a SMAD-associated signaling pathway. Oncotarget 2017, 8, 1508–1528. [Google Scholar] [CrossRef]
- You, G.R.; Chang, J.T.; Li, Y.L.; Huang, C.W.; Tsai, Y.L.; Fan, K.H.; Kang, C.J.; Huang, S.F.; Chang, P.H.; Cheng, A.J. MYH9 Facilitates Cell Invasion and Radioresistance in Head and Neck Cancer via Modulation of Cellular ROS Levels by Activating the MAPK-Nrf2-GCLC Pathway. Cells 2022, 11, 2855. [Google Scholar] [CrossRef]
- Cao, X.; Wen, P.; Fu, Y.; Gao, Y.; Qi, X.; Chen, B.; Tao, Y.; Wu, L.; Xu, A.; Lu, H.; et al. Radiation induces apoptosis primarily through the intrinsic pathway in mammalian cells. Cell. Signal. 2019, 62, 109337. [Google Scholar] [CrossRef]
- Birkinshaw, R.W.; Czabotar, P.E. The BCL-2 family of proteins and mitochondrial outer membrane permeabilisation. Semin. Cell Dev. Biol. 2017, 72, 152–162. [Google Scholar] [CrossRef]
- Penninckx, S.; Pariset, E.; Cekanaviciute, E.; Costes, S.V. Quantification of radiation-induced DNA double strand break repair foci to evaluate and predict biological responses to ionizing radiation. NAR Cancer 2021, 3, zcab046. [Google Scholar] [CrossRef]
- Georgescu, M.M. PTEN Tumor Suppressor Network in PI3K-Akt Pathway Control. Genes Cancer 2010, 1, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Zimta, A.A.; Cenariu, D.; Irimie, A.; Magdo, L.; Nabavi, S.M.; Atanasov, A.G.; Berindan-Neagoe, I. The Role of Nrf2 Activity in Cancer Development and Progression. Cancers 2019, 11, 1755. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.T.; Gao, J.Y.; Wang, H.L.; Wang, Y.; Wang, H.; Li, P.L. Downregulation of microRNA-630 inhibits cell proliferation and invasion and enhances chemosensitivity in human ovarian carcinoma. Genet. Mol. Res. 2015, 14, 8766–8777. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, X.; Zhou, W.; Li, D.; Chang, A.; Wang, Y.; Wu, Y.; Zhou, Q. Plasma microRNA expression signature involving miR-548q, miR-630 and miR-940 as biomarkers for nasopharyngeal carcinoma detection. Cancer Biomark. 2018, 23, 579–587. [Google Scholar] [CrossRef]
- Chu, D.; Zhao, Z.; Li, Y.; Li, J.; Zheng, J.; Wang, W.; Zhao, Q.; Ji, G. Increased microRNA-630 expression in gastric cancer is associated with poor overall survival. PLoS ONE 2014, 9, e90526. [Google Scholar] [CrossRef]
- Rupaimoole, R.; Ivan, C.; Yang, D.; Gharpure, K.M.; Wu, S.Y.; Pecot, C.V.; Previs, R.A.; Nagaraja, A.S.; Armaiz-Pena, G.N.; McGuire, M.; et al. Hypoxia-upregulated microRNA-630 targets Dicer, leading to increased tumor progression. Oncogene 2016, 35, 4312–4320. [Google Scholar] [CrossRef]
- Wu, Y.; Song, Y.; Wang, R.; Wang, T. Molecular mechanisms of tumor resistance to radiotherapy. Mol. Cancer 2023, 22, 96. [Google Scholar] [CrossRef]
- Jan, R.; Chaudhry, G.E. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef]
- Luna-Vargas, M.P.A.; Chipuk, J.E. Physiological and Pharmacological Control of BAK, BAX, and beyond. Trends Cell Biol. 2016, 26, 906–917. [Google Scholar] [CrossRef]
- Zhao, J.J.; Chen, P.J.; Duan, R.Q.; Li, K.J.; Wang, Y.Z.; Li, Y. miR-630 functions as a tumor oncogene in renal cell carcinoma. Arch. Med. Sci. 2016, 12, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.X.; Zhou, P.K. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal. Transduct. Target. Ther. 2020, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Taneja, N.; Davis, M.; Choy, J.S.; Beckett, M.A.; Singh, R.; Kron, S.J.; Weichselbaum, R.R. Histone H2AX phosphorylation as a predictor of radiosensitivity and target for radiotherapy. J. Biol. Chem. 2004, 279, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Morselli, E.; Vitale, I.; Kepp, O.; Senovilla, L.; Criollo, A.; Servant, N.; Paccard, C.; Hupe, P.; Robert, T.; et al. miR-181a and miR-630 regulate cisplatin-induced cancer cell death. Cancer Res. 2010, 70, 1793–1803. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.D.; Wang, F.Z.; Lee, C.Y.; Nien, C.Y.; Tseng, Y.K.; Yao, C.L.; Chen, S.C. 4-Aminobiphenyl inhibits the DNA homologous recombination repair in human liver cells: The role of miR-630 in downregulating RAD18 and MCM8. Toxicology 2020, 440, 152441. [Google Scholar] [CrossRef] [PubMed]
- Rana, J.N.; Mumtaz, S.; Choi, E.H.; Han, I. ROS production in response to high-power microwave pulses induces p53 activation and DNA damage in brain cells: Radiosensitivity and biological dosimetry evaluation. Front. Cell Dev. Biol. 2023, 11, 1067861. [Google Scholar] [CrossRef] [PubMed]
- Diehn, M.; Cho, R.W.; Lobo, N.A.; Kalisky, T.; Dorie, M.J.; Kulp, A.N.; Qian, D.; Lam, J.S.; Ailles, L.E.; Wong, M.; et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009, 458, 780–783. [Google Scholar] [CrossRef]
- Kawamura, K.; Qi, F.; Kobayashi, J. Potential relationship between the biological effects of low-dose irradiation and mitochondrial ROS production. J. Radiat. Res. 2018, 59, ii91–ii97. [Google Scholar] [CrossRef]
- Lee, D.Y.; Song, M.Y.; Kim, E.H. Role of Oxidative Stress and Nrf2/KEAP1 Signaling in Colorectal Cancer: Mechanisms and Therapeutic Perspectives with Phytochemicals. Antioxidants 2021, 10, 743. [Google Scholar] [CrossRef]
- Gorrini, C.; Baniasadi, P.S.; Harris, I.S.; Silvester, J.; Inoue, S.; Snow, B.; Joshi, P.A.; Wakeham, A.; Molyneux, S.D.; Martin, B.; et al. BRCA1 interacts with Nrf2 to regulate antioxidant signaling and cell survival. J. Exp. Med. 2013, 210, 1529–1544. [Google Scholar] [CrossRef]
- Vidotto, T.; Melo, C.M.; Lautert-Dutra, W.; Chaves, L.P.; Reis, R.B.; Squire, J.A. Pan-cancer genomic analysis shows hemizygous PTEN loss tumors are associated with immune evasion and poor outcome. Sci. Rep. 2023, 13, 5049. [Google Scholar] [CrossRef] [PubMed]
- Eze, N.; Lee, J.W.; Yang, D.H.; Zhu, F.; Neumeister, V.; Sandoval-Schaefer, T.; Mehra, R.; Ridge, J.A.; Forastiere, A.; Chung, C.H.; et al. PTEN loss is associated with resistance to cetuximab in patients with head and neck squamous cell carcinoma. Oral Oncol. 2019, 91, 69–78. [Google Scholar] [CrossRef]
- Snietura, M.; Jaworska, M.; Mlynarczyk-Liszka, J.; Goraj-Zajac, A.; Piglowski, W.; Lange, D.; Wozniak, G.; Nowara, E.; Suwinski, R. PTEN as a prognostic and predictive marker in postoperative radiotherapy for squamous cell cancer of the head and neck. PLoS ONE 2012, 7, e33396. [Google Scholar] [CrossRef] [PubMed]
- Rojo, A.I.; Rada, P.; Mendiola, M.; Ortega-Molina, A.; Wojdyla, K.; Rogowska-Wrzesinska, A.; Hardisson, D.; Serrano, M.; Cuadrado, A. The PTEN/NRF2 axis promotes human carcinogenesis. Antioxid. Redox Signal. 2014, 21, 2498–2514. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Nambiar, D.K.; Cao, H.; Viswanathan, V.; Kwok, S.; Hui, A.B.; Hou, Y.; Hildebrand, R.; von Eyben, R.; Holmes, B.J.; et al. NFE2L2 Mutations Enhance Radioresistance in Head and Neck Cancer by Modulating Intratumoral Myeloid Cells. Cancer Res. 2023, 83, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.L.; Jang, H.; Kim, E.H.; Shin, D. Targeting of the Glutathione, Thioredoxin, and Nrf2 Antioxidant Systems in Head and Neck Cancer. Antioxid. Redox Signal. 2017, 27, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Chen, B.; Jiao, N.L.; Liu, Y.H.; Sun, S.Y.; Zhang, Y.W. Elevated Glutathione Peroxidase 2 Expression Promotes Cisplatin Resistance in Lung Adenocarcinoma. Oxid. Med. Cell Longev. 2020, 2020, 7370157. [Google Scholar] [CrossRef]
- Kotowska-Zimmer, A.; Pewinska, M.; Olejniczak, M. Artificial miRNAs as therapeutic tools: Challenges and opportunities. Wiley Interdiscip. Rev. RNA 2021, 12, e1640. [Google Scholar] [CrossRef]
- Gurbuz, N.; Ozpolat, B. MicroRNA-based Targeted Therapeutics in Pancreatic Cancer. Anticancer Res. 2019, 39, 529–532. [Google Scholar] [CrossRef]
- Shah, M.Y.; Ferrajoli, A.; Sood, A.K.; Lopez-Berestein, G.; Calin, G.A. microRNA Therapeutics in Cancer—An Emerging Concept. EBioMedicine 2016, 12, 34–42. [Google Scholar] [CrossRef]
- Lima, J.F.; Cerqueira, L.; Figueiredo, C.; Oliveira, C.; Azevedo, N.F. Anti-miRNA oligonucleotides: A comprehensive guide for design. RNA Biol. 2018, 15, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Barata, P.; Sood, A.K.; Hong, D.S. RNA-targeted therapeutics in cancer clinical trials: Current status and future directions. Cancer Treat. Rev. 2016, 50, 35–47. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, G.-R.; Cheng, A.-J.; Shen, E.Y.-L.; Fan, K.-H.; Huang, Y.-F.; Huang, Y.-C.; Chang, K.-P.; Chang, J.T. MiR-630 Promotes Radioresistance by Induction of Anti-Apoptotic Effect via Nrf2–GPX2 Molecular Axis in Head–Neck Cancer. Cells 2023, 12, 2853. https://doi.org/10.3390/cells12242853
You G-R, Cheng A-J, Shen EY-L, Fan K-H, Huang Y-F, Huang Y-C, Chang K-P, Chang JT. MiR-630 Promotes Radioresistance by Induction of Anti-Apoptotic Effect via Nrf2–GPX2 Molecular Axis in Head–Neck Cancer. Cells. 2023; 12(24):2853. https://doi.org/10.3390/cells12242853
Chicago/Turabian StyleYou, Guo-Rung, Ann-Joy Cheng, Eric Yi-Liang Shen, Kang-Hsing Fan, Yi-Fang Huang, Yu-Chen Huang, Kai-Ping Chang, and Joseph T. Chang. 2023. "MiR-630 Promotes Radioresistance by Induction of Anti-Apoptotic Effect via Nrf2–GPX2 Molecular Axis in Head–Neck Cancer" Cells 12, no. 24: 2853. https://doi.org/10.3390/cells12242853




