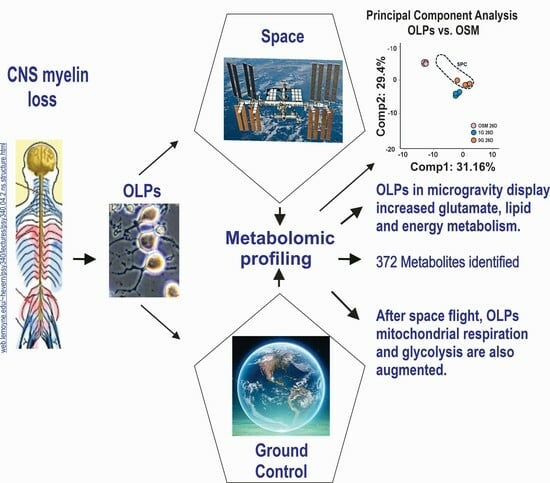
Metabolomics Profile of the Secretome of Space-Flown Oligodendrocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture System for OLPs and Pre-Myelinating OLs
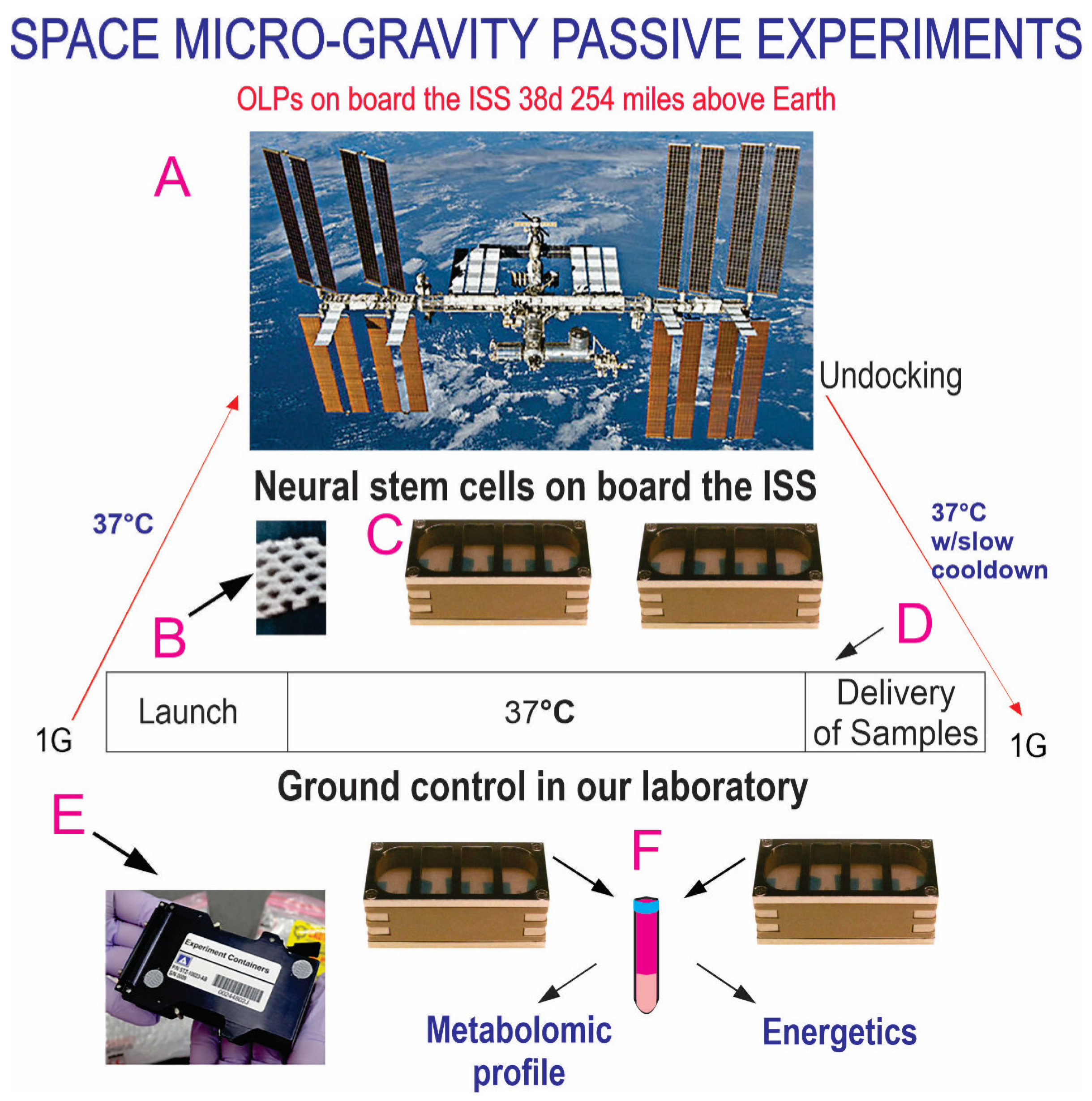
2.2. Hardware and Space Flight
2.3. Secretome Metabolomics
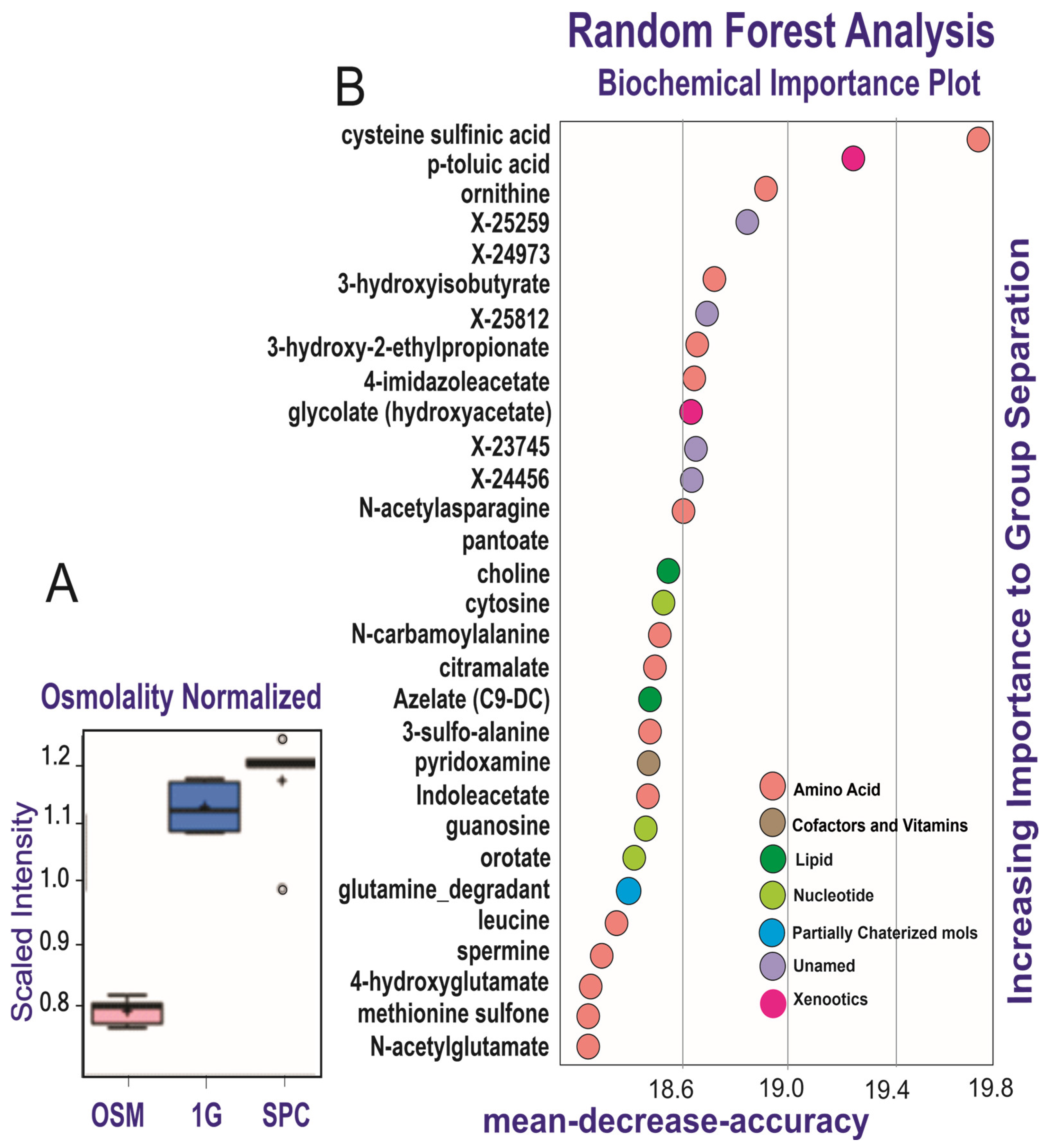
2.4. Statistical Analysis for the Metabolomics Study
2.5. Launch to the ISS
2.6. Cellular Bioenergetics
3. Results
3.1. Global Profile of the Secretome from the Different Treatments
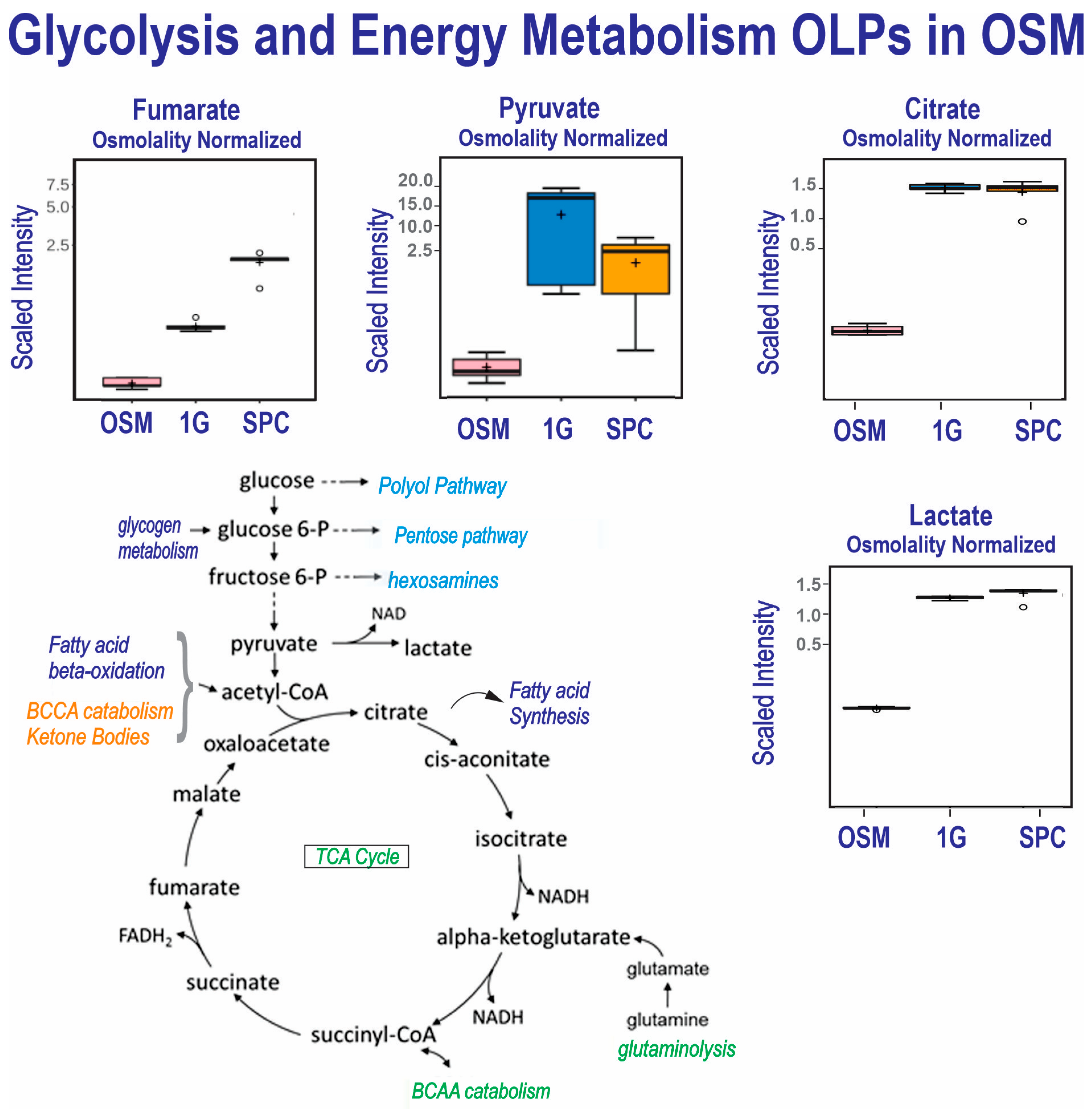
3.2. Glycolysis and Energy-Related Metabolism
3.3. Differences in Glutamate Metabolism
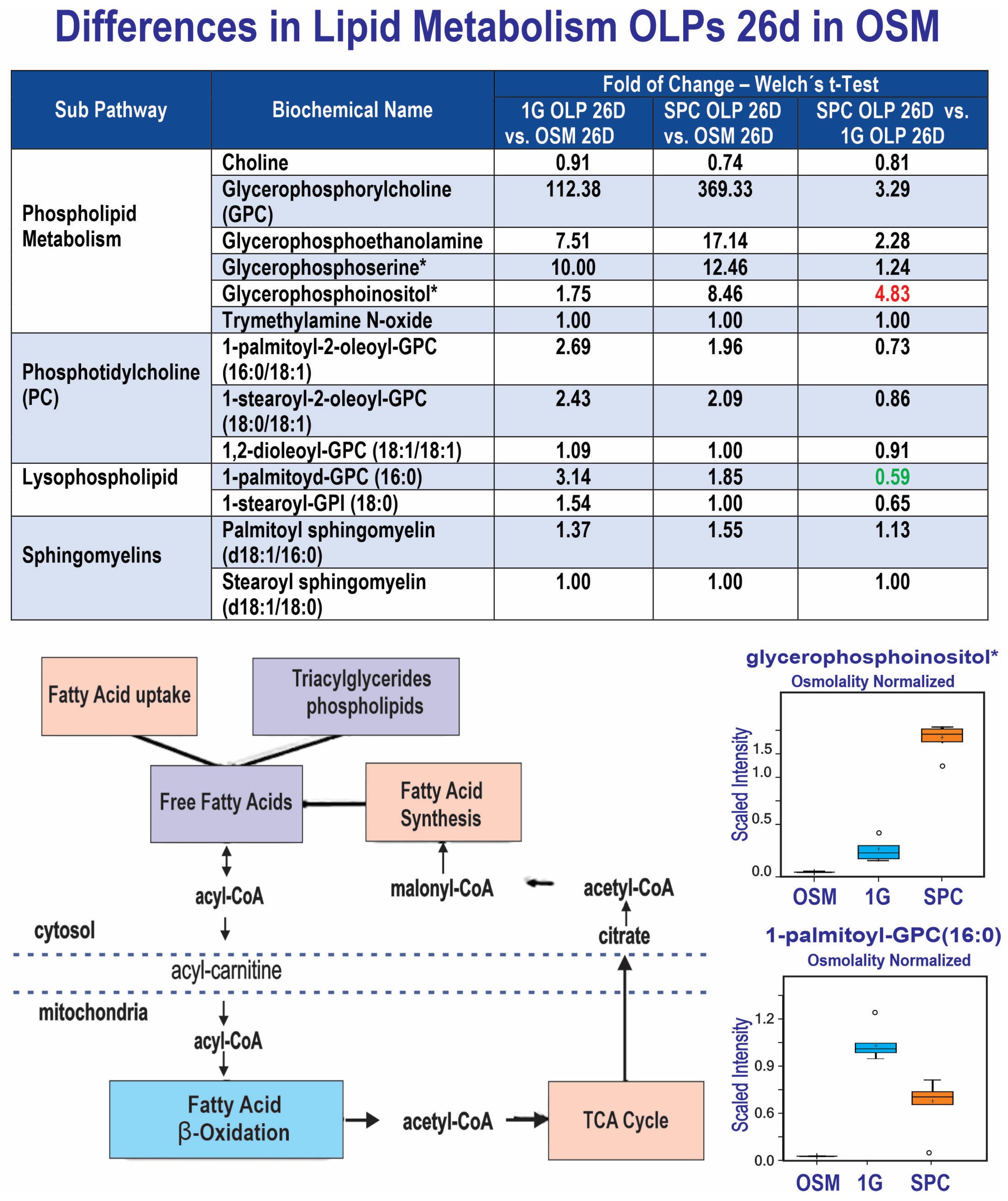
3.4. Differences in Lipid Metabolism
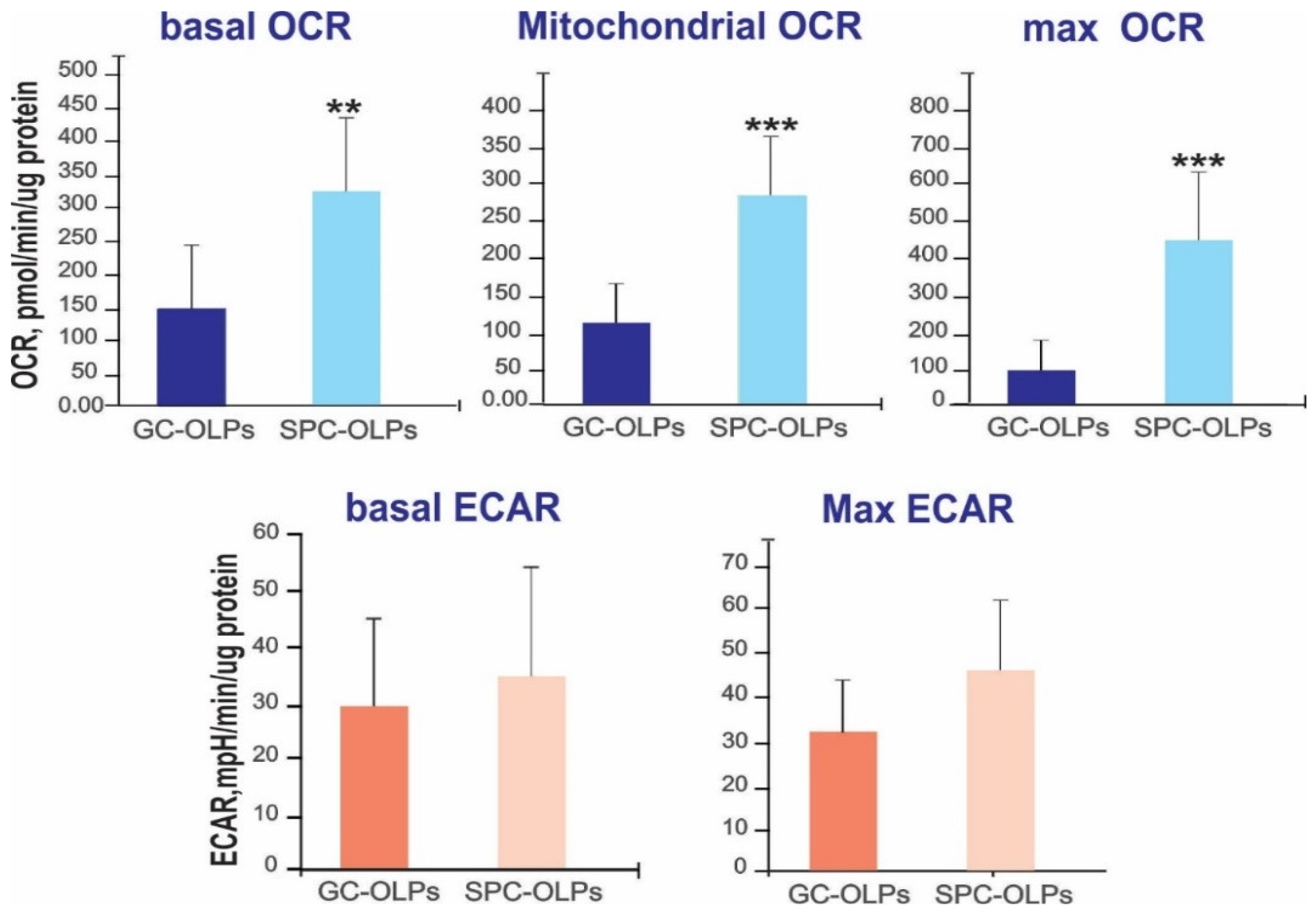
3.5. Space Flight Elicits Higher Respiration Rates in hiPS-Derived OLPs
4. Discussion
4.1. Glycolysis and Energy Related Metabolites
4.2. Glutamate Metabolism
4.3. Lipid Metabolism
4.4. Post-Flight Energetics
4.5. General Considerations
4.6. Significance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ICP | Intracranial hypertension |
| VIIP | Visual impairment intracranial pressure |
| µG | Microgravity |
| OLPs | Oligodendrocyte progenitors |
| SPC OLPs | Space flight oligodendrocyte progenitors |
| sim-µG | Simulated microgravity |
| OLs | Oligodendrocytes |
| CNS | Central nervous system |
| MS | Multiple sclerosis |
| BrdU | Bromodeoxyuridine |
| ISS | International Space Station |
| hiPS | Human induced pluripotent stem cells |
| CS83iCTR-33-n1 | Fibroblasts |
| N2 | A supplement containing recombinant human insulin, human transferrin sodium selenite, putrescine and progesterone |
| B-27 | Trademark |
| OSM | Oligodendrocyte specification medium |
| IGF-1 | Insulin Growth Factor 1 |
| DMEM/F12 | Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 |
| n = 5 | Five biological replicate specimens per group |
| 1G | Earth Gravity |
| UHPLC/MS | Ultra-High-Performance Liquid Chromatography/Mass Spectrometry |
| Q-value | False discovery rate |
| PCA | Principal Component Analysis |
| HCA | Hierarchical Clustering Analysis |
| STAaRS | Space Technology and Advanced Research System Experiment Facility 1 |
| NASA | National Aeronautics and Space Administration |
| mSv | Millisieverts |
| mGy | Milligray |
| OCR | Oxygen consumption rate |
| ECAR | Extracellular acidification rate |
| mM | Millimolar |
| 1G OLP 26D | Earth gravity Oligodendrocyte Progenitors kept for 26 days |
| SPC OLP 26D | Space flight Oligodendrocyte Progenitors kept for 26 days |
| OSM 26D | Oligodendrocyte specification medium kept for 26 days |
| TCA Cycle | Tricarboxylic Acid Cycle |
| BCAA | Branched-Chain Amino Acid |
| NAD | Nicotinamide adenine dinucleotide |
| FADH2 | Flavin adenine dinucleotide |
| α-KG | α-Ketoglutarate |
| NH4 | Ammonium |
| ATP | Adenosine tri-phosphate |
| DMF | Dimethyl-fumarate |
| MTR | Magnetization Transfer Ratio |
| NADH | Nicotinamide adenine dinucleotide (NAD) + hydrogen (H) |
| GS | Glutamate Synthetase |
| CSF | Cerebrospinal fluid |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| NMDA | N-methyl-D-aspartate |
| FAS | Fatty acids |
| MTA | Material transfer agreement |
References
- Studer, M.; Bradacs, G.; Hilliger, A.; Hürlimann, E.; Engeli, S.; Thiel, C.S.; Zeitner, P.; Denier, B.; Binggeli, M.; Syburra, T.; et al. Parabolic maneuvers of the Swiss Air Force fighter jet F-5E as a research platform for cell culture experiments in microgravity. Acta Astronaut. 2011, 68, 1729–1741. [Google Scholar] [CrossRef]
- Grimm, D.; Bauer, J.; Kossmehl, P.; Shakibaei, M.; Schönberger, J.; Pickenhahn, H.; Schulze-Tanzil, G.; Vetter, R.; Eilles, C.; Paul, M.; et al. Simulated microgravity alters differentiation and increases apoptosis in human follicular thyroid carcinoma cells. FASEB J. 2002, 16, 604–606. [Google Scholar] [CrossRef]
- Grimm, D.; Schulz, H.; Krüger, M.; Cortés-Sánchez, J.L.; Egli, M.; Kraus, A.; Sahana, J.; Corydon, T.J.; Hemmersbach, R.; Wise, P.M.; et al. The Fight against Cancer by Microgravity: The Multicellular Spheroid as a Metastasis Model. Int. J. Mol. Sci. 2022, 23, 3073. [Google Scholar] [CrossRef]
- Medha, M.; Roy, A. Microgravity: New aspect for breast cancer treatment, a review. Acta Astronaut. 2022, 190, 62–73. [Google Scholar] [CrossRef]
- Baumann, N.; Pham-Dinh, D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol. Rev. 2001, 81, 871–927. [Google Scholar] [CrossRef]
- Simons, M.; Nave, K.A. Oligodendrocytes: Myelination and axonal support. Cold Spring Harb. Perspect. Biol. 2016, 8, a020479. [Google Scholar] [CrossRef]
- Haines, J.D.; Inglese, M.; Casaccia, P. Axonal damage in multiple sclerosis. Mt. Sinai J. Med. J. Transl. Pers. Med. 2011, 78, 231–243. [Google Scholar] [CrossRef]
- Espinosa-Jeffrey, A.; Paez, P.M.; Cheli, V.T.; Spreuer, V.; Wanner, I.; de Vellis, J. Impact of simulated microgravity on oligodendrocyte development: Implications for central nervous system repair. PLoS ONE 2013, 8, e76963. [Google Scholar] [CrossRef]
- Tran, V.; Carpo, N.; Cepeda, C.; Espinosa-Jeffrey, A. Oligodendrocyte Progenitors Display Enhanced Proliferation and Autophagy after Space Flight. Biomolecules 2023, 13, 201. [Google Scholar] [CrossRef]
- Tran, V.; Carpo, N.; Shaka, S.; Zamudio, J.; Choi, S.; Cepeda, C.; Espinosa-Jeffrey, A. Delayed Maturation of Oligodendrocyte Progenitors by Microgravity: Implications for Multiple Sclerosis and Space Flight. Life 2022, 12, 797. [Google Scholar] [CrossRef]
- Espinosa-Jeffrey, A.; Wakeman, R.D.; Kim, U.S.; Snyder, E.; de Vellis, J. Culture System for Rodent and Human Oligodendrocyte Specification, Lineage Progression and Maturation. Curr. Protoc. Stem Cell Biol. 2009, 10, 2D.4.1–2D.4.26. [Google Scholar] [CrossRef]
- Espinosa-Jeffrey, A.; Blanchi, B.; Biancotti, J.C.; Kumar, S.; Hirose, M.; Mandefro, B.; Talavera-Adame, D.; Benvenisty, N.; de Vellis, J. Efficient generation of viral and integration-free human induced pluripotent stem cell-derived oligodendrocytes. Curr. Protoc. Stem Cell Biol. 2016, 38, 2D.18.1–2D.18.27. [Google Scholar] [CrossRef]
- Kuhn, S.; Gritti, L.; Crooks, D.; Dombrowski, Y. Oligodendrocytes in Development, Myelin Generation and Beyond. Cells 2019, 8, 1424. [Google Scholar] [CrossRef]
- Narine, M.; Colognato, H. Current Insights into Oligodendrocyte Metabolism and Its Power to Sculpt the Myelin Landscape. Front. Cell. Neurosci. 2022, 16, 892968. [Google Scholar] [CrossRef]
- Espinosa-Jeffrey, A.; Becker-Catania, S.G.; Zhao, P.M.; Cole, R.; Edmond, J.; de Vellis, J. Selective specification of CNS stem cells into oligodendroglial or neuronal cell lineage: Cell culture and transplant studies. J. Neurosci. Res. 2002, 69, 810–825. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Jeffrey, A.; Nguyen, K.; Kumar, S.; Toshimasa, O.; Hirose, R.; Reue, K.; Vergnes, L.; Kinchen, J.; de Vellis, J. Simulated microgravity enhances oligodendrocyte mitochondrial function and lipid metabolism. J. Neurosci. Res. 2016, 94, 1434–1450. [Google Scholar] [CrossRef]
- De Haven, M.J. Multimorbidity, chronic disease, and community health science. J. Eval. Clin. Pract. 2016, 23, 219–221. [Google Scholar] [CrossRef]
- Evans, A.M.; De Haven, C.D.; Barrett, T.; Mitchell, M.; Milgram, E. Integrated, nontargeted ultra-high performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological system. Anal. Chem. 2009, 81, 6656–6667. [Google Scholar] [CrossRef]
- De Haven, C.D.; Evans, A.M.; Dai, H.; Lawton, K.A. Organization of GC/MS and LC/MS metabolomics data into chemical libraries. J. Cheminformatics 2010, 2, 9. [Google Scholar] [CrossRef]
- Weissgerber, T.L.; Milic, N.M.; Winham, S.J.; Garovic, V.D. Beyond Bar and Line Graphs: Time for a New Data Presentation Paradigm. PLoS Biol. 2015, 13, e1002128. [Google Scholar] [CrossRef]
- Rosko, L.; Smith, V.N.; Yamazaki, R.; Huang, J.K. Oligodendrocyte bioenergetics in health and disease. Neuroscientist 2019, 25, 334–343. [Google Scholar] [CrossRef]
- Pantoni, L.; Garcia, J.H.; Gutierrez, J.A. Cerebral white matter is highly vulnerable to ischemia. Stroke 1996, 27, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.J.; Ha, S.-K.; Sati, P.; Absinta, M.; Luciano, N.J.; Lefeuvre, J.A.; Schindler, M.K.; Leibovitch, E.C.; Ryu, J.K.; Petersen, M.A.; et al. Spatiotemporal distribution of fibrinogen in marmoset and human inflammatory demyelination. Brain 2018, 141, 1637–1649. [Google Scholar] [CrossRef] [PubMed]
- Mahad, D.; Ziabreva, I.; Lassmann, H.; Turnbull, D. Mitochondrial defects in acute multiple sclerosis lesions. Brain 2008, 131, 1722–1735. [Google Scholar] [CrossRef] [PubMed]
- Lucchinetti, C.; Brück, W.; Parisi, J.; Scheithauer, B.; Rodriguez, M.; Lassmann, H. Heterogeneity of multiple sclerosis lesions: Implications for the pathogenesis of demyelination. Ann. Neurol. 2000, 47, 707–717. [Google Scholar] [CrossRef]
- Phillips, J.T.; Fox, R.J. BG-12 in multiple sclerosis. Semin. Neurol. 2013, 33, 56–65. [Google Scholar] [CrossRef]
- Harris, J.J.; Attwell, D. The energetics of CNS white matter. J. Neurosci. 2012, 32, 356–371. [Google Scholar] [CrossRef]
- Huang, H.; Taraboletti, A.; Shriver, L.P. Dimethyl fumarate modulates antioxidant and lipid metabolism in oligodendrocytes. Redox Biol. 2015, 5, 169–175. [Google Scholar] [CrossRef]
- Arnold, D.L.; Gold, R.; Kappos, L.; Bar-Or, A.; Giovannoni, G.; Selmaj, K.; Yang, M.; Zhang, R.; Stephan, M.; Sheikh, S.I.; et al. Effects of delayed-release dimethyl fumarate on MRI measures in the Phase 3 DEFINE study. J. Neurol. 2014, 261, 1794–1802. [Google Scholar] [CrossRef]
- McKenna, M.C.; Dienel, G.; Sonnewald, U.; Waagepetersen, H.S.; Schousboe, A. Energy metabolism of the brain. In Basic Neurochemistry; Brady, S., Siegel, G., Albers, R.W., Price, D.E., Eds.; Academic Press: Amsterdam, The Netherlands, 2012; pp. 200–234. [Google Scholar] [CrossRef]
- Armbruster, M.; Hanson, E.; Dulla, C.G. Glutamate clearance is locally modulated by presynaptic neuronal activity in the cerebral cortex. J. Neurosci. 2016, 36, 10404–10415. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Mironova, Y.A.; Shen, H.; Marino, R.A.M.; Waisman, A.; Lamers, W.H.; Bergles, D.E.; Bonci, A. Oligodendrocytes Support Neuronal Glutamatergic Transmission via Expression of Glutamine Synthetase. Cell Rep. 2019, 27, 2262–2271.e5. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hakvoort, T.B.M.; Vermeulen, J.L.M.; Labruyère, W.T.; De Waart, D.R.; Van Der Hel, W.S.; Ruijter, J.M.; Uylings, H.B.M.; Lamers, W.H. Glutamine Synthetase Deficiency in Murine Astrocytes Results in Neonatal Death. Glia 2010, 58, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Kaelin, W.G., Jr.; McKnight, S.L. Influence of metabolism on epigenetics and disease. Cell 2013, 153, 56–69. [Google Scholar] [CrossRef]
- TeSlaa, T.; Chaikovsky, A.C.; Lipchina, I.; Escobar, S.L.; Hochedlinger, K.; Huang, J.; Graeber, T.G.; Braas, D.; Teitell, M.A. α-Ketoglutarate Accelerates the Initial Differentiation of Primed Human Pluripotent Stem Cells. Cell Metab. 2016, 24, 485–493. [Google Scholar] [CrossRef]
- Vardhana, S.A.; Arnold, P.K.; Rosen, B.P.; Chen, Y.; Carey, B.W.; Huangfu, D.; Carmona-Fontaine, C.; Thompson, C.B.; Finley, L.W.S. Glutamine independence is a selectable feature of pluripotent stem cells. Nat. Metab. 2019, 1, 676–687. [Google Scholar] [CrossRef]
- Al Gawwam, G.; Sharquie, I.K. Serum Glutamate Is a Predictor for the Diagnosis of Multiple Sclerosis. Sci. World J. 2017, 2017, 9320802. [Google Scholar] [CrossRef]
- Westall, F.C. A response to “Association of an EAAT2 polymorphism with higher glutamate concentration in relapsing multiple sclerosis”. J. Neuroimmunol. 2008, 203, 116–117. [Google Scholar] [CrossRef]
- Sarchielli, P.; Greco, L.; Floridi, A.; Floridi, A.; Gallai, V. Excitatory amino acids and multiple sclerosis: Evidence from cerebrospinal fluid. Arch. Neurol. 2003, 60, 1082–1088. [Google Scholar] [CrossRef]
- Cianfoni, A.; Niku, S.; Imbesi, S.G. Metabolite findings in tumefactive demyelinating lesions utilizing short echo time proton magnetic resonance spectroscopy. Am. J. Neuroradiol. 2007, 28, 272–277. [Google Scholar]
- Kuzmina, U.S.; Zainullina, L.F.; Vakhitov, V.A.; Bakhtiyarova, K.Z.; Vakhitova, Y.V. The Role of Glutamate in the Pathogenesis of Multiple Sclerosis. Neurosci. Behav. Phys. 2020, 50, 669–675. [Google Scholar] [CrossRef]
- Newcombe, J.; Uddin, A.; Dove, R.; Patel, B.; Turski, L.; Nishizawa, Y.; Smith, T. Glutamate receptor expression in multiple sclerosis lesions. Brain Pathol. 2008, 18, 52–61. [Google Scholar] [CrossRef]
- Bano, D.; Young, K.W.; Guerin, C.J.; LeFeuvre, R.; Rothwell, N.J.; Naldini, L.; Rizzuto, R.; Carafoli, E.; Nicotera, P. Cleavage of the Plasma Membrane Na+/Ca2+ Exchanger in Excitotoxicity. Cell 2005, 12, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Armada-Moreira, A.; Gomes, J.I.; Pina, C.C.; Savchak, O.K.; Gonçalves-Ribeiro, J.; Rei, N.; Pinto, S.; Morais, T.P.; Martins, R.S.; Ribeiro, F.F.; et al. Going the Extra (Synaptic) Mile: Excitotoxicity as the Road Toward Neurodegenerative Diseases. Front. Cell. Neurosci. 2020, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Marangon, D.; Boccazzi, M.; Lecca, D.; Fumagalli, M. Regulation of Oligodendrocyte Functions: Targeting Lipid Metabolism and Extracellular Matrix for Myelin Repair. J. Clin. Med. 2020, 9, 470. [Google Scholar] [CrossRef] [PubMed]
- Harayama, T.; Riezman, H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 281–296. [Google Scholar] [CrossRef]
- O’Brien, J.S.; Sampson, E.L. Lipid composition of the normal human brain. J. Lipid Res. 1965, 6, 537–544. [Google Scholar] [CrossRef]
- Montani, L. Lipids in regulating oligodendrocyte structure and function. Semin. Cell Dev. Biol. 2021, 112, 114–122. [Google Scholar] [CrossRef]


| Reagents | OSM | BS1 |
|---|---|---|
| Insulin | 10 µg/mL | 10 µg/mL |
| Transferrin | 100 µg/mL | 100 µg/mL |
| Putrescine | 32.2 µg/mL | 32.2 µg/mL |
| Na-bicarbonate | 4.4 µg/mL | 4.4 µg/mL |
| D(+) galactose | 9.2 mg/mL | 9.2 mg/mL |
| Kanamycin | 16 ng/mL | 16 ng/mL |
| Na-selenite | 6.4 ng/mL | 6.4 ng/mL |
| N2 | 2% | 0 |
| B-27 | 2% | 0 |
| IGF-1 | 160 ng/µL |
| Number of Metabolites Measured in the Secretome’s Sample | 1G OLP 26 D vs. OSM 26 D | SPC OLP 26 D vs. OSM 26 D | SPC OLP 26 D vs. 1G OLP 26 D |
|---|---|---|---|
| Total number of metabolites measured (secretome or medium) | 247 | 237 | 198 |
| Number of metabolites higher in the secretome than in the medium | 157 | 182 | 136 |
| Number of metabolites lower in the secretome than in the medium | 90 | 55 | 62 |
| Number of metabolites that decreased in the secretome, % of total | 36 | 23 | 31 |
| Sub Pathway | Biochemical Name | 1 G OLP 26 D (GC)/ OSM 26 D | SPACE OLP 26 D/ OSM 26 D | SPACE OLP 26 D/ 1 G OLP 26 D (GC) |
|---|---|---|---|---|
| Glycolysis, Glyconeogenesis and pyruvate metabolism | Pyruvate | 17.56 | 7.1 | 0.4 |
| Lactate | 28.71 | 32.84 | 1.14 | |
| Glycerate | 0.49 | 0.96 | 1.97 | |
| TCA Cycle | Citrate | 7.55 | 7.06 | 0.94 |
| Aconitate (cis or trans) | 13.78 | 14.06 | 1.02 | |
| Alppha keto-glutarate | 1.40 | 1.93 | 1.38 | |
| Succinate | 2.45 | 4.07 | 1.66 | |
| Fumarate | 2.8 | 8.84 | 3.16 | |
| Malate | 3.81 | 4.69 | 1.23 | |
| Itaconate | 1.0 | 1.65 | 1.24 | |
| Tricarballylate | 0.96 | 1.06 | 1.11 | |
| 2-Methylcitrate/Honocitrate | 0.73 | 1.13 | 1.44 | |
| Mesaconate | 0.96 | 2.0 | 2.09 | |
| Citraconate/gluconate | 0.88 | 2.02 | 2.31 | |
| Oxydative Phosphorylation | Phosphate | 3.59 | 1.3 | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vergnes, L.; Foucaud, B.; Cepeda, C.; Espinosa-Jeffrey, A. Metabolomics Profile of the Secretome of Space-Flown Oligodendrocytes. Cells 2023, 12, 2249. https://doi.org/10.3390/cells12182249
Vergnes L, Foucaud B, Cepeda C, Espinosa-Jeffrey A. Metabolomics Profile of the Secretome of Space-Flown Oligodendrocytes. Cells. 2023; 12(18):2249. https://doi.org/10.3390/cells12182249
Chicago/Turabian StyleVergnes, Laurent, Bernard Foucaud, Carlos Cepeda, and Araceli Espinosa-Jeffrey. 2023. "Metabolomics Profile of the Secretome of Space-Flown Oligodendrocytes" Cells 12, no. 18: 2249. https://doi.org/10.3390/cells12182249