Aquaglyceroporins in Human Breast Cancer
Abstract
:1. Introduction
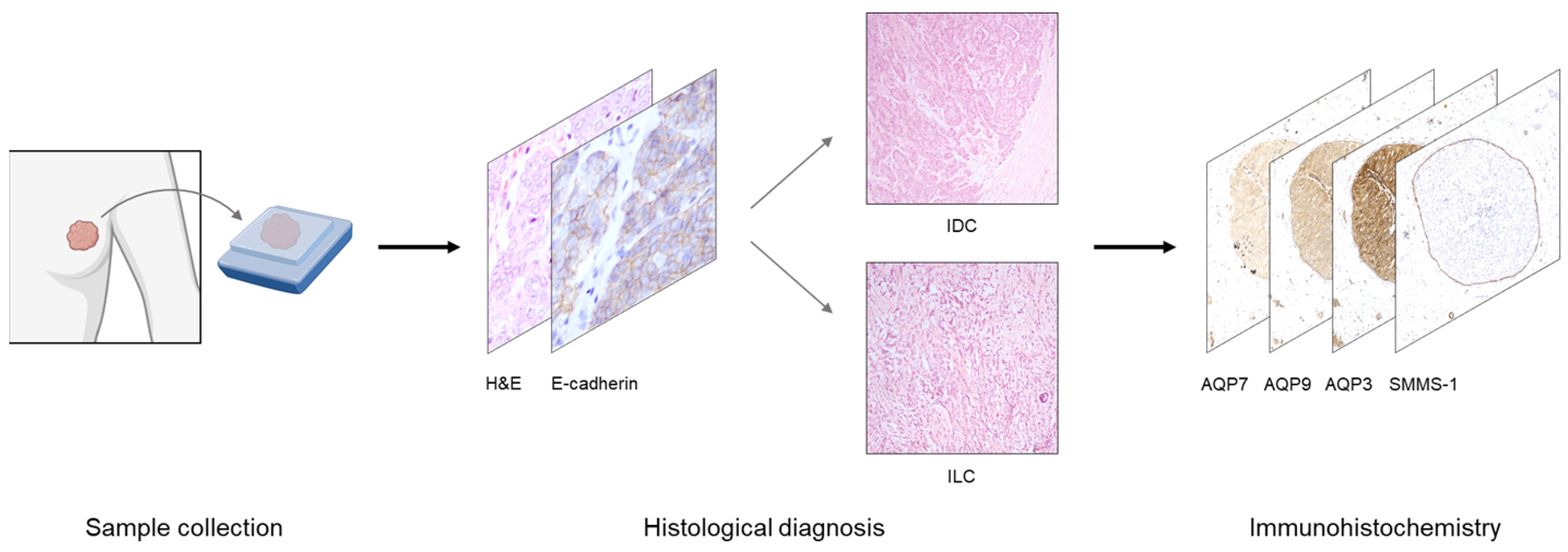
2. Materials and Methods
2.1. Patient Samples
2.2. Immunohistochemistry
2.3. Microscopy and Image Analysis
3. Results
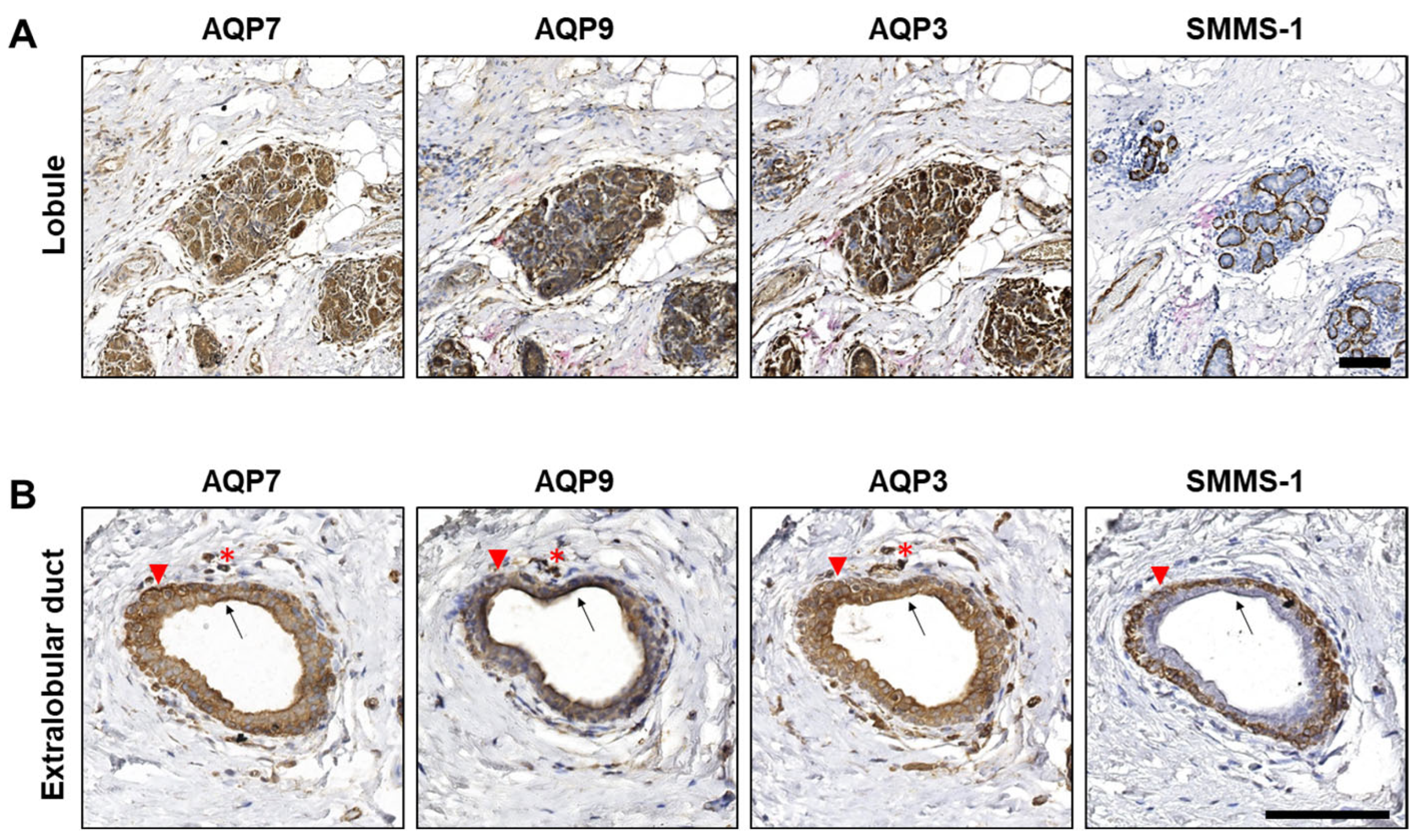
3.1. AQP3, AQP7 and AQP9 Are Expressed in Epithelial Cells of Normal Lobules and Extralobular Ducts in Benign Structures Adjacent to Tumor Tissue
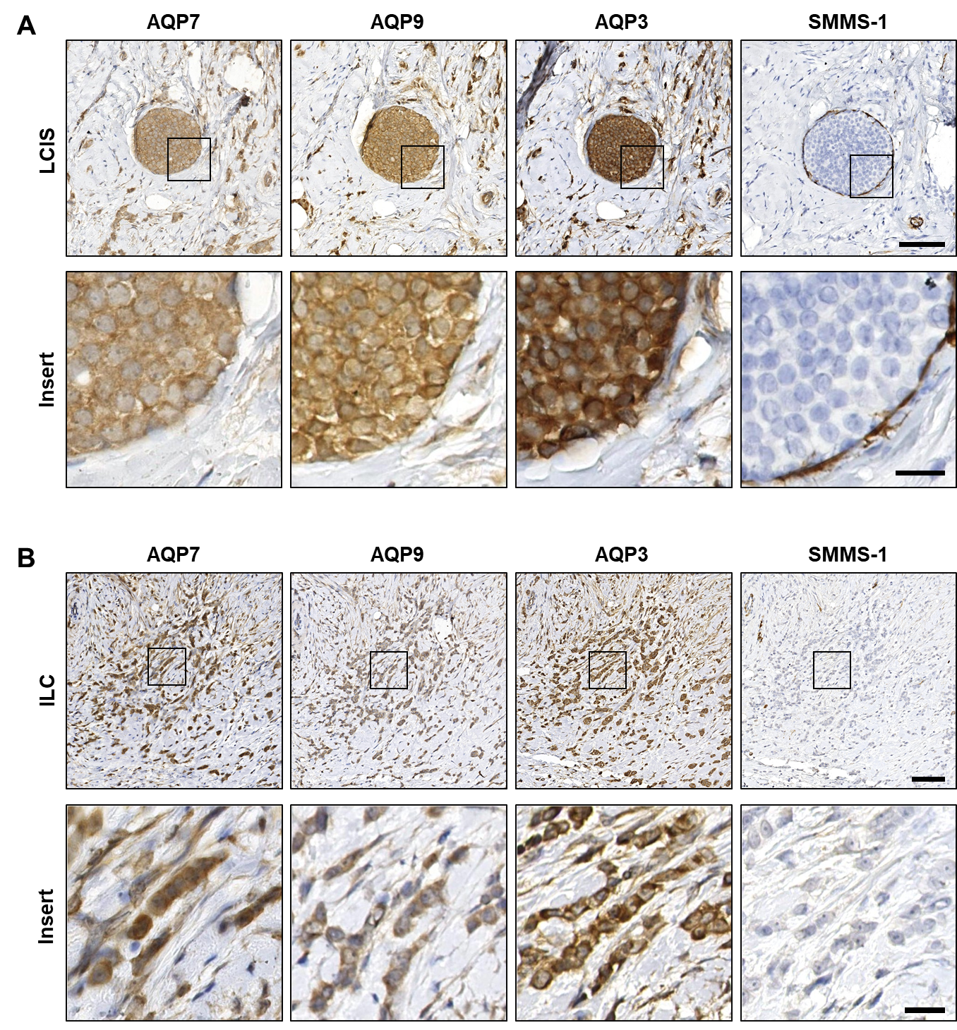
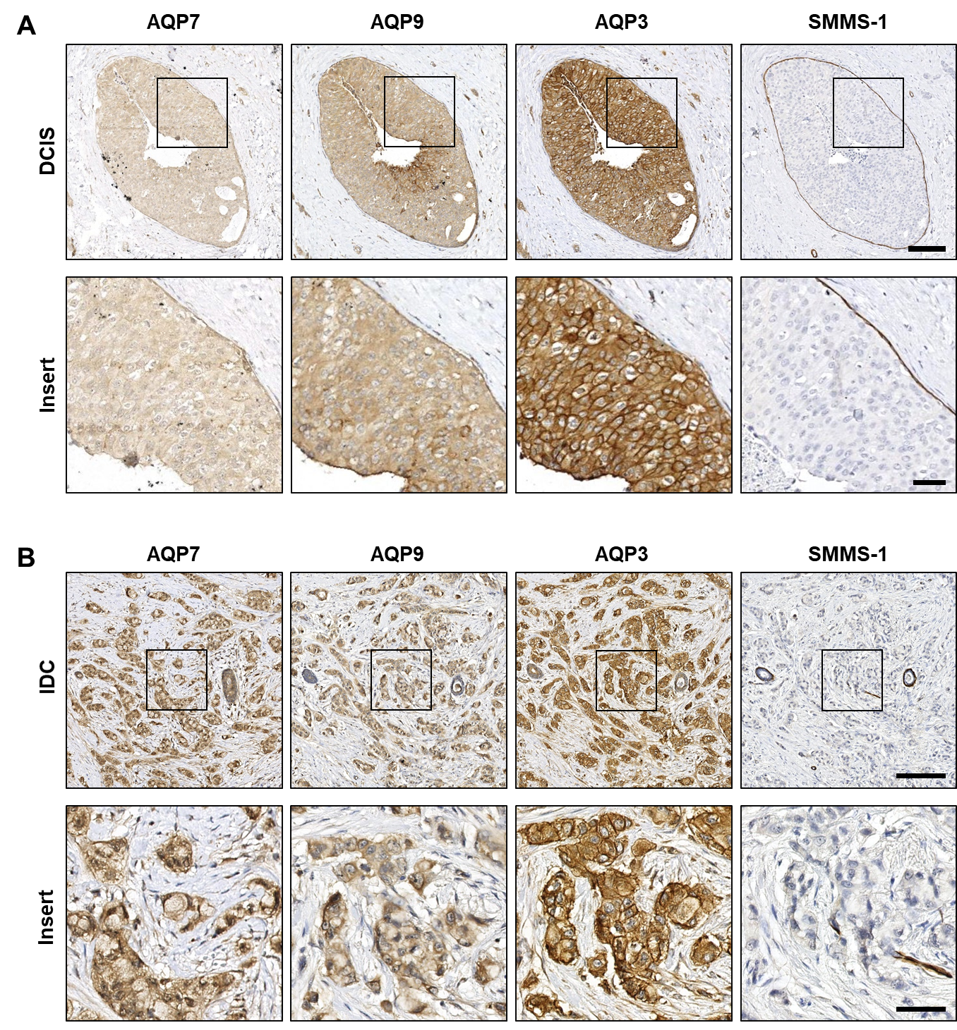
3.2. AQP3, AQP7 and AQP9 Are Homogeneously Distributed in Both Premalignant In Situ Regions and in Invasive Breast Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Login, F.H.; Nejsum, L.N. Aquaporin water channels: Roles beyond renal water handling. Nat. Rev. Nephrol. 2023, 19, 604–618. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, D.; Xia, Y.; Zhou, F.; Zhang, Q.; Wang, Q.; Qin, A.; Zhao, J.; Li, D.; Li, Y.; et al. The structural basis for glycerol permeation by human AQP7. Sci. Bull. 2021, 66, 1550–1558. [Google Scholar] [CrossRef]
- Gotfryd, K.; Mosca, A.F.; Missel, J.W.; Truelsen, S.F.; Wang, K.; Spulber, M.; Krabbe, S.; Helix-Nielsen, C.; Laforenza, U.; Soveral, G.; et al. Human adipose glycerol flux is regulated by a pH gate in AQP10. Nat. Commun. 2018, 9, 4749. [Google Scholar] [CrossRef]
- Montiel, V.; Bella, R.; Michel, L.Y.M.; Esfahani, H.; De Mulder, D.; Robinson, E.L.; Deglasse, J.P.; Tiburcy, M.; Chow, P.H.; Jonas, J.C.; et al. Inhibition of aquaporin-1 prevents myocardial remodeling by blocking the transmembrane transport of hydrogen peroxide. Sci. Transl. Med. 2020, 12, eaay2176. [Google Scholar] [CrossRef]
- Wang, H.; Schoebel, S.; Schmitz, F.; Dong, H.; Hedfalk, K. Characterization of aquaporin-driven hydrogen peroxide transport. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183065. [Google Scholar] [CrossRef]
- Miller, E.W.; Dickinson, B.C.; Chang, C.J. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 15681–15686. [Google Scholar] [CrossRef]
- Rodrigues, C.; Pimpao, C.; Mosca, A.F.; Coxixo, A.S.; Lopes, D.; da Silva, I.V.; Pedersen, P.A.; Antunes, F.; Soveral, G. Human Aquaporin-5 Facilitates Hydrogen Peroxide Permeation Affecting Adaption to Oxidative Stress and Cancer Cell Migration. Cancers 2019, 11, 932. [Google Scholar] [CrossRef]
- Pellavio, G.; Martinotti, S.; Patrone, M.; Ranzato, E.; Laforenza, U. Aquaporin-6 May Increase the Resistance to Oxidative Stress of Malignant Pleural Mesothelioma Cells. Cells 2022, 11, 1892. [Google Scholar] [CrossRef]
- Bienert, G.P.; Moller, A.L.; Kristiansen, K.A.; Schulz, A.; Moller, I.M.; Schjoerring, J.K.; Jahn, T.P. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 2007, 282, 1183–1192. [Google Scholar] [CrossRef]
- Bertolotti, M.; Bestetti, S.; Garcia-Manteiga, J.M.; Medrano-Fernandez, I.; Dal Mas, A.; Malosio, M.L.; Sitia, R. Tyrosine kinase signal modulation: A matter of H2O2 membrane permeability? Antioxid. Redox Signal. 2013, 19, 1447–1451. [Google Scholar] [CrossRef]
- Watanabe, S.; Moniaga, C.S.; Nielsen, S.; Hara-Chikuma, M. Aquaporin-9 facilitates membrane transport of hydrogen peroxide in mammalian cells. Biochem. Biophys. Res. Commun. 2016, 471, 191–197. [Google Scholar] [CrossRef]
- Bestetti, S.; Galli, M.; Sorrentino, I.; Pinton, P.; Rimessi, A.; Sitia, R.; Medrano-Fernandez, I. Human aquaporin-11 guarantees efficient transport of H2O2 across the endoplasmic reticulum membrane. Redox Biol. 2020, 28, 101326. [Google Scholar] [CrossRef]
- Dai, C.; Charlestin, V.; Wang, M.; Walker, Z.T.; Miranda-Vergara, M.C.; Facchine, B.A.; Wu, J.; Kaliney, W.J.; Dovichi, N.J.; Li, J.; et al. Aquaporin-7 Regulates the Response to Cellular Stress in Breast Cancer. Cancer Res. 2020, 80, 4071–4086. [Google Scholar] [CrossRef] [PubMed]
- Deen, P.M.; Verdijk, M.A.; Knoers, N.V.; Wieringa, B.; Monnens, L.A.; van-Os, C.H.; van-Oost, B.A. Requirement of human renal water channel aquaporin-2 for vasopressin-dependent concentration of urine. Science 1994, 264, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Terris, J.; Andersen, D.; Ecelbarger, C.; Frokiaer, J.; Jonassen, T.; Marples, D.; Knepper, M.A.; Petersen, J.S. Congestive heart failure in rats is associated with increased expression and targeting of aquaporin-2 water channel in collecting duct. Proc. Natl. Acad. Sci. USA 1997, 94, 5450–5455. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.L.; Martin, P.Y.; Ohara, M.; St John, J.; Pattison, T.; Meng, X.; Morris, K.; Kim, J.K.; Schrier, R.W. Upregulation of aquaporin-2 water channel expression in chronic heart failure rat. J. Clin. Investig. 1997, 99, 1500–1505. [Google Scholar] [CrossRef]
- Marples, D.; Christensen, S.; Christensen, E.I.; Ottosen, P.D.; Nielsen, S. Lithium-induced downregulation of aquaporin-2 water channel expression in rat kidney medulla. J. Clin. Investig. 1995, 95, 1838–1845. [Google Scholar] [CrossRef]
- Traberg-Nyborg, L.; Login, F.H.; Edamana, S.; Tramm, T.; Borgquist, S.; Nejsum, L.N. Aquaporin-1 in breast cancer. APMIS 2022, 130, 3–10. [Google Scholar] [CrossRef]
- Bystrup, M.; Login, F.H.; Edamana, S.; Borgquist, S.; Tramm, T.; Kwon, T.H.; Nejsum, L.N. Aquaporin-5 in breast cancer. APMIS 2022, 130, 253–260. [Google Scholar] [CrossRef]
- Bruun-Sorensen, A.S.; Edamana, S.; Login, F.H.; Borgquist, S.; Nejsum, L.N. Aquaporins in pancreatic ductal adenocarcinoma. APMIS 2021, 129, 700–705. [Google Scholar] [CrossRef]
- Tomita, Y.; Dorward, H.; Yool, A.J.; Smith, E.; Townsend, A.R.; Price, T.J.; Hardingham, J.E. Role of Aquaporin 1 Signalling in Cancer Development and Progression. Int. J. Mol. Sci. 2017, 18, 299. [Google Scholar] [CrossRef]
- Marlar, S.; Jensen, H.H.; Login, F.H.; Nejsum, L.N. Aquaporin-3 in Cancer. Int. J. Mol. Sci. 2017, 18, 2106. [Google Scholar] [CrossRef] [PubMed]
- Jensen, H.H.; Login, F.H.; Koffman, J.S.; Kwon, T.H.; Nejsum, L.N. The role of aquaporin-5 in cancer cell migration: A potential active participant. Int. J. Biochem. Cell Biol. 2016, 79, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Verkman, A.S. Prevention of skin tumorigenesis and impairment of epidermal cell proliferation by targeted aquaporin-3 gene disruption. Mol. Cell. Biol. 2008, 28, 326–332. [Google Scholar] [CrossRef]
- Matsumura, K.; Chang, B.H.; Fujimiya, M.; Chen, W.; Kulkarni, R.N.; Eguchi, Y.; Kimura, H.; Kojima, H.; Chan, L. Aquaporin 7 is a beta-cell protein and regulator of intraislet glycerol content and glycerol kinase activity, beta-cell mass, and insulin production and secretion. Mol. Cell. Biol. 2007, 27, 6026–6037. [Google Scholar] [CrossRef] [PubMed]
- Login, F.H.; Jensen, H.H.; Pedersen, G.A.; Koffman, J.S.; Kwon, T.H.; Parsons, M.; Nejsum, L.N. Aquaporins differentially regulate cell-cell adhesion in MDCK cells. FASEB J. 2019, 33, 6980–6994. [Google Scholar] [CrossRef]
- Huang, P.; Venskutonyte, R.; Prasad, R.B.; Ardalani, H.; de Mare, S.W.; Fan, X.; Li, P.; Spegel, P.; Yan, N.; Gourdon, P.; et al. Cryo-EM structure supports a role of AQP7 as a junction protein. Nat. Commun. 2023, 14, 600. [Google Scholar] [CrossRef]
- Edamana, S.; Login, F.H.; Yamada, S.; Kwon, T.H.; Nejsum, L.N. Aquaporin water channels as regulators of cell-cell adhesion proteins. Am. J. Physiol. Cell Physiol. 2021, 320, C771–C777. [Google Scholar] [CrossRef]
- Papadopoulos, M.C.; Saadoun, S.; Verkman, A.S. Aquaporins and cell migration. Pflugers Arch. 2008, 456, 693–700. [Google Scholar] [CrossRef]
- Smith, I.M.; Stroka, K.M. The multifaceted role of aquaporins in physiological cell migration. Am. J. Physiol. Cell Physiol. 2023, 325, C208–C223. [Google Scholar] [CrossRef]
- Jung, H.J.; Park, J.Y.; Jeon, H.S.; Kwon, T.H. Aquaporin-5: A marker protein for proliferation and migration of human breast cancer cells. PLoS ONE 2011, 6, e28492. [Google Scholar] [CrossRef]
- Edamana, S.; Login, F.H.; Riishede, A.; Dam, V.S.; Tramm, T.; Nejsum, L.N. The cell polarity protein Scribble is downregulated by the water channel aquaporin-5 in breast cancer cells. Am. J. Physiol. Cell Physiol. 2023, 324, C307–C319. [Google Scholar] [CrossRef]
- Oishi, M.; Munesue, S.; Harashima, A.; Nakada, M.; Yamamoto, Y.; Hayashi, Y. Aquaporin 1 elicits cell motility and coordinates vascular bed formation by downregulating thrombospondin type-1 domain-containing 7A in glioblastoma. Cancer Med. 2020, 9, 3904–3917. [Google Scholar] [CrossRef]
- McCoy, E.; Sontheimer, H. Expression and function of water channels (aquaporins) in migrating malignant astrocytes. Glia 2007, 55, 1034–1043. [Google Scholar] [CrossRef]
- Xu, H.; Xu, Y.; Zhang, W.; Shen, L.; Yang, L.; Xu, Z. Aquaporin-3 positively regulates matrix metalloproteinases via PI3K/AKT signal pathway in human gastric carcinoma SGC7901 cells. J. Exp. Clin. Cancer Res. 2011, 30, 86. [Google Scholar] [CrossRef]
- Roche, J.V.; Tornroth-Horsefield, S. Aquaporin Protein-Protein Interactions. Int. J. Mol. Sci. 2017, 18, 2255. [Google Scholar] [CrossRef]
- Login, F.H.; Palmfeldt, J.; Cheah, J.S.; Yamada, S.; Nejsum, L.N. Aquaporin-5 regulation of cell-cell adhesion proteins: An elusive “tail” story. Am. J. Physiol. Cell Physiol. 2021, 320, C282–C292. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Nolan, E.; Lindeman, G.J.; Visvader, J.E. Deciphering breast cancer: From biology to the clinic. Cell 2023, 186, 1708–1728. [Google Scholar] [CrossRef]
- Makki, J. Diversity of Breast Carcinoma: Histological Subtypes and Clinical Relevance. Clin. Med. Insights Pathol. 2015, 8, 23–31. [Google Scholar] [CrossRef]
- Chow, P.H.; Bowen, J.; Yool, A.J. Combined Systematic Review and Transcriptomic Analyses of Mammalian Aquaporin Classes 1 to 10 as Biomarkers and Prognostic Indicators in Diverse Cancers. Cancers 2020, 12, 1911. [Google Scholar] [CrossRef]
- Lee, S.J.; Chae, Y.S.; Kim, J.G.; Kim, W.W.; Jung, J.H.; Park, H.Y.; Jeong, J.Y.; Park, J.Y.; Jung, H.J.; Kwon, T.H. AQP5 expression predicts survival in patients with early breast cancer. Ann. Surg. Oncol. 2014, 21, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Zhang, H.; Shao, Y.; Liu, X.; Yang, L.; Huang, Y.; Fu, L.; Gu, F.; Ma, Y. Expression of aquaporin1, a water channel protein, in cytoplasm is negatively correlated with prognosis of breast cancer patients. Oncotarget 2016, 7, 8143–8154. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Jiao, L.; Li, T.; Wang, H.; Wei, W.; Qian, H. Expression of AQP3 and AQP5 as a prognostic marker in triple-negative breast cancer. Oncol. Lett. 2018, 16, 2661–2667. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Ma, N.; Wang, B.; Wang, L.; Zhou, C.; Yan, Y.; He, J.; Ren, Y. Significant prognostic values of aquaporin mRNA expression in breast cancer. Cancer Manag. Res. 2019, 11, 1503–1515. [Google Scholar] [CrossRef]
- Jensen, H.H.; Login, F.H.; Park, J.Y.; Kwon, T.H.; Nejsum, L.N. Immunohistochemical evalulation of activated Ras and Rac1 as potential downstream effectors of aquaporin-5 in breast cancer in vivo. Biochem. Biophys. Res. Commun. 2017, 493, 1210–1216. [Google Scholar] [CrossRef]
- Lebeck, J.; Ostergard, T.; Rojek, A.; Fuchtbauer, E.M.; Lund, S.; Nielsen, S.; Praetorius, J. Gender-specific effect of physical training on AQP7 protein expression in human adipose tissue. Acta Diabetol. 2012, 49 (Suppl. S1), S215–S226. [Google Scholar] [CrossRef]
- Lindskog, C.; Asplund, A.; Catrina, A.; Nielsen, S.; Rutzler, M. A Systematic Characterization of Aquaporin-9 Expression in Human Normal and Pathological Tissues. J. Histochem. Cytochem. 2016, 64, 287–300. [Google Scholar] [CrossRef]
- Elkjaer, M.; Vajda, Z.; Nejsum, L.N.; Kwon, T.; Jensen, U.B.; Amiry-Moghaddam, M.; Frokiaer, J.; Nielsen, S. Immunolocalization of AQP9 in liver, epididymis, testis, spleen, and brain. Biochem. Biophys. Res. Commun. 2000, 276, 1118–1128. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernandez, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Polyak, K. Heterogeneity in breast cancer. J. Clin. Investig. 2011, 121, 3786–3788. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Wray, S.; Marples, D. Distribution of AQP2 and AQP3 water channels in human tissue microarrays. J. Mol. Histol. 2005, 36, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Z.; Xu, D.; Liu, Y.; Gao, Y. Aquaporin 3 promotes prostate cancer cell motility and invasion via extracellular signal-regulated kinase 1/2-mediated matrix metalloproteinase-3 secretion. Mol. Med. Rep. 2015, 11, 2882–2888. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Hayashi, S.; Matumoto, T.; Hashimoto, S.; Takayama, K.; Chinzei, N.; Kihara, S.; Haneda, M.; Kirizuki, S.; Kuroda, Y.; et al. Downregulation of aquaporin 9 decreases catabolic factor expression through nuclear factor-kappaB signaling in chondrocytes. Int. J. Mol. Med. 2018, 42, 1548–1558. [Google Scholar] [CrossRef]
- Edamana, S.; Pedersen, S.F.; Nejsum, L.N. Aquaporin water channels affect the response of conventional anticancer therapies of 3D grown breast cancer cells. Biochem. Biophys. Res. Commun. 2023, 639, 126–133. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirkegaard, T.; Riishede, A.; Tramm, T.; Nejsum, L.N. Aquaglyceroporins in Human Breast Cancer. Cells 2023, 12, 2185. https://doi.org/10.3390/cells12172185
Kirkegaard T, Riishede A, Tramm T, Nejsum LN. Aquaglyceroporins in Human Breast Cancer. Cells. 2023; 12(17):2185. https://doi.org/10.3390/cells12172185
Chicago/Turabian StyleKirkegaard, Teresa, Andreas Riishede, Trine Tramm, and Lene N. Nejsum. 2023. "Aquaglyceroporins in Human Breast Cancer" Cells 12, no. 17: 2185. https://doi.org/10.3390/cells12172185
APA StyleKirkegaard, T., Riishede, A., Tramm, T., & Nejsum, L. N. (2023). Aquaglyceroporins in Human Breast Cancer. Cells, 12(17), 2185. https://doi.org/10.3390/cells12172185





