How Light Modulates the Growth of Cyanidioschyzon merolae Cells by Changing the Function of Phycobilisomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Cyanidioschyzon merolae Growth Rate Study
2.3. SDS-PAGE and Immunoblotting Analysis
2.4. Production and Purification of Protein Standards
2.5. Determination of ATP and ADP Content in Whole Cells
2.6. Starch Concentration Measurements
2.7. Determination of Chlorophyll a Concentration
2.8. Oxygen Exchange Activity of Cells (Photosynthesis and Respiration Rate)
2.9. 77 K Fluorescence
3. Results
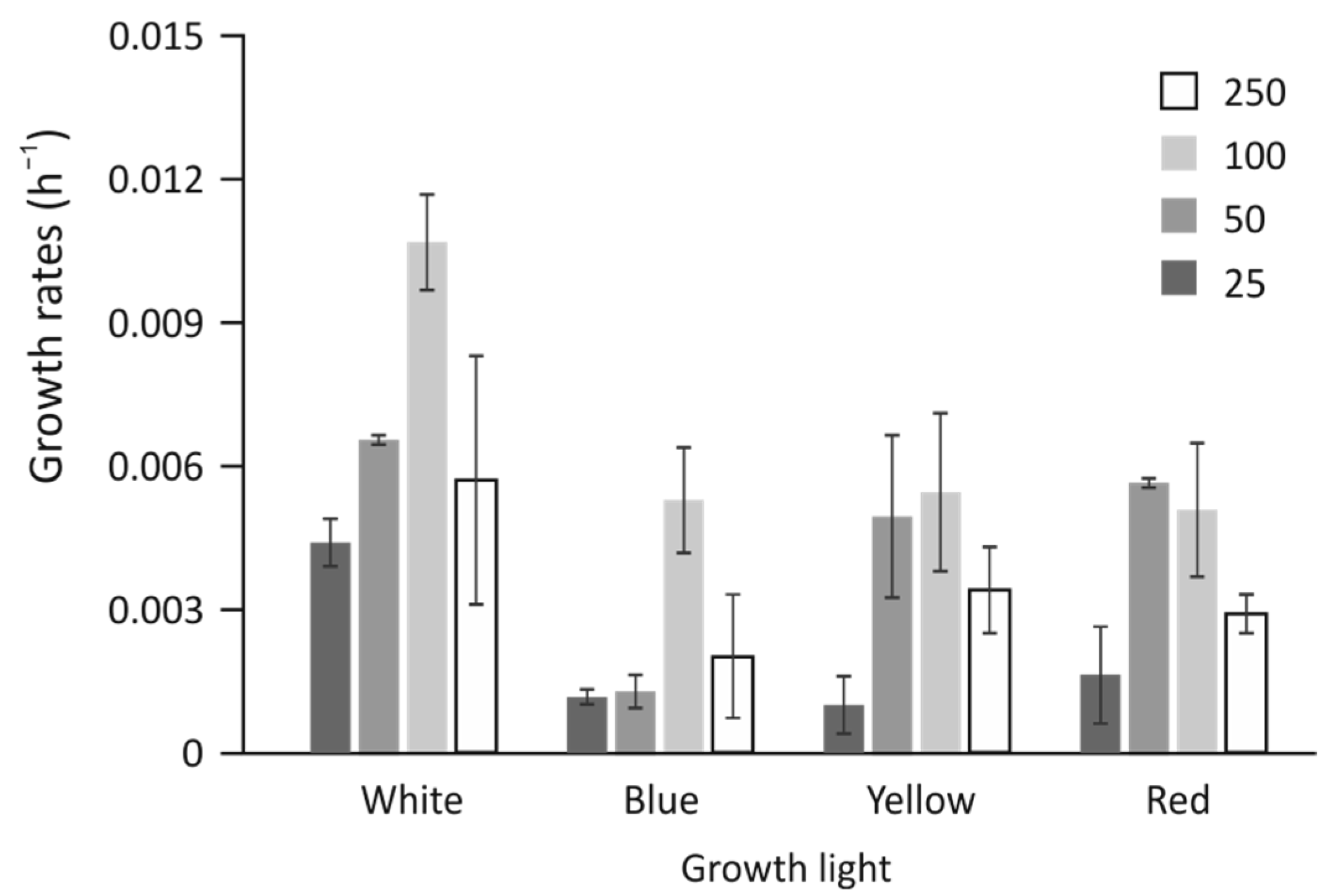
3.1. Cell Growth Rates
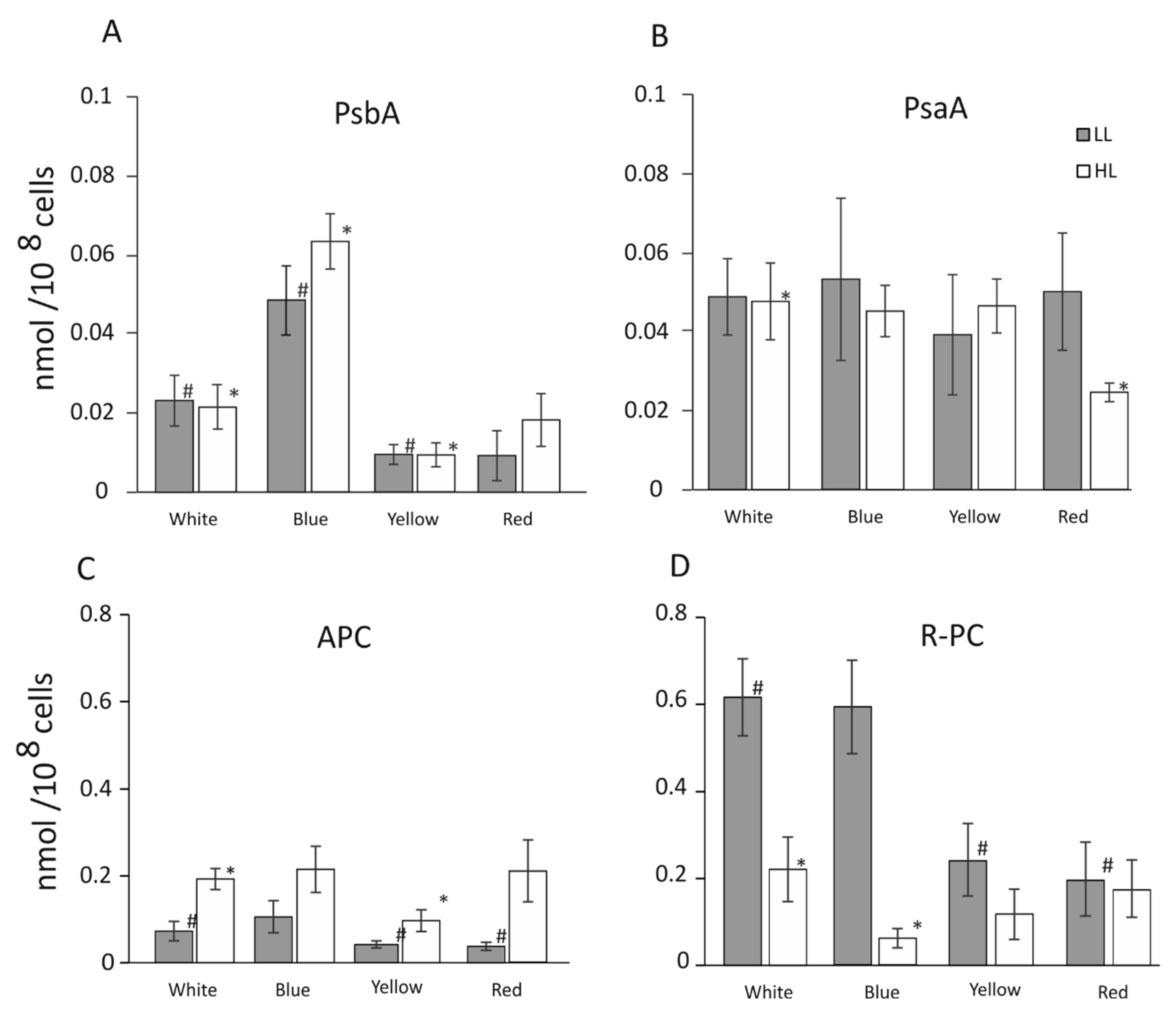
3.2. Level of PsaA, PsbA, APC, and R-PC Proteins
3.3. Initial Photosynthesis and Respiration of C. merolae Cells
3.4. The Concentration of Chlorophyll a in C. merolae Cells
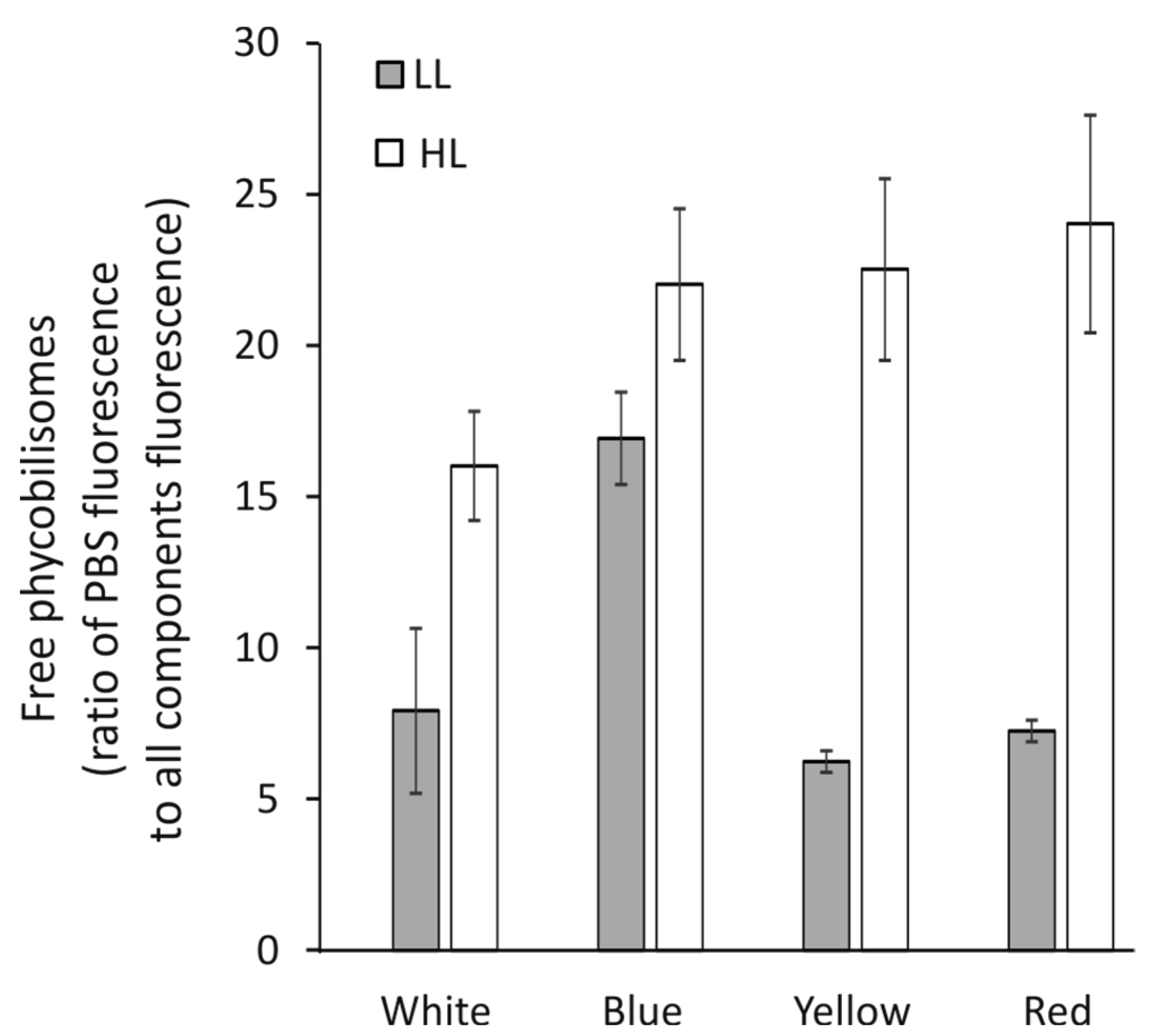
3.5. Fluorescence of Free Phycobilisomes
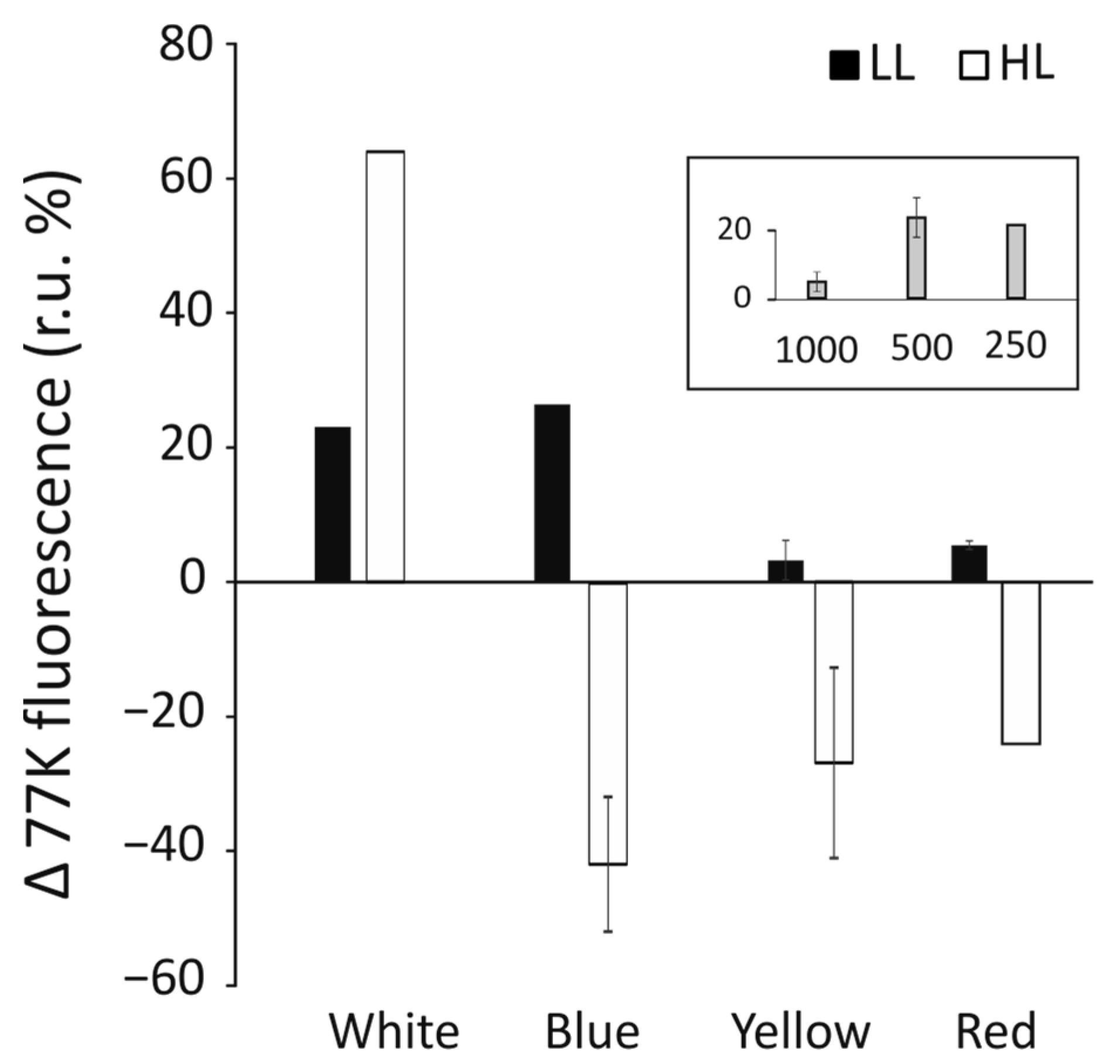
3.6. Induction of Phycobilisome Detachment by Short Exposure to High Light Intensity
3.7. Cell Energization, and ATP and ADP Concentration
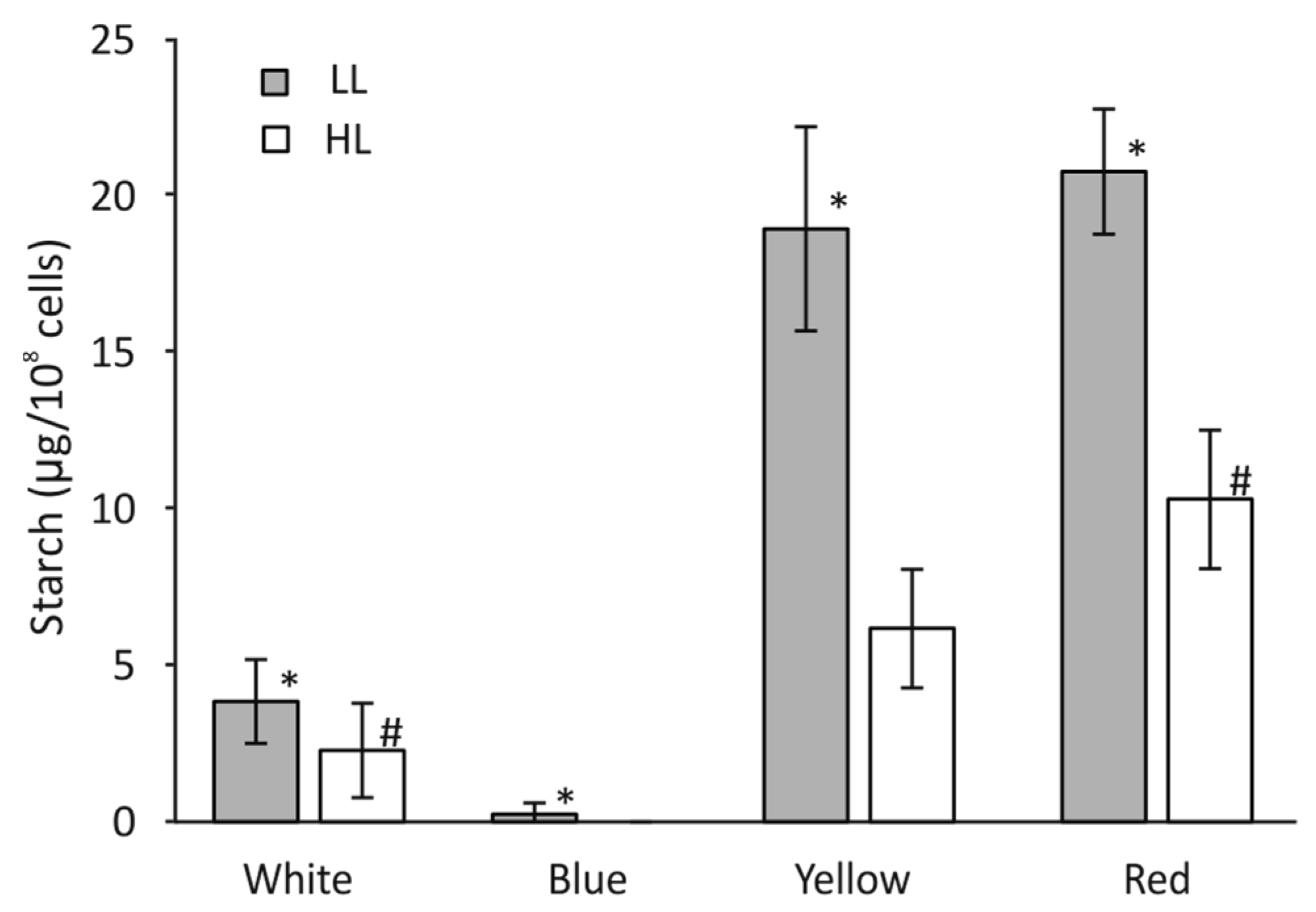
3.8. Starch Accumulation in C. merolae Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Merola, A.; Castaldo, R.; De Luca, P.; Gambardella, R.; Mussachio, A.; Taddei, R. Revision of Cyanidium caldarium. Three species of acidophilic algae. G Bot. Ital. 2009, 115, 189–195. [Google Scholar] [CrossRef]
- Ohta, N.; Sato, N.; Kuroiwa, T. Structure and organization of the mitochondrial genome of the unicellular red alga Cyanidioschyzon merolae deduced from the complete nucleotide sequence. Nucleic Acids Res. 1998, 26, 5190–5198. [Google Scholar] [CrossRef]
- Ohta, N.; Matsuzaki, M.; Misumi, O.; Miyagishima, S.; Nozaki, H.; Tanaka, K.; Shin, I.T.; Kohara, Y.; Kuroiwa, T. Complete sequence and analysis of the plastid genome of the unicellular red alga Cyanidioschyzon merolae. DNA Res. 2003, 10, 67–77. [Google Scholar] [CrossRef]
- Matsuzaki, M.; Misumi, O.; Shin, I.T.; Maruyama, S.; Takahara, M.; Miyagishima, S.-Y.; Mori, T.; Nishida, K.; Yagisawa, F.; Nishida, K.; et al. Genome sequence of the ultrasmall unicellular red alga Cyanidioschyzon merolae 10D. Nature 2004, 428, 653–657. [Google Scholar] [CrossRef]
- Parys, E.; Krupnik, T.; Kułak, I.; Kania, K.; Romanowska, E. Photosynthesis of the Cyanidioschyzon merolae cells in blue, red, and white light. Photosynth. Res. 2021, 147, 61–73. [Google Scholar] [CrossRef]
- Li, M.; Ma, J.; Li, X.; Sui, S.F. In situ cryo-ET structure of phycobilisome-photosystem II supercomplex from red alga. eLife 2021, 10, e69635. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.B.; Houghton, J.D.; Vernon, D.I. Biosynthesis of phycobilins. Formation of the chromophore of phytochrome, phycocyanin and phycoerythrin. J. Photochem. Photobiol. B 1990, 5, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Noy, D.; Moser, C.C.; Dutton, P.L. Design and engineering of photosynthetic light-harvesting and electron transfer using length, time, and energy scales. Biochim. Biophys. Acta 2006, 1757, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Beck, W.F.; Sauer, K. Energy-transfer and exciton-state relaxation processes in allophycocyanin. J. Phys. Chem. 2002, 96, 4658–4666. [Google Scholar] [CrossRef]
- Ueno, Y.; Aikawa, S.; Kondo, A.; Akimoto, S. Light adaptation of the unicellular red alga, Cyanidioschyzon merolae, probed by time-resolved fluorescence spectroscopy. Photosynth. Res. 2015, 125, 211–218. [Google Scholar] [CrossRef]
- Fujiwara, T.; Hirooka, S.; Miyagishima, S. A cotransformation system of the unicellular red alga Cyanidioschyzon merolae with blasticidin S deaminase and chloramphenicol acetyltransferase selectable markers. BMC Plant Biol. 2021, 21, 573. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Lauersen, K.J. Gene Delivery Technologies with Applications in Microalgal Genetic Engineering. Biology 2021, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Calderon, R.H. More than just a pair of blue genes: How cyanobacteria adapt to changes in their light environment. Physiol. Plant. 2020, 170, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Luimstra, V.M.; Schuurmans, J.M.; Hellingwerf, K.J.; Matthijs, H.C.P.; Huisman, J. Blue light induces major changes in the gene expression profile of the cyanobacterium Synechocystis sp. PCC 6803. Physiol. Plant. 2020, 170, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Minoda, A.; Sakagami, R.; Yagisawa, F.; Kuroiwa, T.; Tanaka, K. Improvement of culture conditions and evidence for nuclear transformation by homologous recombination in a red alga, Cyanidioschyzon merolae 10D. Plant Cell Physiol. 2004, 45, 667–671. [Google Scholar] [CrossRef]
- Hanahan, D. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 1983, 166, 557–580. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef]
- Krupnik, T.; Kotabová, E.; van Bezouwen, L.S.; Mazur, R.; Garstka, M.; Nixon, P.J.; Barber, J.; Kaňa, R.; Boekema, E.J.; Kargul, J. A reaction center-dependent photoprotection mechanism in a highly robust photosystem II from an extremophilic red alga, Cyanidioschyzon merolae. J. Biol. Chem. 2013, 288, 2329–2342. [Google Scholar] [CrossRef]
- Gardeström, P.; Wigge, B. Influence of photorespiration on ATP/ADP ratios in the chloroplasts, mitochondria and cytosol, studied by rapid fractionation of barley (Hordeum vulgare) protoplasts. Plant Physiol. 1988, 88, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.H. Carotenoids. In Chemistry and Biochemistry of Plants Pigments; Goodwin, T.W., Ed.; Academic Press: London, UK, 1976; Volume 2, pp. 38–165. [Google Scholar]
- Zienkiewicz, M.; Krupnik, T.; Drożak, A.; Golke, A.; Romanowska, E. Transformation of the Cyanidioschyzon merolae chloroplast genome: Prospects for understanding chloroplast function in extreme environments. Plant Mol. Biol. 2017, 93, 171–183. [Google Scholar] [CrossRef]
- Hellingwerf, K.J. The molecular basis of sensing and responding to light in microorganisms. Antonie Van Leeuwenhoek 2002, 81, 51–59. [Google Scholar] [CrossRef]
- Asimgil, H.; Kavakli, I.H. Purification and characterization of five members of photolyase/cryptochrome family from Cyanidioschyzon merolae. Plant Sci. 2012, 185–186, 190–198. [Google Scholar] [CrossRef]
- Wang, W.; Yu, L.J.; Xu, C.; Tomizaki, T.; Zhao, S.; Umena, Y.; Chen, X.; Qin, X.; Xin, Y.; Suga, M.; et al. Structural basis for blue-green light harvesting and energy dissipation in diatoms. Science 2019, 363, eaav0365. [Google Scholar] [CrossRef] [PubMed]
- Wada, M. Light-dependent spatiotemporal control of plant cell development and organelle movement in fern gametophytes. Microscopy 2019, 68, 13–36. [Google Scholar] [CrossRef]
- Huang, H.; Yoo, C.Y.; Bindbeutel, R.; Goldsworthy, J.; Tielking, A.; Alvarez, S.; Naldrett, M.J.; Evans, B.S.; Chen, M.; Nusinow, D.A. PCH1 integrates circadian and light-signaling pathways to control photoperiod-responsive growth in Arabidopsis. elife 2016, 5, e13292. [Google Scholar] [CrossRef] [PubMed]
- Essen, L.O.; Franz, S.; Banerjee, A. Structural and evolutionary aspects of algal blue light receptors of the cryptochrome and aureochrome type. J. Plant Physiol. 2017, 217, 27–37. [Google Scholar] [CrossRef]
- Lin, C. Plant blue-light receptors. Trends Plant Sci. 2000, 5, 337–342. [Google Scholar] [CrossRef]
- Ahmad, M.; Cashmore, A.R. Seeing blue: The discovery of cryptochrome. Plant Mol. Biol. 1996, 30, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Beel, B.; Prager, K.; Spexard, M.; Sasso, S.; Weiss, D.; Müller, N.; Heinnickel, M.; Dewez, D.; Ikoma, D.; Grossman, A.R.; et al. A flavin binding cryptochrome photoreceptor responds to both blue and red light in Chlamydomonas reinhardtii. Plant Cell 2012, 24, 2992–3008. [Google Scholar] [CrossRef]
- Rockwell, N.C.; Duanmu, D.; Martin, S.S.; Bachy, C.; Price, D.C.; Bhattacharya, D.; Worden, A.Z.; Lagarias, J.C. Eukaryotic algal phytochromes span the visible spectrum. Proc. Natl. Acad. Sci. USA 2014, 111, 3871–3876. [Google Scholar] [CrossRef]
- Zou, Y.; Wenzel, S.; Müller, N.; Prager, K.; Jung, E.M.; Kothe, E.; Kottke, T.; Mittag, M. An animal-like cryptochrome controls the Chlamydomonas sexual cycle. Plant Physiol. 2017, 174, 1334–1347. [Google Scholar] [CrossRef]
- Brudler, R.; Hitomi, K.; Daiyasu, H.; Toh, H.; Kucho, K.; Ishiura, M.; Kanehisa, M.; Roberts, V.A.; Todo, T.; Tainer, J.A.; et al. Identification of a new cryptochrome class: Structure function, and evolution. Mol. Cell 2003, 11, 59–67. [Google Scholar] [CrossRef]
- Kiontke, S.; Göbel, T.; Brych, A.; Batschauer, A. DASH-type cryptochromes—Solved and open questions. Biol. Chem. 2020, 40, 1487–1493. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, A.E.; Annunziata, R.; Jaubert, M.; Bouly, J.P.; Falciatore, A. Dealing with light: The widespread and multitasking cryptochrome/photolyase family in photosynthetic organisms. J. Plant Physiol. 2015, 172, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Terui, S.; Suzuki, K.; Takahashi, H.; Itoh, R.; Kuroiwa, T. Synchronization of chloroplast division in the ultramicroalga Cyanidioschyzon merolae (Rhodophyta) by treatment with light and aphidicolin. J. Phycol. 1995, 31, 958–961. [Google Scholar] [CrossRef]
- Nishida, K.; Yagisawa, F.; Kuroiwa, H.; Nagata, T.; Kuroiwa, T. Cell cycle-regulated, microtubule-independent organelle division in Cyanidioschyzon merolae. Mol. Biol. Cell 2005, 16, 2493–2502. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.K.; Fribourgh, J.L.; Van Gelder, R.N.; Partch, C.L. Animal cryptochromes: Divergent roles in light perception, circadian timekeeping and beyond. Photochem. Photobiol. 2017, 93, 128–140. [Google Scholar] [CrossRef]
- Darko, E.; Heydarizadeh, P.; Schoefs, B.; Sabzalian, M. Photosynthesis under artificial light: The shift in primary and secondary metabolism. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130243. [Google Scholar] [CrossRef]
- Cruz, J.A.; Avenson, T.J.; Kanazawa, A.; Takizawa, K.; Edwards, G.E.; Kramer, D.K. Plasticity in light reactions of photosynthesis for energy production and photoprotection. J. Exp. Bot. 2005, 56, 395–406. [Google Scholar] [CrossRef]
- Hernández-Zamora, M.; Martínez-Jerónimo, F. Exposure to the azo dye Direct blue 15 produces toxic effects on microalgae, cladocerans, and zebrafish embryos. Ecotoxicology 2019, 28, 890–902. [Google Scholar] [CrossRef]
- Luimstra, V.M.; Schuurmans, J.M.; de Carvalho, C.F.M.; Matthijs, H.C.P.; Hellingwerf, K.J.; Huisman, J. Exploring the low photosynthetic efficiency of cyanobacteria in blue light using a mutant lacking phycobilisomes. Photosynth. Res. 2019, 141, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Samsonoff, W.A.; MacColl, R. Biliproteins and phycobilisomes from cyanobacteria and red algae at the extremes of habitat. Arch. Microbiol. 2001, 176, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Kaňa, R.; Kotabová, E.; Lukeš, M.; Papáček, S.; Matonoha, C.; Liu, L.N.; Prášil, O.; Mullineaux, C.W. Phycobilisome mobility and its role in the regulation of light harvesting in red algae. Plant Physiol. 2014, 165, 1618–1631. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krupnik, T.; Zienkiewicz, M.; Wasilewska-Dębowska, W.; Drożak, A.; Kania, K. How Light Modulates the Growth of Cyanidioschyzon merolae Cells by Changing the Function of Phycobilisomes. Cells 2023, 12, 1480. https://doi.org/10.3390/cells12111480
Krupnik T, Zienkiewicz M, Wasilewska-Dębowska W, Drożak A, Kania K. How Light Modulates the Growth of Cyanidioschyzon merolae Cells by Changing the Function of Phycobilisomes. Cells. 2023; 12(11):1480. https://doi.org/10.3390/cells12111480
Chicago/Turabian StyleKrupnik, Tomasz, Maksymilian Zienkiewicz, Wioleta Wasilewska-Dębowska, Anna Drożak, and Kinga Kania. 2023. "How Light Modulates the Growth of Cyanidioschyzon merolae Cells by Changing the Function of Phycobilisomes" Cells 12, no. 11: 1480. https://doi.org/10.3390/cells12111480





