Innate, High Tolerance to Zinc and Lead in Violets Confirmed at the Suspended Cell Level
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Development of Tissue Culture on Solid Media
2.3. Development of Cell Suspension Cultures and Their Treatment with Heavy Metals
2.4. Quantification of Low Molecular Weight Organic Acids and Thiols
2.5. Localization and Content of Heavy Metals
2.6. Statistical Analysis
3. Results
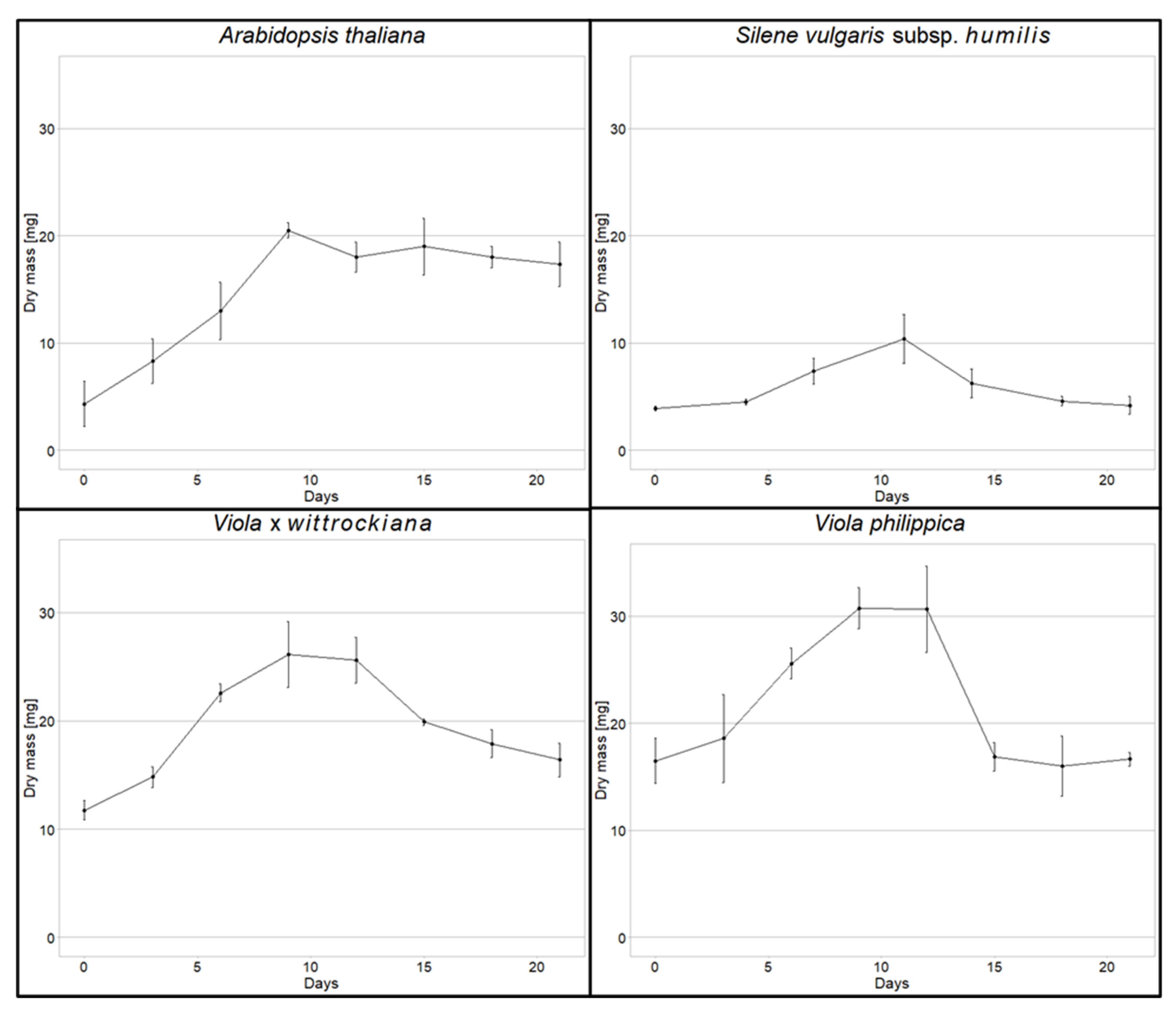
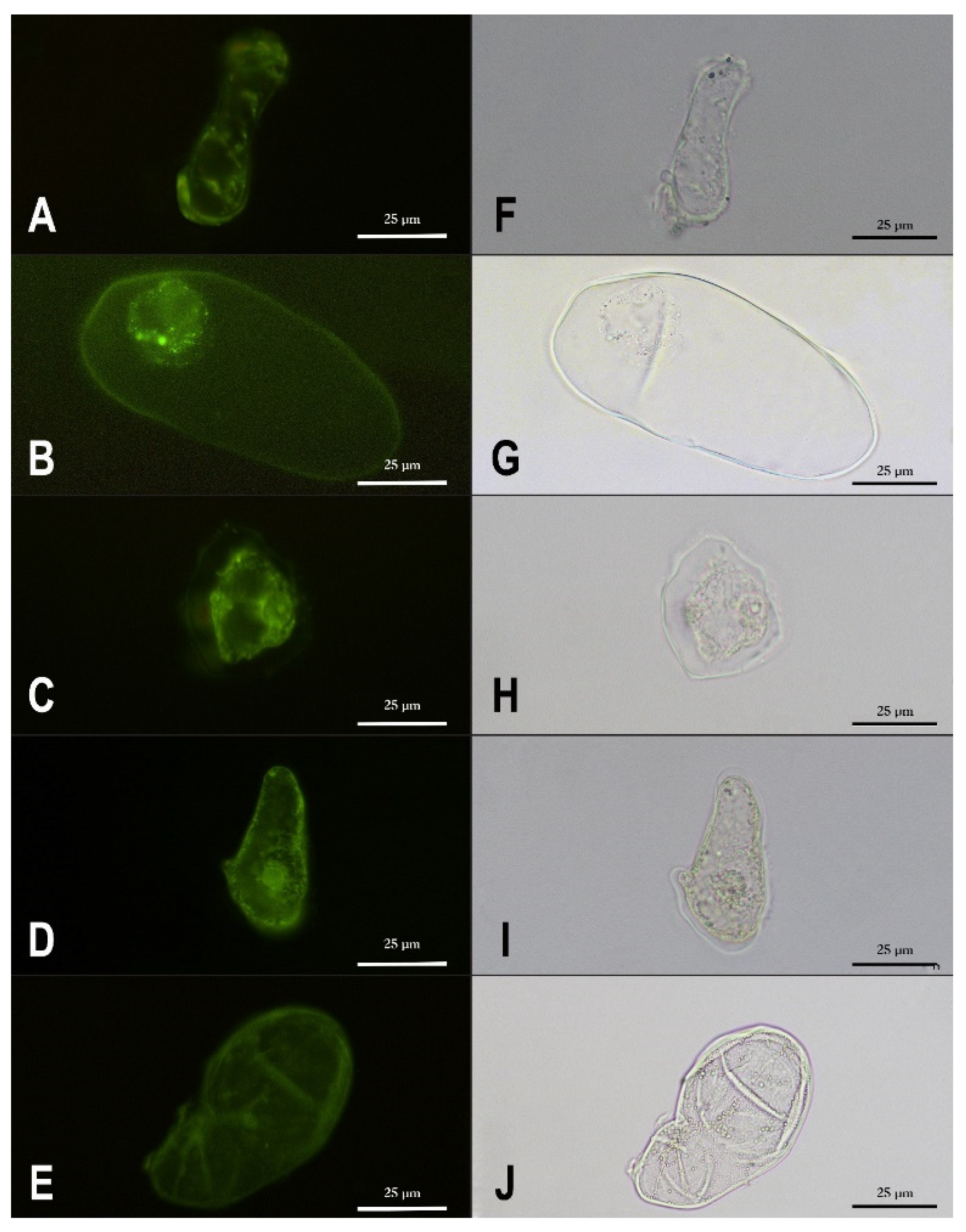
3.1. Cell Suspension Cultures Development
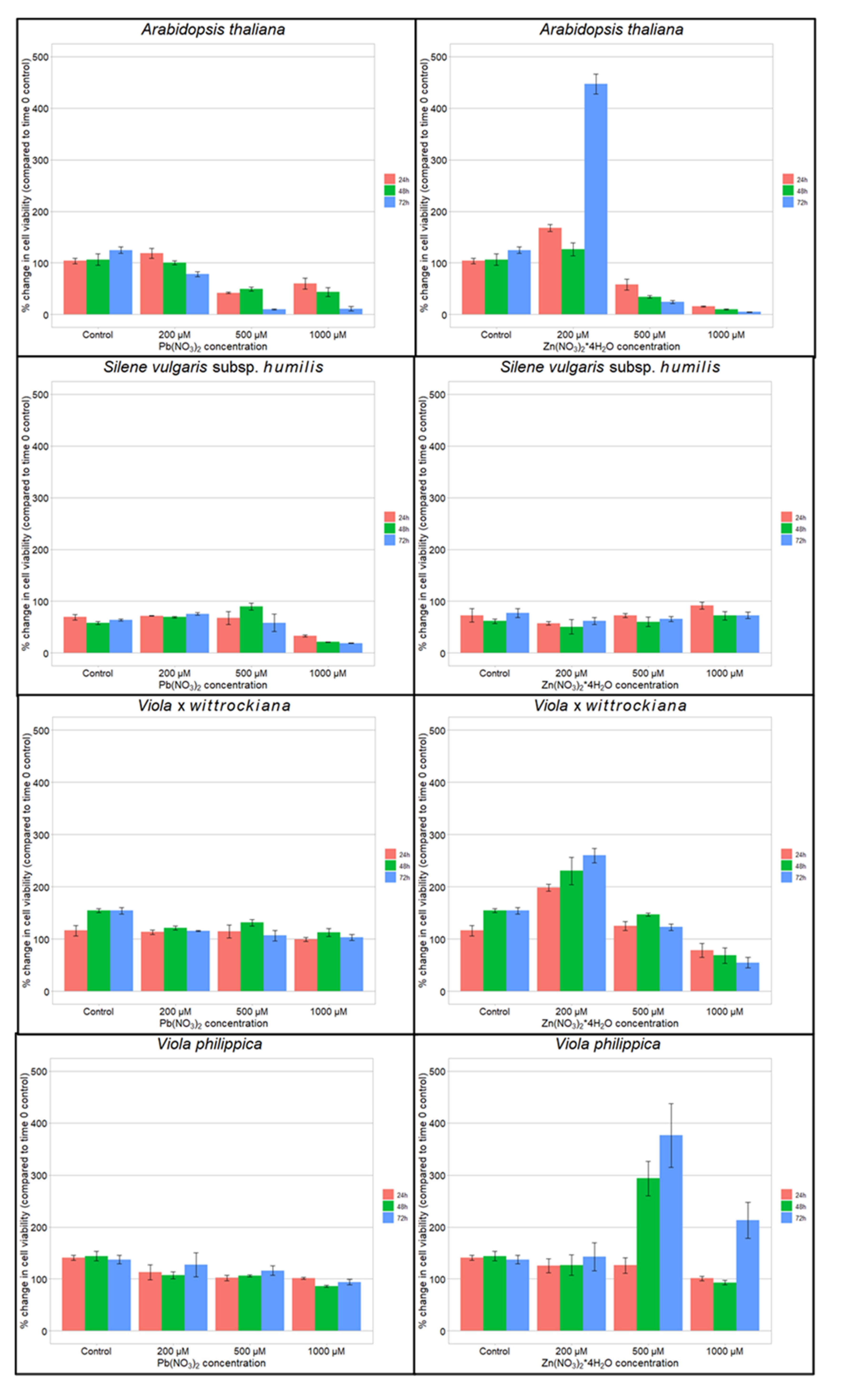
3.2. Cell Viability after Heavy Metal Treatment
3.3. Non-Enzymatic Antioxidants of the Cells after Heavy Metal Treatment
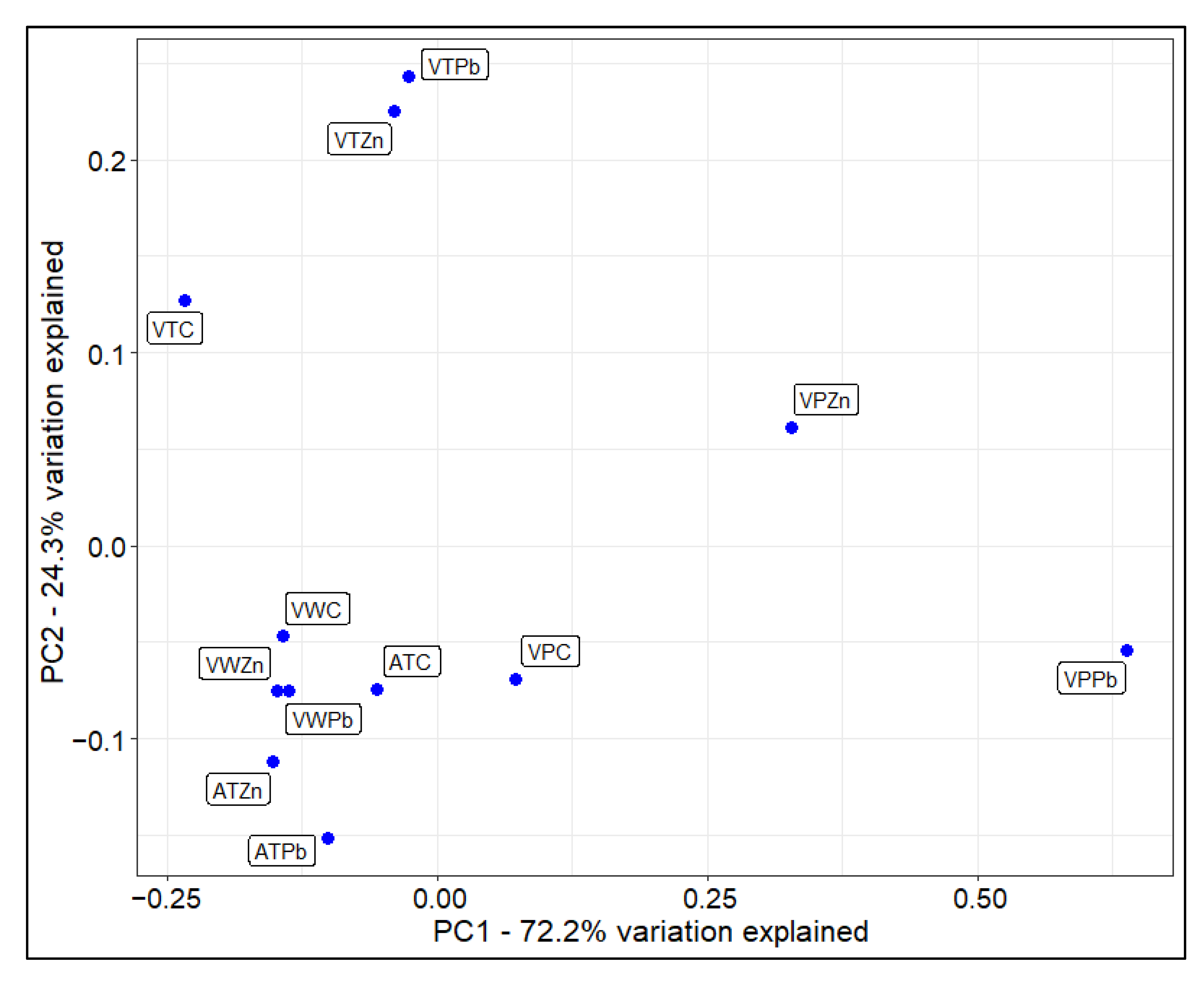
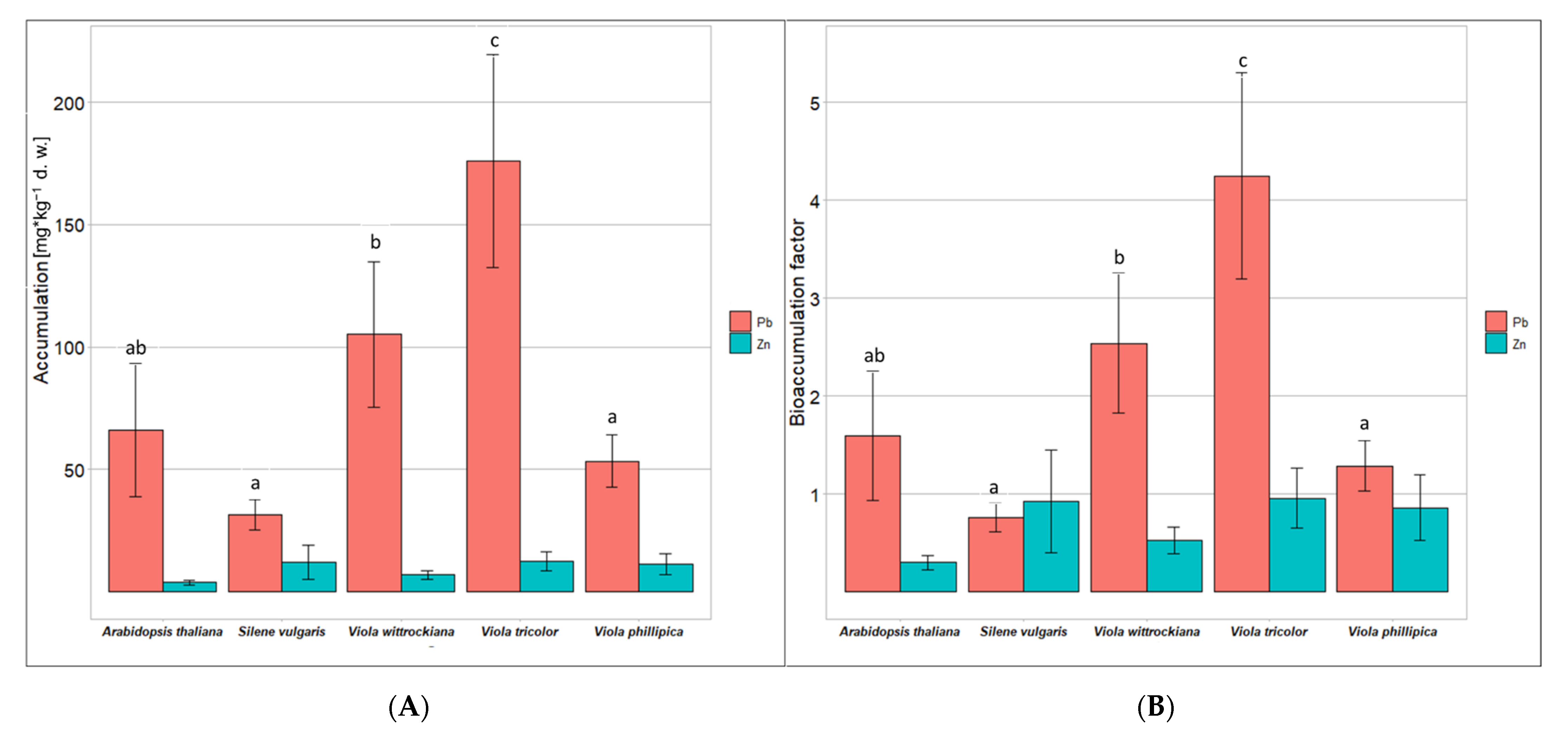
3.4. Accumulation of Heavy Metals by Cells
4. Discussion
4.1. Cells of Viola Species Show High Tolerance to Pb and Zn
4.2. Metallophyte Violets Produce High Content of Non-Enzymatic Antioxidants
4.3. Moderate Accumulation of Zn and Pb in Cells of Violets
4.4. Innate Tolerance to Heavy Metals of Viola Species
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alford, É.R.; Pilon-Smits, E.A.; Paschke, M.W. Metallophytes—A view from the rhizosphere. Plant Soil 2010, 337, 33–50. [Google Scholar] [CrossRef]
- Cobbett, C.S. Phytochelatins and their roles in heavy metal detoxification. Plant Physiol. 2000, 123, 825–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, J.L. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef]
- Riyazuddin, R.; Nisha, N.; Ejaz, B.; Khan, M.I.R.; Kumar, M.; Ramteke, P.W.; Gupta, R. A Comprehensive review on the heavy M=metal toxicity and sequestration in plants. Biomolecules 2021, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef] [Green Version]
- Tamás, L.; Mistrík, I.; Zelinová, V. Heavy metal-induced reactive oxygen species and cell death in barley root tip. Environ. Exp. Bot. 2017, 140, 34–40. [Google Scholar] [CrossRef]
- Sychta, K.; Dubas, E.; Yamada, K.; Słomka, A.; Krzewska, M.; Kuta, E. Papain-like cysteine proteases are involved in pro-grammed cell death in plants under heavy metal stress. Environ. Exp. Bot. 2020, 174, 104041. [Google Scholar] [CrossRef]
- Sychta, K.; Słomka, A.; Kuta, E. Insights into plant programmed cell death induced by heavy metals—Discovering a terra incognita. Cells 2021, 10, 65. [Google Scholar] [CrossRef]
- Sychta, K.; Słomka, A.; Kuta, E. Garden pansy (Viola × wittrockiana Gams.)—A good candidate for the revitalisation of pol-luted areas. Plant Soil Environ. 2020, 66, 272–280. [Google Scholar] [CrossRef]
- Sychta, K.; Słomka, A.; Kuta, E. Kultury zawiesinowe komórek jako model do badania tolerancji roślin na metale ciężkie. Kosmos 2018, 67, 335–346. [Google Scholar] [CrossRef] [Green Version]
- Sychta, K.; Słomka, A.; Suski, S.; Fiedor, E.; Gregoraszczuk, E.; Kuta, E. Suspended cells of metallicolous and nonmetal-licolous Viola species tolerate, accumulate and detoxify zinc and lead. Plant Physiol. Biochem. 2018, 132, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Bothe, H.; Vogel-Mikuš, K.; Pongrac, P.; Likar, M.; Stepic, N.; Pelicon, P.; Vavpetič, P.; Jeromel, L.; Regvar, M. Metallophyte status of violets of the section Melanium. Chemosphere 2013, 93, 1844–1855. [Google Scholar]
- Bothe, H.; Słomka, A. Divergent biology of facultative heavy metal plants. J. Plant Physiol. 2017, 219, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Mullaj, A.; Shehu, J.; Tan, K.; Imeraj, A. New records for the Albanian flora. Bot. Serb. 2010, 34, 163–167. [Google Scholar]
- Psaras, G.K.; Constantinidis, T. Two new nickel hyperaccumulators from the Greek serpentine flora. Fresenius Environ. Bull. 2009, 18, 798–803. [Google Scholar]
- Słomka, A.; Godzik, B.; Szarek-Łukaszewska, G.; Shuka, L.; Hoef-Emden, K.; Bothe, H. Albanian violets of the section Mela-nium, their morphological variability, genetic similarity and their adaptations to serpentine or chalk soils. J. Plant Physiol. 2015, 174, 110–123. [Google Scholar] [CrossRef]
- Stevanovic, V.; Tan, K.; Iatrou, G. Distribution of the endemic Balkan flora on serpentine I. – obligate serpentine endemics. Plant Syst. Evol. 2003, 242, 149–170. [Google Scholar] [CrossRef]
- Stevanović, B.; Dražić, G.; Tomović, G.; Šinžar-Sekulić, J.; Melovski, L.; Novović, I.; Marković, D.M. Accumulation of arsenic and heavy metals in some Viola species from an abandoned mine, Alchar, Republic of Macedonia (FYROM). Plant Biosyst. 2010, 144, 644–655. [Google Scholar] [CrossRef]
- Bidwell, S.D.; Crawford, S.A.; Woodrow, I.E.; Sommer-Knudsen, J.; Marshall, A.T. Sub-cellular localization of Ni in the hy-peraccumulator, Hybanthus floribundus (Lindley) F. Muell. Plant Cell Environ. 2004, 27, 705–716. [Google Scholar] [CrossRef]
- Brooks, R.; Wither, E. Nickel accumulation by Rinorea bengalensis (Wall.) O.K. J. Geochem. Explor. 1977, 7, 295–300. [Google Scholar] [CrossRef]
- Gao, J.; Luo, M.; Zhu, Y.; He, Y.; Wang, Q.; Zhang, C. Transcriptome sequencing and differential gene expression analysis in Viola yedoensis Makino (Fam. Violaceae) responsive to cadmium (Cd) pollution. Biochem. Biophys. Res. Commun. 2015, 459, 60–65. [Google Scholar] [CrossRef]
- Liu, W.; Shu, W.; Lan, C. Viola baoshanensis, a plant that hyperaccumulates cadmium. Chin. Sci. Bull. 2004, 49, 29–32. [Google Scholar] [CrossRef]
- Shu, H.; Zhang, J.; Liu, F.; Bian, C.; Liang, J.; Lin, Z.; Li, J.; Shi, Q.; Liao, B. Comparative sranscriptomic Studies on a cadmium hyperaccumulator Viola baoshanensis and its non-tolerant counterpart V. inconspicua. Int. J. Mol. Sci. 2019, 20, 1906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bothe, H. Plants in heavy metal soils. In Detoxification of Heavy Metals. Soil Biology, 1st ed.; Sherameti, I., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 30, pp. 35–58. [Google Scholar]
- Słomka, A.; Kuta, E.; Szarek-Łukaszewska, G.; Godzik, B.; Kapusta, P.; Tylko, G.; Bothe, H. Violets of the section Melanium, their colonization by arbuscular mycorrhizal fungi and their occurrence on heavy metal heaps. J. Plant Physiol. 2011, 168, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Papadopulos, A.S.T.; Helmstetter, A.J.; Osborne, O.G.; Comeault, A.A.; Wood, D.P.; A Straw, E.; Mason, L.; Fay, M.F.; Parker, J.; Dunning, L.T.; et al. Rapid parallel adaptation to anthropogenic heavy metal pollution. Mol. Biol. Evol. 2021, 38, 3724–3736. [Google Scholar] [CrossRef]
- Pauwels, M.; Frérot, H.; Bonnin, I.; Saumitou-Laprade, P. A broad-scale analysis of population differentiation for Zn toler-ance in an emerging model species for tolerance study: Arabidopsis halleri (Brassicaceae). J. Evol. Biol. 2006, 19, 1838–1850. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, U.; Hoef-Emden, K.; Backhausen, S.; Bothe, H.; Bożek, M.; Siuta, A.; Kuta, E. The rare, endemic zinc violets of Central Europe originate from Viola lutea Huds. Plant Syst. Evol. 2006, 257, 205–222. [Google Scholar] [CrossRef]
- Kuta, E.; Bohdanowicz, J.; Słomka, A.; Pilarska, M.; Bothe, H. Floral structure and pollen morphology of two zinc violets (Viola lutea ssp. calaminaria and V. lutea ssp. westfalica) indicate their taxonomic affinity to Viola lutea. Oesterreichische Bot. Z. 2011, 298, 445–455. [Google Scholar] [CrossRef] [Green Version]
- Kuta, E.; Jędrzejczyk-Korycińska, M.; Cieślak, E.; Rostański, A.; Szczepaniak, M.; Migdałek, G.; Wąsowicz, P.; Suda, J.; Combik, M.; Słomka, A. Morphological versus genetic diversity of Viola reichenbachiana and V. riviniana (sect. Viola, Violaceae) from soils differing in heavy metal content. Plant Biol. 2014, 16, 924–934. [Google Scholar] [CrossRef]
- Migdałek, G.; Woźniak, M.; Słomka, A.; Godzik, B.; Jędrzejczyk-Korycińska, M.; Rostański, A.; Kuta, E. Morphological dif-ferences between violets growing at heavy metal polluted and non-polluted sites. Flora. Morphol. Distrib. Funct. Ecol. Plants 2013, 208, 87–96. [Google Scholar] [CrossRef]
- Neuffer, B.; Auge, H.; Mesch, H.; Amarell, U.; Brandl, R. Spread of violets in polluted pine forests: Morphological and mo-lecular evidence for the ecological importance of interspecific hybridization. Mol. Ecol. 1999, 8, 365–377. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Sychta, K.; Szklarzewicz, J.; Słomka, A.; Gregoraszczuk, E.; Kuta, E. Critical factors besides treatment dose and duration need to be controlled in Pb toxicity tests in plant cell suspension cultures. Acta Soc. Bot. Pol. 2017, 86, 3555. [Google Scholar] [CrossRef] [Green Version]
- Dresler, S.; Hanaka, A.; Bednarek, W.; Maksymiec, W. Accumulation of low-molecular-weight organic acids in roots and leaf segments of Zea mays plants treated with cadmium and copper. Acta Physiol. Plant. 2014, 36, 1565–1575. [Google Scholar] [CrossRef] [Green Version]
- Dresler, S.; Hawrylak-Nowak, B.; Kováčik, J.; Pochwatka, M.; Hanaka, A.; Strzemski, M.; Sowa, I.; Wójciak-Kosior, M. Al-lantoin attenuates cadmium-induced toxicity in cucumber plants. Ecotoxicol. Environ. Saf. 2019, 170, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Mackey, E.A.; Becker, D.A.; Oflaz, R.D.; Paul, R.L.; Greenberg, R.R.; Lindstrom, R.M.; Yu, L.L.; Wood, L.J.; Long, S.E.; Kelley, W.R.; et al. Certification of NIST Standard Reference Material 1575a pine needles and results of an international laboratory comparison. J. Res. 2004. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R foundation for statistical computing: Vienna, Austria, 2021. [Google Scholar]
- Paradis, E.; Schliep, K. Ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2018, 35, 526–528. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community ecology package. R package version 2019, 14, 732–740. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Arnold, J.B. Ggthemes: Extra Themes, Scales and Geoms for Ggplot2’, R package Version 4.2.4; R Foundation for statistical computing: Vienna, Austria, 2021. [Google Scholar]
- Kawano, T.; Muto, S. Mechanism of peroxidase actions for salicylic acid-induced generation of active oxygen species and an increase in cytosolic calcium in tobacco cell suspension culture. J. Exp. Bot. 2000, 51, 685–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smetanska, I. Production of secondary metabolites using plant cell cultures. Food Biotechnol. Adv. Biochem. Eng. Biotechnol. 2008, 111, 187–228. [Google Scholar] [CrossRef]
- Moscatiello, R.; Baldan, B.; Navazio, L. Plant cell suspension culture. Plant Mineral Nutr.: Method. Protocol. 2013, 953, 77–93. [Google Scholar]
- Maldonado-Magaña, A.; Orozco-Villafuerte, J.; Buendia-González, L.; Estrada-Zuňiga, M.E.; Bernabé-Antonio, A.; Cruz-Sosa, F. Establishment of cell suspension cultures of Prosopis laevigata (Humb.&Bonpl. ex Willd) M.C. Johnst to determine the effect of zinc on the uptake and accumulation of lead. Rev. Mex. Ing. Quim. 2013, 12, 489–498. [Google Scholar]
- Bernabe-Antonio, A.; Alvarez, L.W.; Buendía-González, L.; Maldonado-Magaña, A.; Cruz-Sosa, F. Accumulation and tolerance of Cr and Pb using a cell suspension culture system of Jatropha curcas. Plant Cell Tissue Organ Cult. 2014, 120, 221–228. [Google Scholar] [CrossRef]
- Castillo-González, J.; Ojeda-Barrios, D.; Hernández-Rodríguez, A.; González-Franco, A.C.; Robles-Hernández, L.; López-Ochoa, G.R. Zinc metalloenzymes in plants. Interciencia 2018, 43, 242–248. [Google Scholar]
- Rellán-Álvarez, R.; Giner-Martínez-Sierra, J.; Orduna, J.; Orera, I.; Rodríguez-Castrillón, J.Á.; García-Alonso, J.I.; Abadía, J.; Alvarez-Fernández, A. Identification of a tri-iron (III), tri-citrate complex in the xylem sap of iron-deficient tomato resupplied with iron: New insights into plant iron long-distance transport. Plant Cell Physiol. 2010, 51, 91–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.; Ye, Z.H.; Wang, X.R.; Wong, M.H. Increase of glutathione in mine population of Sedum alfredii: A Zn hyperaccu-mulator and Pb accumulator. Phytochemistry 2005, 66, 2549–2556. [Google Scholar] [CrossRef] [PubMed]
- Schat, H.; Mercè, L.; Riet, V.; Hartley-Whitaker, J.; Bleeker, P.M. The role of phytochelatins in constitutive and adaptive heavy metal tolerances in hyperaccumulator and non-hyperaccumulator metallophytes. J. Exp. Bot. 2002, 53, 2381–2392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harmens, H.; Hartog, P.R.D.; Bookum, W.; Verkleij, J. Increased zinc tolerance in Silene vulgaris (Moench) Garcke is not due to increased production of phytochelatins. Plant Physiol. 1993, 103, 1305–1309. [Google Scholar] [CrossRef] [Green Version]
- Semane, B.; Cuypers, A.; Smeets, K.; Van Belleghem, F.; Horemans, N.; Schat, H.; Vangronsveld, J. Cadmium responses in Arabidopsis thaliana: Glutathione metabolism and antioxidative defence system. Physiol. Plant. 2007, 129, 519–528. [Google Scholar] [CrossRef]
- Audet, P.; Charest, C. Heavy metal phytoremediation from a meta-analytical perspective. Environ. Pollut. 2007, 147, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Kopittke, P.M.; Asher, C.J.; Blamey, F.P.C.; Auchterlonie, G.J.; Guo, Y.N.; Menzies, N.W. Localization and chemical speciation of Pb in roots of signal grass (Brachiaria decumbens) and rhodes grass (Chloris gayana). Environ. Sci. Technol. 2008, 42, 4595–4599. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.P.; Holmberg, J.A.; Osborne, O.G.; Helmstetter, A.J.; Dunning, L.T.; Ellison, A.R.; Smith, R.J.; Lighten, J.; Papadopu-los, A.S.T. Genetic assimilation of ancestral plasticity during parallel adaptation. BioRxiv 2021. [CrossRef]
- Jedrzejczyk, M.; Rostanski, A.; Malkowski, E. Accumulation of zinc and lead in selected taxa of the genus Viola L. Acta Biol. Cracov. Ser. Bot. 2002, 44, 49–55. [Google Scholar]
- Jaguś, A.; Skrzypiec, M. Toxic elements in mountain soils (Little Beskids, Polish Carpathians). J. Ecol. Eng. 2019, 20, 197–202. [Google Scholar] [CrossRef]
- Bačeva, K.; Stafilov, T.; Matevski, V. Bioaccumulation of heavy metals by endemic Viola species from the soil in the vicinity of the As-Sb-Tl mine “Allchar”, Republic of Macedonia. Int. J. Phytorem. 2014, 16, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Erben, M. The significance of hybridization on the forming of species in the genus Viola. Bocconea 1996, 5, 113–118. [Google Scholar]
| Species | Treatment | ALLA mg/g | L-AA mg/g | GSH nmol/g | PC2 nmol/g | PC3 nmol/g | PC4 nmol/g | Tartrate mg/g | Malate mg/g | Citrate mg/g |
|---|---|---|---|---|---|---|---|---|---|---|
| Arabidopsis thaliana | 0 μM | 0.024 [±0.005] | 0.293 [±0.089] | 16.437 [±0.6335] | ND | ND | ND | 0.024 [±0.002] | 0.113 [±0.035] | 0.007 [±0.002] |
| 200 μM Pb | 0.024 [±0.003] | 0.108 [±0.064] | 8.216 [±1.2716] | 3.741 [±0.607] | ND | ND | 0.027 [±0.002] | 0.177 [±0.029] | 0.083 [±0.050] | |
| 200 μM Zn | 0.017 [±0.004] | 0.096 [±0.062] | 5.330 [±0.4109] | 3.377 [±1.086] | ND | ND | 0.027 [±0.006] | 0.131 [±0.021] | 0.036 [±0.023] | |
| Viola · wittrockiana | 0 μM | 0.015 [±0.001] | 0.124 [±0.021] | 173.353 [±63.9950] | ND | ND | ND | 0.024 [±0.003] | 0.057 [±0.020] | 0.001 [±0.000] |
| 200 μM Pb | 0.018 [±0.002] | 0.075 [±0.026] | 203.789 [±16.6196] | 3.246 [±0.694] | 2.098 [±0.150] | ND | 0.021 [±0.002] | 0.083 [±0.032] | 0.005 [±0.001] | |
| 200 μM Zn | 0.016 [±0.004] | 0.045 [±0.015] | 212.254 [±6.8863] | 3.649 [±0.207] | ND | ND | 0.022 [±0.005] | 0.108 [±0.084] | 0.003 [±0.001] | |
| V. tricolor | 0 μM | ND | 0.133 [±0.052] | 479.745 [±7.0620] | ND | ND | ND | 0.025 [±0.001] | 0.006 [±0.001] | 0.020 [±0.004] |
| 200 μM Pb | ND | 0.316 [±0.025] | 407.607 [±25.1181] | 2.169 [±0.478] | ND | ND | 0.075 [±0.062] | 0.012 [±0.003] | 0.038 [±0.009] | |
| 200 μM Zn | ND | 0.293 [±0.033] | 432.550 [±13.3967] | 5.386 [±1.317] | 2.312 [±0.279] | ND | 0.065 [±0.039] | 0.013 [±0.001] | 0.055 [±0.035] | |
| V. philippica | 0 μM | 0.019 [±0.001] | 0.214 [±0.069] | 202.427 [±31.9216] | ND | ND | ND | 0.045 [±0.002] | 0.431 [±0.068] | 0.465 [±0.051] |
| 200 μM Pb | 0.025 [±0.003] | 0.290 [±0.050] | 234.810 [±86.1177] | 82.744 [±99.973] | 141.209 [±82.618] | 63.54 [±31.060] | 0.083 [±0.012] | 0.696 [±0.151] | 1.046 [±0.224] | |
| 200 μM Zn | 0.023 [±0.003] | 0.279 [±0.059] | 342.980 [±28.3279] | 56.363 [±14.759] | 13.034 [±1.775] | ND | 0.075 [±0.013] | 0.678 [±0.217] | 0.751 [±0.204] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miszczak, S.; Sychta, K.; Dresler, S.; Kurdziel, A.; Hanaka, A.; Słomka, A. Innate, High Tolerance to Zinc and Lead in Violets Confirmed at the Suspended Cell Level. Cells 2022, 11, 2355. https://doi.org/10.3390/cells11152355
Miszczak S, Sychta K, Dresler S, Kurdziel A, Hanaka A, Słomka A. Innate, High Tolerance to Zinc and Lead in Violets Confirmed at the Suspended Cell Level. Cells. 2022; 11(15):2355. https://doi.org/10.3390/cells11152355
Chicago/Turabian StyleMiszczak, Szymon, Klaudia Sychta, Sławomir Dresler, Agnieszka Kurdziel, Agnieszka Hanaka, and Aneta Słomka. 2022. "Innate, High Tolerance to Zinc and Lead in Violets Confirmed at the Suspended Cell Level" Cells 11, no. 15: 2355. https://doi.org/10.3390/cells11152355







