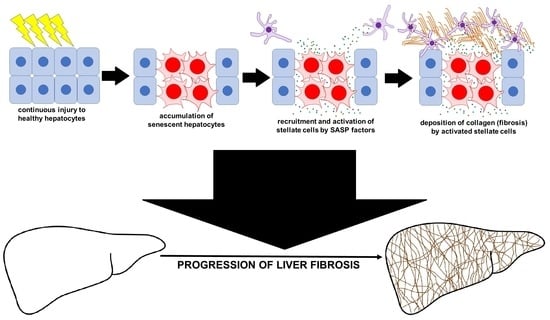
Role of Hepatocyte Senescence in the Activation of Hepatic Stellate Cells and Liver Fibrosis Progression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Liver Biopsy Specimens and Histological Quantification
2.2. Induction of Cellular Senescence and Hepatic Stellate Cell Culture
2.3. Determination of Fibroblast Activating Factors in Culture Media and Serum Samples
2.4. Statistical Analysis
3. Results
3.1. Association between Hepatocyte Senescence, Activation of Hepatic Stellate Cells, and Liver Fibrosis
3.2. Induction of Senescence in Hepg2 Cells
3.3. Induction of Hepatc Stellate Cells
3.4. SASP Factors
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aravinthan, A. Cellular senescence: A hitchhiker’s guide. Hum. Cell 2015, 28, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Toussaint, O.; Royer, V.; Salmon, M.; Remacle, J. Stress-induced premature senescence and tissue ageing. Biochem. Pharmacol. 2002, 64, 1007–1009. [Google Scholar] [CrossRef]
- Coppe, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basisty, N.; Kale, A.; Jeon, O.H.; Kuehnemann, C.; Payne, T.; Rao, C.; Holtz, A.; Shah, S.; Sharma, V.; Ferrucci, L.; et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020, 18, e3000599. [Google Scholar] [CrossRef] [Green Version]
- Marshall, A.; Rushbrook, S.; Davies, S.E.; Morris, L.S.; Scott, I.S.; Vowler, S.L.; Coleman, N.; Alexander, G. Relation between hepatocyte G1 arrest, impaired hepatic regeneration, and fibrosis in chronic hepatitis C virus infection. Gastroenterology 2005, 128, 33–42. [Google Scholar] [CrossRef]
- Aravinthan, A.; Scarpini, C.; Tachtatzis, P.; Verma, S.; Penrhyn-Lowe, S.; Harvey, R.; Davies, S.E.; Allison, M.; Coleman, N.; Alexander, G. Hepatocyte senescence predicts progression in non-alcohol-related fatty liver disease. J. Hepatol. 2013, 58, 549–556. [Google Scholar] [CrossRef]
- Aravinthan, A.; Pietrosi, G.; Hoare, M.; Jupp, J.; Marshall, A.; Verrill, C.; Davies, S.; Bateman, A.; Sheron, N.; Allison, M.; et al. Hepatocyte expression of the senescence marker p21 is linked to fibrosis and an adverse liver-related outcome in alcohol-related liver disease. PLoS ONE 2013, 8, e72904. [Google Scholar] [CrossRef] [Green Version]
- Mela, M.; Smeeton, W.; Davies, S.E.; Miranda, E.; Scarpini, C.; Coleman, N.; Alexander, G.J. The Alpha-1 Antitrypsin Polymer Load Correlates With Hepatocyte Senescence, Fibrosis Stage and Liver-Related Mortality. Chronic. Obstr. Pulm. Dis. 2020, 7, 151–162. [Google Scholar] [CrossRef]
- Tachtatzis, P.M.; Marshall, A.; Aravinthan, A.; Verma, S.; Penrhyn-Lowe, S.; Mela, M.; Scarpini, C.; Davies, S.E.; Coleman, N.; Alexander, G.J. Chronic Hepatitis B Virus Infection: The Relation between Hepatitis B Antigen Expression, Telomere Length, Senescence, Inflammation and Fibrosis. PLoS ONE 2015, 10, e0127511. [Google Scholar]
- Friedman, S.L. Hepatic stellate cells: Protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev. 2008, 88, 125–172. [Google Scholar] [CrossRef] [PubMed]
- Lee, U.E.; Friedman, S.L. Mechanisms of hepatic fibrogenesis. Best Pract. Res. Clin. Gastroenterol. 2011, 25, 195–206. [Google Scholar] [CrossRef] [PubMed]
- McKee, C.; Sigala, B.; Soeda, J.; Mouralidarane, A.; Morgan, M.; Mazzoccoli, G.; Rappa, F.; Cappello, F.; Cabibi, D.; Pazienza, V.; et al. Amphiregulin activates human hepatic stellate cells and is upregulated in non-alcoholic steatohepatitis. Sci. Rep. 2015, 5, 8812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, S.; Sharpless, N.E. Senescence in Health and Disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef]
- Deroulers, C.; Ameisen, D.; Badoual, M.; Gerin, C.; Granier, A.; Lartaud, M. Analyzing huge pathology images with open source software. Diagn. Pathol. 2013, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Arganda-Carreras, I.; Kaynig, V.; Rueden, C.; Eliceiri, K.W.; Schindelin, J.; Cardona, A.; Sebastian Seung, H. Trainable Weka Segmentation: A machine learning tool for microscopy pixel classification. Bioinformatics 2017, 33, 2424–2426. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Aravinthan, A.; Shannon, N.; Heaney, J.; Hoare, M.; Marshall, A.; Alexander, G.J. The senescent hepatocyte gene signature in chronic liver disease. Exp. Gerontol. 2014, 60, 37–45. [Google Scholar] [CrossRef]
- Aravinthan, A.; Challis, B.; Shannon, N.; Hoare, M.; Heaney, J.; Alexander, G.J. Selective insulin resistance in hepatocyte senescence. Exp. Cell Res. 2015, 331, 38–45. [Google Scholar] [CrossRef]
- Sabater, L.; Locatelli, L.; Oakley, F.; Hardy, T.; French, J.; Robinson, S.M.; Sen, G.; Mann, D.A.; Mann, J. RNA sequencing reveals changes in the microRNAome of transdifferentiating hepatic stellate cells that are conserved between human and rat. Sci. Rep. 2020, 10, 21708. [Google Scholar] [CrossRef]
- Donato, M.T.; Tolosa, L.; Gomez-Lechon, M.J. Culture and Functional Characterization of Human Hepatoma HepG2 Cells. Methods Mol. Biol. 2015, 1250, 77–93. [Google Scholar] [PubMed]
- Deevska, G.; Dotson, P.P.; Mitov, M.; Butterfield, D.A.; Nikolova-Karakashian, M. Onset of Senescence and Steatosis in Hepatocytes as a Consequence of a Shift in the Diacylglycerol/Ceramide Balance at the Plasma Membrane. Cells 2021, 10, 1278. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.; Kang, H.; Choi, H.; Choi, W.; Jun, H.S. Reactive oxygen species-induced changes in glucose and lipid metabolism contribute to the accumulation of cholesterol in the liver during aging. Aging Cell 2019, 18, e12895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuilman, T.; Peeper, D.S. Senescence-messaging secretome: SMS-ing cellular stress. Nat. Rev. Cancer 2009, 9, 81–94. [Google Scholar] [CrossRef]
- Davalos, A.R.; Coppe, J.P.; Campisi, J.; Desprez, P.Y. Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev. 2010, 29, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Hoare, M.; Young, A.R.; Narita, M. Autophagy in cancer: Having your cake and eating it. Semin. Cancer Biol. 2011, 21, 397–404. [Google Scholar] [CrossRef]
- Laberge, R.M.; Sun, Y.U.; Orjalo, A.V.; Patil, C.K.; Freund, A.; Zhou, L.; Curran, S.C.; Davalos, A.R.; Wilson-Edell, K.A.; Liu, S.U.; et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat. Cell Biol. 2015, 17, 1049–1061. [Google Scholar] [CrossRef]
- Herranz, N.; Gallage, S.; Mellone, M.; Wuestefeld, T.; Klotz, S.; Hanley, C.J.; Raguz, S.; Acosta, J.C.; Innes, A.J.; Banito, A.; et al. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat. Cell Biol. 2015, 17, 1205–1217. [Google Scholar] [CrossRef] [Green Version]
- Hillel, A.T.; Gelbard, A. Unleashing rapamycin in fibrosis. Oncotarget 2015, 6, 15722–15723. [Google Scholar] [CrossRef]
- Neef, M.; Ledermann, M.; Saegesser, H.; Schneider, V.; Reichen, J. Low-dose oral rapamycin treatment reduces fibrogenesis, improves liver function, and prolongs survival in rats with established liver cirrhosis. J. Hepatol. 2006, 45, 786–796. [Google Scholar] [CrossRef]
- Wang, W.; Yan, J.; Wang, H.; Shi, M.; Zhang, M.; Yang, W.; Peng, C.; Li, H. Rapamycin ameliorates inflammation and fibrosis in the early phase of cirrhotic portal hypertension in rats through inhibition of mTORC1 but not mTORC2. PLoS ONE 2014, 9, e83908. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Shankar, A.; Gee, I.; Nash, K.; Hoare, M.; Gibbs, P.; Davies, S.; Alexander, G.J.M. A retrospective 15-year review: Survival advantage after switching to sirolimus in hepatitis C virus infected liver graft recipients. Aliment. Pharmacol. Ther. 2015, 41, 379–392. [Google Scholar] [PubMed] [Green Version]
- Sakaida, I.; Nagatomi, A.; Hironaka, K.; Uchida, K.; Okita, K. Quantitative analysis of liver fibrosis and stellate cell changes in patients with chronic hepatitis C after interferon therapy. Am. J. Gastroenterol. 1999, 94, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Aravinthan, A.D.; Alexander, G.J. Hepatocyte senescence explains conjugated bilirubinaemia in chronic liver failure. J. Hepatol. 2015, 63, 532–533. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.S.; Dreesen, O. Biomarkers of Cellular Senescence and Skin Aging. Front. Genet. 2018, 9, 247. [Google Scholar] [CrossRef]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of Cellular Senescence. Trends. Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef]
- Moncsek, A.; Al-Suraih, M.S.; Trussoni, C.E.; O’Hara, S.P.; Splinter, P.L.; Zuber, C.; Patsenker, E.; Valli, P.V.; Fingas, C.D.; Weber, A.; et al. Targeting senescent cholangiocytes and activated fibroblasts with B-cell lymphoma-extra large inhibitors ameliorates fibrosis in multidrug resistance 2 gene knockout (Mdr2(−/−) ) mice. Hepatology 2018, 67, 247–259. [Google Scholar] [CrossRef] [Green Version]
- Schafer, M.J.; White, T.A.; Iijima, K.; Haak, A.J.; Ligresti, G.; Atkinson, E.J.; Oberg, A.L.; Birch, J.; Salmonowicz, H.; Zhu, Y.I.; et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017, 8, 14532. [Google Scholar] [CrossRef]





| Primer | Sequence (5′ to 3′) | |
|---|---|---|
| GAPDH | F | GAAGGTGAAGGTCGGAGTC |
| R | GAAGATGGTGATGGGATTTC | |
| IL-6 | F | AAATTCGGTACATCCTCGACGG |
| R | GGAAGGTTCAGGTTGTTTTCTGC | |
| TNFα | F | AACCTCCTCTCTGCCATCAA |
| R | CCAAAGTAGACCTGCCCAGA | |
| IL-1β | F | TTCCATTTTGTTTGCCTTAT |
| R | TTATGGGAAAGTCTCAAAAC | |
| TIMP1 | F | TTATCCATCCCCTGCAAACTG |
| R | CAAGGTGACGGGACTGGAA | |
| αSMA | F | GCGTGGCTATTCCTTCGTTACT |
| R | CCGATGAAGGATGGCTGGAACA | |
| ProCollagen I | F | CAAGAGGAAGGCCAAGTCGAG |
| R | CGTTGTCGCAGACGCAGAT | |
| TGFβ1 | F | TGACAGCAGGGATAACACACT |
| R | CGCACGCAGCAGTTCTTCTCC | |
| All (n = 50) Median (IQR) or Number (%) | ||
|---|---|---|
| Age at biopsy | 57 (48–67) | |
| Female sex | 20 (40%) | |
| Body Mass Index (kg/m2) | 36.8 (30.7–40.4) | |
| Metabolic Comorbidities | Type 2 DM | 26 (52%) |
| Hypertension | 20 (40%) | |
| Dyslipidaemia | 27 (54%) | |
| Blood tests at biopsy | AST | 37 (31–56) |
| ALT | 39 (29–59) | |
| ALP | 90 (73–117) | |
| Bilirubin | 10 (8–13) | |
| Albumin | 42 (40–44) | |
| AST/ALT ratio | 1.0 (0.8–1.1) | |
| Platelets | 255 (197–302) | |
| PT | 11.0 (10.8–11.3) | |
| HbA1c | 50 (39–63) | |
| Biopsy Steatosis | Mild (grade 1) | 5 (10%) |
| Moderate (grade 2) | 22 (44%) | |
| Severe (grade 3) | 23 (46%) | |
| Steatohepatitis | 41 (82%) | |
| Biopsy Fibrosis Stage | 0 (no fibrosis) | 7 (14%) |
| 1 | 9 (18%) | |
| 2 | 11 (22%) | |
| 3 | 11 (22%) | |
| 4 (cirrhosis) | 12 (24%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijayasiri, P.; Astbury, S.; Kaye, P.; Oakley, F.; Alexander, G.J.; Kendall, T.J.; Aravinthan, A.D. Role of Hepatocyte Senescence in the Activation of Hepatic Stellate Cells and Liver Fibrosis Progression. Cells 2022, 11, 2221. https://doi.org/10.3390/cells11142221
Wijayasiri P, Astbury S, Kaye P, Oakley F, Alexander GJ, Kendall TJ, Aravinthan AD. Role of Hepatocyte Senescence in the Activation of Hepatic Stellate Cells and Liver Fibrosis Progression. Cells. 2022; 11(14):2221. https://doi.org/10.3390/cells11142221
Chicago/Turabian StyleWijayasiri, Pramudi, Stuart Astbury, Philip Kaye, Fiona Oakley, Graeme J. Alexander, Timothy J. Kendall, and Aloysious D. Aravinthan. 2022. "Role of Hepatocyte Senescence in the Activation of Hepatic Stellate Cells and Liver Fibrosis Progression" Cells 11, no. 14: 2221. https://doi.org/10.3390/cells11142221