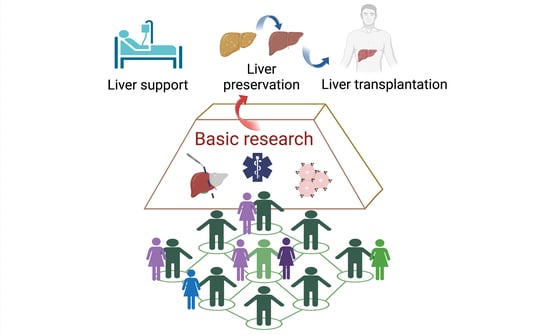
The Influence of Interdisciplinary Work towards Advancing Knowledge on Human Liver Physiology
Abstract
:1. Introduction
2. Interdisciplinary Models Applied to Translational Medicine
2.1. Animal Models of Liver Regeneration
2.2. Models Applied to Predict Different Scenarios after Liver Resection in Humans
3. Strategies to Face Current Challenges in Clinical Practice
3.1. Extracorporeal Liver Support and Liver Preservation Techniques
3.2. Tissue Engineering Techniques and Biotechnological Advances
3.3. Liver and Hepatocyte Xenotrasplantation
3.3.1. Liver Function by Hepatocyte Xenotransplants
3.3.2. Liver Function by Liver Xenotransplants
4. Current and Future Ethical Challenges
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tiniakos, D.G.; Kandilis, A.; Geller, S.A. Tityus: A forgotten myth of liver regeneration. J. Hepatol. 2010, 53, 357–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felekouras, E.S.; Kaparelos, D.C.; Papalampros, E. The history of liver surgery, hepatectomy and haemostasis. Hellenic J. Surg. 2010, 82, 280–296. [Google Scholar] [CrossRef]
- Smith, K.A. Louis Pasteur, the father of immunology? Front. Immunol. 2012, 3, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunschwig, A. Hepatic lobectomy for metastatic cancer. Cancer 1963, 16, 277–282. [Google Scholar] [CrossRef]
- Higgins, G.M.; Anderson, R.M. Experimental pathology of the liver. I. Restoration of the liver of the liver of the white rat following partial surgical removal. Arch. Pathol. 1931, 12, 186–202. [Google Scholar]
- Bucher, N.L.R.; Scott, J.F.; Aub, J.C. Regeneration of the liver in parabiotic rats. Cancer Res. 1951, 11, 457–465. [Google Scholar] [PubMed]
- Wenneker, A.S.; Sussman, N. Regeneration of liver tissue following partial hepatectomy in parabiotic rats. Proc. Soc. Exp. Biol. Med. 1951, 76, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, A.; Ove, P.; Polimeno, L.; Coetzee, M.; Makowka, L.; Barone, D.H.; Thiel, V.; Starzl, T.E. Regulation of liver size and regeneration: Importance in liver transplantation. Transplant. Proc. 1988, 20, 494–497. [Google Scholar]
- Starzl, T.E.; Fung, J.; Tzakis, A.; Todo, S.; Demetris, A.J.; Marino, I.R.; Doyle, H.; Zeevi, A.; Warty, V.; Michaels, M.; et al. Baboon-to-human liver transplantation. Lancet 1993, 341, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Michalopoulos, G.K. Liver regeneration. J. Cell Physiol. 2007, 213, 286–300. [Google Scholar] [CrossRef]
- Miyaoka, Y.; Ebato, K.; Kato, H.; Arakawa, S.; Shimizu, S.; Miyajima, A. Hypertrophy and unconventional cell division of hepatocytes underlie liver regeneration. Curr. Biol. 2012, 22, 1166–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyaoka, Y.; Miyajima, A. To divide or not to divide: Revisiting liver regeneration. Cell Div. 2013, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Fausto, N.; Riehle, K. Mechanisms of liver regeneration and their clinical implications. J. Hepatobiliary Pancreat. Surg. 2005, 12, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Han, N.; Du, L.; Wang, M.; Chen, L.; Tang, H. A narrative review of liver regeneration-from models to molecular basis. Ann. Transl. Med. 2021, 9, 1705. [Google Scholar] [CrossRef] [PubMed]
- Hadjittofi, C.; Feretis, M.; Martin, J.; Harper, S.; Huguet, E. Liver regeneration biology: Implications for liver tumour therapies. W. J. Clin. Oncol. 2021, 12, 1101–1156. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhao, L.; Fish, M.; Logan, C.Y.; Nusse, R. Self-renewing diploid Axin2+ cells fuel homeostatic renewal of the liver. Nature 2015, 524, 180–185. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Jimenez, R.J.; Sharma, K.; Luu, H.Y.; Hsu, B.Y.; Ravindranathan, A.; Stohr, B.A.; Willenbring, H. Broad distribution of hepatocyte proliferation in liver homeostasis and regeneration. Cell Stem Cell 2020, 26, 27–33. [Google Scholar] [CrossRef]
- So, J.; Kim, A.; Lee, S.-H.; Shin, D. Liver progenitor cell-driven liver regeneration. Exp. Mol. Med. 2020, 52, 1230–1238. [Google Scholar] [CrossRef]
- Fausto, N. Liver regeneration: From laboratory to clinic. Liver Transpl. 2001, 7, 835–844. [Google Scholar] [CrossRef]
- Ku, Y.; Tominaga, M.; Sugimoto, T.; Iwakasi, T.; Fukumoto, T.; Takahashi, T.; Suzuki, Y.; Kuroda, Y. Preoperative hepatic venous embolization for partial hepatectomy combined with segmental resection of major hepatic vein. Br. J. Surg. 2002, 89, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.; Hickman, R.; Terblanche, J. A porcine model for the study of liver regeneration. J. Investig. Surg. 1988, 1, 139–142. [Google Scholar] [CrossRef]
- Gaglio, P.J.; Baskin, G.; Bohm, R.; Blanchard, J.; Cheng, S.; Dunne, B.; Davidson, J.; Liu, H.; Dash, S. Hepatectomy and laparoscopic-guided liver biopsy in Rhesus Macaques (Macaca mulatta): Novel approach for study of liver regeneration. Comp. Med. 2000, 50, 363–368. [Google Scholar]
- Khan, D.; Hickman, R.; Terblanche, J.; von Sommoggy, S. Partial hepatectomy and liver regeneration in pigs- the response to different resection sizes. J. Surg. Res. 1988, 45, 176–180. [Google Scholar] [CrossRef]
- Iguchi, K.; Hatano, E.; Yamanaka, K.; Sato, M.; Yamamoto, G.; Kasai, Y.; Okamoto, T.; Okuno, M.; Taura, K.; Fukumoto, K.; et al. Hepatoprotective effect or pretreatment with olprinone in a swine partial hepatectomy model. Liver Transpl. 2014, 20, 838–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darnis, B.; Mohkam, K.; Schmitt, Z.; Ledochowski, S.; Vial, J.-P.; Duperret, S.; Vogt, S.; Demian, C.; Golse, N.; Mezoughi, S.; et al. Subtotal hepatectomy in swine for studying small-for-size syndrome and portal inflow modulation: It is reliable? HPB (Oxford) 2015, 17, 881–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Navarro-Alvarez, N.; Yang, C.; Markmann, J.F.; Dong, J. A reliable scoring system after major liver resection in mice. J. Surg. Res. 2016, 204, 75–82. [Google Scholar] [CrossRef]
- Taub, R. Liver regeneration: From myth to mechanism. Nat. Rev. Mol. Cell. Biol. 2004, 5, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Fausto, N.; Campbell, J.S.; Riehle, K.J. Liver regeneration. Hepatology 2006, 43, S45–S53. [Google Scholar] [CrossRef]
- Michalopoulous, G.K.; Bhushan, B. Liver regeneration: Biological and pathological mechanisms and implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 40–55. [Google Scholar] [CrossRef]
- Borowiak, M.; Garratt, A.N.; Wustefeld, T.; Strehle, M.; Trautwein, C.; Birchmeier, C. Met provides essential signals for liver regeneration. Proc. Natl. Acad. Sci. USA 2004, 101, 10608–10613. [Google Scholar] [CrossRef] [Green Version]
- Michalopoulos, G.K. Principles of liver regeneration and growth homeostasis. Compr. Physiol. 2013, 3, 485–513. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Wang, M.; Chen, E.; Tang, H. Liver regeneration: Analysis of the main relevant signaling molecules. Mediators Inflamm. 2017, 2017, 4256352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Lu, L.; Cai, X. Liver regeneration and cell transplantation for end-stage liver disease. Biomolecules 2021, 11, 1907. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.-A.; Petrowsky, H.; DeOliveira, M.L.; Graf, R. Strategies for liver surgery and partial liver transplantation. N. Engl. J. Med. 2007, 356, 1545–1559. [Google Scholar] [CrossRef]
- Müller, P.C.; Linecker, M.; Kirimker, E.O.; Oberkofler, C.E.; Clavien, P.-A.; Balci, D.; Petrowsky, H. Induction of liver hypertrophy for extended liver surgery and partial liver transplantation: State of the art of parenchyma augmentation-assisted liver surgery. Langenbecks Arch. Surg. 2021, 406, 2201–2215. [Google Scholar] [CrossRef]
- Rous, P.; Larimore, L.D. Relation of the portal blood to liver maintenance. J. Exp. Med. 1920, 31, 609–632. [Google Scholar] [CrossRef] [Green Version]
- Yaprak, O.; Guler, N.; Altaca, G.; Dayanga, M.; Demirbas, T.; Akyildiz, M.; Ulusoy, L.; Tokat, Y.; Yuzer, Y. Ratio of remnant to total liver volume or remnant to body weight: Which one is more predictive on donor outcomes? HPB (Oxford) 2012, 14, 476–482. [Google Scholar] [CrossRef] [Green Version]
- Abdalla, E.K.; Denys, A.; Chevalier, P.; Nemr, R.A.; Vautney, J.N. Total and segmental liver volume variations: Implications for liver surgery. Surgery 2004, 135, 404–410. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Nagino, M.; Nimura, Y. Mechanisms of hepatic regeneration following portal vein embolization and partial hepatectomy: A review. World J. Surg. 2007, 31, 367–374. [Google Scholar] [CrossRef]
- Tashiro, S. Mechanism of liver regeneration after liver resection and portal vein embolization (ligation) is different? J. Hepatobiliary Pancreat. Surg. 2009, 16, 292–299. [Google Scholar] [CrossRef]
- Moris, D.; Vernadakis, S.; Papalampros, A.; Vailas, M.; Dimitrokallis, N.; Petrou, A.; Dimitroulis, D. Mechanistic insights of rapid liver regeneration after associating liver partition and portal vein ligation for stage hepatectomy. World J. Gastroenterol. 2016, 22, 7613–7624. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Lesurtel, M.; Melloul, E.; Limani, P.; Tschuor, C.; Graf, R.; Humar, B.; Clavien, P.A. ALPPS: From human to mice highlighting accelerated and novel mechanisms of liver regeneration. Ann. Surg. 2014, 260, 839–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, K.; Murakami, T.; Kawaguchi, D.; Hiroshima, Y.; Koda, K.; Yamazaki, K.; Ishida, Y.; Tanaka, K. Histologic features after surgery associating liver partition and portal vein ligation for staged hepatectomy versus those after hepatectomy with portal vein embolization. Surgery 2016, 159, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.G.; Goessling, W. The lure of zebrafish in liver research: Regulation of hepatic growth in development and regeneration. Curr. Opin. Genet. Dev. 2015, 32, 153–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado-Coello, B. Liver regeneration observed across the different classes of vertebrates from an evolutionary perspective. Heliyon 2021, 7, e06449. [Google Scholar] [CrossRef]
- Michalopoulos, G.K. Liver regeneration after partial hepatectomy. Critical analysis of mechanistic dilemmas. Am. J. Pathol. 2010, 176, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Martínez de la Maza, L.; Prado, V.; Hessheimer, A.J.; Muñoz, J.; García-Valdecasas, J.C.; Fondevila, C.A. novel and simple formula to predict liver mass in porcine experimental models. Sci. Rep. 2019, 9, 12459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christ, B.; Collatz, M.; Dahmen, U.; Herrmann, K.-H.; Höpfl, S.; König, M.; Lambers, L.; Marz, M.; Meyer, D.; Radde, N.; et al. Hepatectomy-induced alterations in hepatic perfusion and function- toward multi-scale computational modeling for a better prediction of post-hepatectomy liver function. Front. Physiol. 2021, 12, 733868. [Google Scholar] [CrossRef] [PubMed]
- Periwal, V.; Gaillard, J.R.; Needleman, L.; Doria, R. Mathematical model of liver regeneration in human live donors. J. Cell Physiol. 2014, 229, 599–606. [Google Scholar] [CrossRef]
- Yamamoto, K.N.; Ishii, M.; Inoue, Y.; Hirokawa, F.; MacArthur, B.D.; Nakamura, A.; Haeno, H.; Uchiyama, K. Prediction of postoperative liver regeneration from clinical information using a data-led mathematical model. Sci. Rep. 2016, 6, 34214. [Google Scholar] [CrossRef] [Green Version]
- Debbaut, C.; Segers, P.; Cornillie, P.; Casteleyn, C.; Dierick, M.; Laleman, W.; Monbaliu, D. Analyzing the human liver vascular architecture by combining vascular corrosion casting and micro-CT scanning: A feasibility study. J. Anat. 2014, 224, 509–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, R.; Hunter, P.; Cousins, W.; Ho, H.; Bartlett, A.; Safaei, S. Anatomically based simulation of hepatic perfusion in the human liver. Int. J. Numer. Method. Biomed. Eng. 2019, 35, e3229. [Google Scholar] [CrossRef] [PubMed]
- Lorente, S.; Hautefeuille, M.; Sanchez-Cedillo, A. The liver, a functionalized vascular structure. Sci. Rep. 2020, 10, 16194. [Google Scholar] [CrossRef] [PubMed]
- Torres Rojas, A.M.; Lorente, S.; Hautefeuille, M.; Sanchez-Cedillo, A. Hierarchical modeling of the liver vascular system. Front. Physiol. 2021, 12, 733165. [Google Scholar] [CrossRef] [PubMed]
- Mayo Clinic Website. Available online: https://www.mayoclinic.org/tests-procedures/liver-transplant/about/pac-20384842 (accessed on 18 July 2022).
- Struecker, B.; Raschzok, N.; Sauer, I.M. Liver support strategies: Cutting-edge technologies. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 166–176. [Google Scholar] [CrossRef]
- Kanjo, A.; Ocskay, K.; Gede, N.; Kiss, S.; Szakács, Z.; Párniczky, M.S.; Stange, J.; Hegyi, P.; Molnár, Z. Efficacy and safety of liver support devices in acute and hyperacute liver failure: A systematic review and network meta-analysis. Sci. Rep. 2021, 11, 4189. [Google Scholar] [CrossRef]
- Ocskay, K.; Kanjo, A.; Gede, N.; Szakács, Z.; Pár, G.; Eross, B.; Stange, J.; Mitzner, S.; Hegyi, P.; Molnár, Z. Uncertainty in the impact of liver support systems in acute-on-chronic liver failure: A systematic review and network meta-analysis. Ann. Intensive Care 2021, 11, 10. [Google Scholar] [CrossRef]
- Saliba, F.; Bañares, R.; Larsen, F.S.; Wilmer, A.; Parés, A.; Mitzner, S.; Stange, J.; Fuhrmann, V.; Gilg, S.; Hassanein, T.; et al. Artificial liver support in patients with liver failure: A modified DELPHI consensus of international experts. Intensive Care Med. 2022, 48, 1352–1367. [Google Scholar] [CrossRef]
- Narayanan, M.; Vora, R.S.; Flynn, M.M.; Subramanian, R.S. The efficacy of albumin dialysis in the treatment of severe cholestatic drug-induced liver injury. Crit. Care Explor. 2022, 4, e0752. [Google Scholar] [CrossRef]
- Ocskay, K.; Tomescu, D.; Faltlhauser, A.; Jacob, D.; Friesecke, S.; Malbrain, M.; Kogelmann, K.; Bogdanski, R.; Bach, F.; Fritz, H.; et al. Hemoadsorption in ‘liver indication’—Analysis of 109 patients’ data from the CytoSorb International Registry. J. Clin. Med. 2021, 10, 5182. [Google Scholar] [CrossRef]
- Kaps, L.; Schleicher, E.M.; Medina Montano, C.; Bros, M.; Gairing, S.J.; Ahlbrand, C.J.; Michel, M.; Klimpke, P.; Kremer, W.M.; Holtz, S.; et al. Influence of Advanced Organ Support (ADVOS) on cytokine levels in patients with acute-on-chronic liver failure (ACLF). J. Clin. Med. 2022, 11, 2782. [Google Scholar] [CrossRef] [PubMed]
- Klammt, S.; Mitzner, S.R.; Stange, J.; Loock, J.; Heemann, U.; Emmrich, J.; Reisinger, E.C.; Schmidt, R. Improvement of impaired albumin binding capacity in acute-on-chronic liver failure by albumin dialysis. Liver Transpl. 2008, 14, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Chedid, M.F.; Pinto, M.A.; Juchem, J.F.G.; Grezzana-Fljo, T.J.M.; Kruel, C.R.P. Liver preservation prior to transplantation: Past, present, and future. World J. Gastroint. Surg. 2019, 11, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Lascaris, B.; de Meijer, V.E.; Porte, R.J. Liver machine perfusion as a dynamic platform for regenerative purposes: What does the future have in store for us? J. Hepatol. 2022, 77, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Khajavi, M.; Hashemi, M.; Kalalinia, F. Recent advances in optimization of liver decellularization procedures used for liver regeneration. Life Sci. 2021, 281, 119801. [Google Scholar] [CrossRef]
- Vazirzadeh, M.; Azarpira, N.; Davoodi, P.; Vosough, M.; Ghaedi, K. Natural scaffolds used for liver regeneration: A narrative update. Stem Cell Rev. Rep. 2022, 18, 2262–2278. [Google Scholar] [CrossRef]
- Uygun, B.E.; Soto-Gutierrez, A.; Yagi, H.; Izamis, M.L.; Guzzardi, M.A.; Shulman, C.; Milwid, J.; Kobayashi, N.; Tilles, A.; Berthiaume, F.; et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med. 2010, 16, 814–820. [Google Scholar] [CrossRef]
- Yagi, H.; Fukumitsu, K.; Fukuda, K.; Kitago, M.; Shinoda, M.; Obara, H.; Itanu, O.; Kawachi, S.; Tanabe, M.; Coudriet, G.M.; et al. Human- scale whole-organ bioengineering for liver transplantation: A regenerative medicine approach. Cell Transplant. 2013, 22, 231–242. [Google Scholar] [CrossRef] [Green Version]
- Kajbafzadeh, A.M.; Javan-Farazmand, N.; Monajemzadeh, M.; Baghayee, A. Determining the optimal decellularization and sterilization protocol for preparing a tissue scaffold of a human- sized liver tissue. Tissue Eng. Part C Methods. 2013, 19, 642–651. [Google Scholar] [CrossRef]
- Mazza, G.; Rombouts, K.; Rennie Hall, A.; Urbani, L.; Vinh Luong, T.; Al-Akkad, W.; Longato, L.; Brown, D.; Maghsoudlou, P.; Dhillon, A.P.; et al. Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation. Sci. Rep. 2015, 5, 13079. [Google Scholar] [CrossRef] [Green Version]
- Verstegen, M.M.A.; Willemse, J.; van den Hoek, S.; Kremers, G.-J.; Luider, T.M.; van Huizen, N.A.; Willemssen, F.E.J.A.; Metselaar, H.J.; Ijzermans, J.N.M.; van der Laan, L.J.W.; et al. Decellularization of whole human liver grafts using controlled perfusion for transplantable organ bioscaffolds. Stem Cells Dev. 2017, 26, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Tharwat, M.; Larson, E.L.; Felgendreff, P.; Hosseiniasl, S.M.; Rmilah, A.A.; Safwat, K.; Ross, J.J.; Nyberg, S.L. Re-endothelialization of decellularized liver scaffolds: A step for bioengineered liver transplantation. Front. Bioeng. Biotechnol. 2022, 10, 833163. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultured by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [Green Version]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.-R.; Ueno, Y.; Zheng, Y.-W.; Koike, N.; et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499, 481–484. [Google Scholar] [CrossRef]
- Takebe, T.; Sekine, K.; Kimura, M.; Yoshikawa, E.; Ayano, S.; Koido, M.; Funayama, S.; Nakanishi, N.; Hisai, T.; Kobayashi, T.; et al. Massive and reproducible production of liver buds entirely from human pluripotent stem cells. Cell Rep. 2017, 21, 2661–2670. [Google Scholar] [CrossRef] [Green Version]
- Mun, S.J.; Ryu, J.-S.; Lee, M.-O.; Son, Y.S.; Oh, S.J.; Cho, H.-S.; Son, M.-Y.-; Kim, D.-S.; Kim, S.J.; Yoo, H.J.; et al. Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J. Hepatol. 2019, 71, 970–985. [Google Scholar] [CrossRef]
- Kruitwagen, H.S.; Oosterhoff, L.A.; Vernooij, I.G.W.H.; Schrall, I.M.; van Wolferen, M.E.; Bannink, F.; Roesch, C.; van Uden, L.; Molenaar, M.R.; Helms, J.B.; et al. Long-term adult feline liver organoid cultures for disease modeling of hepatic steatosis. Stem Cell Rep. 2017, 8, 822–830. [Google Scholar] [CrossRef] [Green Version]
- Lo Nigro, A.; Gallo, A.; Bulati, M.; Vitale, G.; Paini, D.S.; Pampalone, M.; Galvagno, D.; Conaldi, P.G.; Miceli, V. Amnion-derived mesenchymal stromal/stem cell paracrine signals potentiate human liver organoid differentiation: Translational implications for liver regeneration. Front. Med. 2021, 8, 746298. [Google Scholar] [CrossRef]
- Harrison, S.P.; Baumgarten, S.F.; Verma, R.; Lunov, O.; Dajneka, A.; Sullivan, G.J. Liver organoids: Recent developments, limitations and potential. Front. Med. 2021, 8, 574047. [Google Scholar] [CrossRef]
- Telles-Silva, K.A.; Pacheco, L.; Komatsu, S.; Chianca, F.; Caires-Júnior, L.C.; Silva Araujo, B.H.; Goulart, E.; Zatz, M. Applied hepatic bioengeering: Modeling the human liver using organoid and liver-on-a-chip technologies. Front. Bioeng. Biotechnol. 2022, 10, 845360. [Google Scholar] [CrossRef] [PubMed]
- Lucendo-Villarin, B.; Meseguer-Ripolles, J.; Drew, J.; Fischer, L.; Ma, E.; Flint, O.; Simpson, K.J.; Machesky, L.M.; Mountford, J.C.; Hay, D.C. Development of a cost-effective automated platform to produce human liver spheroids for basic and applied research. Biofabrication 2021, 13, 015009. [Google Scholar] [CrossRef] [PubMed]
- Subba Rao, M.; Sasikala, M.; Reddy, D.N. Thinking outside the liver: Induced pluripotent stem cells for hepatic applications. World J. Gastroenterol. 2013, 19, 3385–3396. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Curran, J.E.; Williams-Blangero, S.; Blangero, J. Efficient generation of functional hepatocytes from human induced pluripotent stem cells for disease modeling and disease gene discovery. Methods Mol. Biol. 2022, 2549, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Kietzman, T. Metabolic zonation of the liver: The oxygen gradient revisited. Redox Biol. 2017, 11, 622–630. [Google Scholar] [CrossRef]
- Leedale, J.A.; Lucendo-Villarin, B.; Meseguer-Ripolles, J.; Kasarinaite, A.; Webb, S.D.; Hay, D.C. Mathematical modelling of oxygen gradients in stem cell-derived liver tissue. PLoS ONE 2021, 16, e0244070. [Google Scholar] [CrossRef]
- Chhabra, A.; Greco Song, H.-H.; Grselak, K.A.; Polacheck, W.J.; Fleming, H.E.; Chen, C.S.; Bhatia, S.N. A vascularized model of the human liver mimics regenerative responses. Proc. Natl. Acad. Sci. USA. 2022, 119, e2115867119. [Google Scholar] [CrossRef]
- Ahrens, H.E.; Petersen, B.; Herrmann, D.; Lucas-Hahn, A.; Hassel, P.; Ziegler, M.; Kues, W.A.; Baulain, U.; Baars, W.; Schwinzer, R.; et al. siRNA mediated knockdown of tissue factor expression in pigs for xenotransplantation. Am. J. Transplant. 2015, 15, 1407–1414. [Google Scholar] [CrossRef]
- Ramirez, P.; Montoya, M.J.; Ríos, A.; García Palenciano, C.; Majado, M.; Chávez, R.; Muñoz, A.; Fernández, O.M.; Sánchez, A.; Segura, B.; et al. Prevention of hyperacute rejection in a model of orthotopic liver xenotransplantation from pig to baboon using polytransgenic pig livers (CD55, CD59, and H-transferase). Transplant. Proc. 2005, 37, 4103–4106. [Google Scholar] [CrossRef]
- Kolber-Simonds, D.; Lai, L.; Watt, S.R.; Denaro, M.; Arn, S.; Augenstein, M.L.; Betthauser, J.; Carter, D.B.; Greenstein, J.L.; Hao, Y.; et al. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc. Natl. Acad. Sci. USA 2004, 101, 7335–7340. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Cooper, D.K. Anti-Gal, alpha-Gal epitopes, and xenotransplantation. Subcell. Biochem. 1999, 32, 229–257. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Schuetz, C.; Elias, N.; Wamala, I.; Varma, M.; Smith, R.N.; Robson, S.C.; Cosimi, A.B.; Sachs, D.H.; Hertl, M. Up to 9-day survival and control of thrombocytopenia following alpha1,3-galactosyl transferase knockout swine liver xenotransplantation in baboons. Xenotransplantation 2012, 19, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.; Machaidze, Z.; Wamala, I.; Fraser, J.W.; Navarro-Alvarez, N.; Kim, K.; Schuetz, C.; Shi, S.; Zhu, A.; Hertl, M.; et al. Increased transfusion-free survival following auxiliary pig liver xenotransplantation. Xenotransplantation 2014, 21, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Yeh, H.; Wei, L.; Enjyoj, K.; Machaidze, Z.; Csizmad, E.; Schuetz, C.; Lee, K.M.; Deng, S.; Robson, S.C.; et al. Mechanisms of xenogeneic baboon platelet aggregation and phagocytosis by porcine liver sinusoidal endothelial cells. PLoS ONE 2012, 7, e47273. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Alvarez, N.; Shah, J.A.; Zhu, A.; Ligocka, J.; Yeh, H.; Elias, N.; Rosales, I.; Colvin, R.; Cosimi, A.B.; Markmann, J.F.; et al. The Effects of exogenous administration of human coagulation factors following pig-to-baboon liver xenotransplantation. Am. J. Transplant. 2016, 16, 1715–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denner, J. Why was PERV not transmitted during preclinical and clinical xenotransplantation trials and after inoculation of animals? Retrovirology 2018, 15, 28. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; He, W.; Ruan, Y.; Geng, Q. First pig-to-human heart transplantation. Innovation (Camb) 2022, 3, 100223. [Google Scholar] [CrossRef]
- Quaglia, A.; Lehec, S.C.; Hughes, R.D.; Mitry, R.R.; Knisely, A.S.; Devereaux, S.; Richards, J.; Rela, M.; Heaton, N.D.; Portmann, B.C.; et al. Liver after hepatocyte transplantation for liver-based metabolic disorders in children. Cell Transplant. 2008, 17, 1403–1414. [Google Scholar] [CrossRef] [Green Version]
- Gunsalus, J.R.; Brady, D.A.; Coulter, S.M.; Gray, B.M.; Edge, A.S. Reduction of serum cholesterol in Watanabe rabbits by xenogeneic hepatocellular transplantation. Nat. Med. 1997, 3, 48–53. [Google Scholar] [CrossRef]
- Nagata, H.; Ito, M.; Cai, J.; Edge, A.S.; Platt, J.L.; Fox, I.J. Treatment of cirrhosis and liver failure in rats by hepatocyte xenotransplantation. Gastroenterology 2003, 124, 422–431. [Google Scholar] [CrossRef]
- Makowka, L.; Rotstein, L.E.; Falk, R.E.; Falk, J.A.; Nossaal, N.A.; Langer, B.; Blendis, L.M.; Phillips, M.J. Allogeneic and xenogeneic hepatocyte transplantation in experimental hepatic failure. Transplantation 1980, 30, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Stefan, A.M.; Coulter, S.; Gray, B.; LaMorte, W.; Nikalaeson, S.; Edge, A.S.; Afdhal, N.H. Xenogeneic transplantation of porcine hepatocytes into the CCl4 cirrhotic rat model. Cell Transplant. 1999, 8, 649–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, T.; Navarro-Alvarez, N.; Soto-Gutierrez, A.; Yuasa, T.; Iwamuro, M.; Kubota, Y.; Seita, M.; Kawamoto, H.; Javed, S.M.; Kondo, E.; et al. Treatment of acute liver failure in mice by hepatocyte xenotransplantation. Cell Transplant. 2010, 19, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Nishitai, R.; Koch, C.A.; Ogata, K.; Knudsen, B.E.; Plummer, T.B.; Butters, K.A.; Platt, J.L. Toward the survival and function of xenogeneic hepatocyte grafts. Liver Transpl. 2005, 11, 39–50. [Google Scholar] [CrossRef]
- Nishitai, R.; Plummer, T.B.; Platt, J.L. Detection of albumin synthesis in transplanted porcine hepatocytes in mice. Liver Transpl. 2002, 8, 972–974. [Google Scholar] [CrossRef]
- Sarkis, R.; Benoist, S.; Honiger, J.; Baudrimont, M.; Delelo, R.; Balladur, P.; Capeau, J.; Nordlinger, B. Transplanted cryopreserved encapsulated porcine hepatocytes are as effective as fresh hepatocytes in preventing death from acute liver failure in rats. Transplantation 2000, 70, 58–64. [Google Scholar]
- Benoist, S.; Sarkis, R.; Barbu, V.; Honiger, J.; Baudrimont, M.; Lakehal, F.; Becquemont, L.; Delelo, R.; Housset, C.; Balladur, P.; et al. Survival and functions of encapsulated porcine hepatocytes after allotransplantation or xenotransplantation without immunosuppression. Surgery 2001, 129, 606–616. [Google Scholar] [CrossRef]
- Baldini, E.; Cursio, R.; De Sousa, G.; Margara, A.; Honiger, J.; Saint-Paul, M.-C.; Bayer, P.; Raimondi, V.; Rahmani, R.; Mouiel, J.; et al. Peritoneal implantation of cryopreserved encapsulated porcine hepatocytes in rats without immunosuppression: Viability and function. Transplant. Proc. 2008, 40, 2049–2052. [Google Scholar] [CrossRef]
- Mei, J.; Sgroi, A.; Mai, G.; Baertschiger, R.; Gonelle-Gispert, C.; Serre-Beinier, V.; Morel, P.; Bühler, L.H. Improved survival of fulminant liver failure by transplantation of microencapsulated cryopreserved porcine hepatocytes in mice. Cell Transplant. 2009, 18, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Joseph, B.; Malhi, H.; Bhargava, K.K.; Palestro, C.J.; McCuskey, R.S.; Gupta, S. Kupffer cells participate in early clearance of syngeneic hepatocytes transplanted in the rat liver. Gastroenterology 2002, 123, 1677–1685. [Google Scholar] [CrossRef]
- Oldenborg, P.A.; Zheleznyak, A.; Fang, Y.F.; Lagenaur, C.F.; Gresham, H.D.; Lindberg, F.P. Role of CD47 as a marker of self on red blood cells. Science 2000, 288, 2051–2054. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Alvarez, N.; Yang, Y.G. CD47: A new player in phagocytosis and xenograft rejection. Cell. Mol. Immunol. 2011, 8, 285–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro-Alvarez, N.; Yang, Y.G. Lack of CD47 on donor hepatocytes promotes innate immune cell activation and graft loss: A potential barrier to hepatocyte xenotransplantation. Cell Transplant. 2014, 23, 345–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machaidze, Z.; Yeh, H.; Wei, L.; Schuetz, C.; Carvello, M.; Sgroi, A.; Smith, R.N.; Schuurman, H.-J.; Sachs, D.H.; Morel, P.; et al. Testing of microencapsulated porcine hepatocytes in a new model of fulminant liver failure in baboons. Xenotransplantation 2017, 24, e12297. [Google Scholar] [CrossRef] [PubMed]
- Nagata, H.; Nishitai, R.; Shirota, C.; Zhang, J.-L.; Koch, C.A.; Cai, J.; Awwad, M.; Schuurman, H.-J.; Christians, U.; Abe, M.; et al. Prolonged survival of porcine hepatocytes in cynomolgus monkeys. Gastroenterology 2007, 132, 321–329. [Google Scholar] [CrossRef]
- Makowa, L.; Cramer, D.V.; Hoffman, A.; Breda, M.; Sher, L.; Eiras-Hreha, G.; Tuso, P.J.; Yasunaga, C.; Cosenza, C.A.; Wu, G.D.; et al. The use of a pig liver xenograft for temporary support of a patient with fulminant hepatic failure. Transplantation 1995, 59, 1654–1659. [Google Scholar] [CrossRef]
- Calne, R.Y.; White, H.J.; Herbertson, B.M.; Millard, P.R.; Davis, D.R.; Salaman, J.R.; Samuel, J.R. Pig-to-baboon liver xenografts. Lancet 1968, 1, 1176–1178. [Google Scholar] [CrossRef]
- Calne, R.Y.; Davis, D.R.; Pena, J.R.; Balner, H.; de Vries, M.; Herbetson, B.M.; Millard, P.R.; Joysey, V.C.; Seaman, M.J.; Samuel, J.R.; et al. Hepatic allografts and xenografts in primates. Lancet 1970, 1, 103–106. [Google Scholar] [CrossRef]
- Powelson, J.; Cosimi, A.B.; Austen, W., Jr.; Bailen, M.; Colvin, R.; Gianello, P.; Savlinski, T.; Lorf, T.; Kawai, T.; Tanaka, M.; et al. Porcine-to-primate orthotopic liver transplantation. Transplant. Proc. 1994, 26, 1353–1354. [Google Scholar]
- Ramirez, P.; Chavez, R.; Majado, M.; Munitiz, V.; Muñoz, A.; Hernandez, Q.; Palenciano, C.G.; Pino-Chavez, G.; Loba, M.; Minguela, A.; et al. Life-supporting human complement regulator decay accelerating factor transgenic pig liver xenograft maintains the metabolic function and coagulation in the nonhuman primate for up to 8 days. Transplantation 2000, 70, 989–998. [Google Scholar] [CrossRef]
- Ekser, B.; Long, C.; Echeverri, G.J.; Hara, H.; Ezzelarab, M.; Lin, C.C.; de Vera, M.E.; Wagner, R.; Klein, E.; Wolff, R.F.; et al. Impact of thrombocytopenia on survival of baboons with genetically modified pig liver transplants: Clinical relevance. Am. J. Transplant. 2010, 10, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.A.; Navarro-Alvarez, N.; DeFazio, M.; Rosales, I.A.; Elias, N.; Yeh, H.; Colvin, R.B.; Cosimi, A.B.; Markmann, J.F.; Hertl, M.; et al. A Bridge to somewhere: 25-day survival after pig-to-baboon liver xenotransplantation. Ann. Surg. 2016, 263, 1069–1071. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.A.; Patel, M.S.; Elias, N.; Navarro-Alvarez, N.; Rosales, I.; Wilkinson, R.A.; Louras, N.J.; Hertl, M.; Fishman, J.A.; Colvin, R.B.; et al. Prolonged survival following pig-to-primate liver xenotransplantation utilizing exogenous coagulation factors and costimulation blockade. Am. J. Transplant. 2017, 17, 2178–2185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro-Alvarez, N.; Machaidze, Z.; Schuetz, C.; Zhu, A.; Liu, W.H.; Shah, J.A.; Vagefi, P.A.; Elias, N.; Buhler, L.; Sachs, D.H.; et al. Xenogeneic heterotopic auxiliary liver transplantation (XHALT) promotes native liver regeneration in a post-hepatectomy liver failure model. PLoS ONE 2018, 13, e0207272. [Google Scholar] [CrossRef] [Green Version]
- Cronin, D.C., II; Millis, J.M.; Siegler, M. Transplantation of liver grafts from living donors into adults?Too much, too soon. N. Engl. J. Med. 2001, 344, 1633–1637. [Google Scholar] [CrossRef]
- Testa, G. Ethical issues regarding related and nonrelated living organ donors. World J. Surg. 2014, 38, 1658–1663. [Google Scholar] [CrossRef]
- Editorial: Liver transplantation in the USA: Ethical issues. Lancet Gastroenterol. Hepatol. 2020, 5, 1. [CrossRef] [Green Version]
- Shaw, D.; Dondorp, W.; de Wert, G. Ethical issues surrounding the transplantation of organs from animals to humans. Rev. Sci. Tech. Off. Int. Epiz. 2018, 37, 123–129. [Google Scholar] [CrossRef]
- Johnson, L.S.M. Existing ethical tensions surrounding in xenotransplantation. Camb. Q. Healthc. Ethics. 2022, 31, 355–367. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, J.; Zhu, W.; Lawrence, N.; Yu, C.; Gou, M.; Chen, S. 3D bioprinting of functional tissue models for personalized drug screening and in vitro disease modeling. Adv. Drug Deliv. Rev. 2018, 132, 235–251. [Google Scholar] [CrossRef]
- Patuzzo, S.; Goracci, G.; Gasperini, L.; Ciliberti, R. 3D bioprinting technology: Scientific aspects and ethical issues. Sci. Eng. Ethics. 2018, 24, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Datta, P.; Cabrera, L.Y.; Ozbolat, I.T. Ethical challenges with 3D bioprinted tissues and organs. Trends Biotechnol. 2022. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado-Coello, B.; Navarro-Alvarez, N.; Mas-Oliva, J. The Influence of Interdisciplinary Work towards Advancing Knowledge on Human Liver Physiology. Cells 2022, 11, 3696. https://doi.org/10.3390/cells11223696
Delgado-Coello B, Navarro-Alvarez N, Mas-Oliva J. The Influence of Interdisciplinary Work towards Advancing Knowledge on Human Liver Physiology. Cells. 2022; 11(22):3696. https://doi.org/10.3390/cells11223696
Chicago/Turabian StyleDelgado-Coello, Blanca, Nalu Navarro-Alvarez, and Jaime Mas-Oliva. 2022. "The Influence of Interdisciplinary Work towards Advancing Knowledge on Human Liver Physiology" Cells 11, no. 22: 3696. https://doi.org/10.3390/cells11223696