Methodological Progress of Stereology in Cardiac Research and Its Application to Normal and Pathological Heart Development
Abstract
:1. Introduction
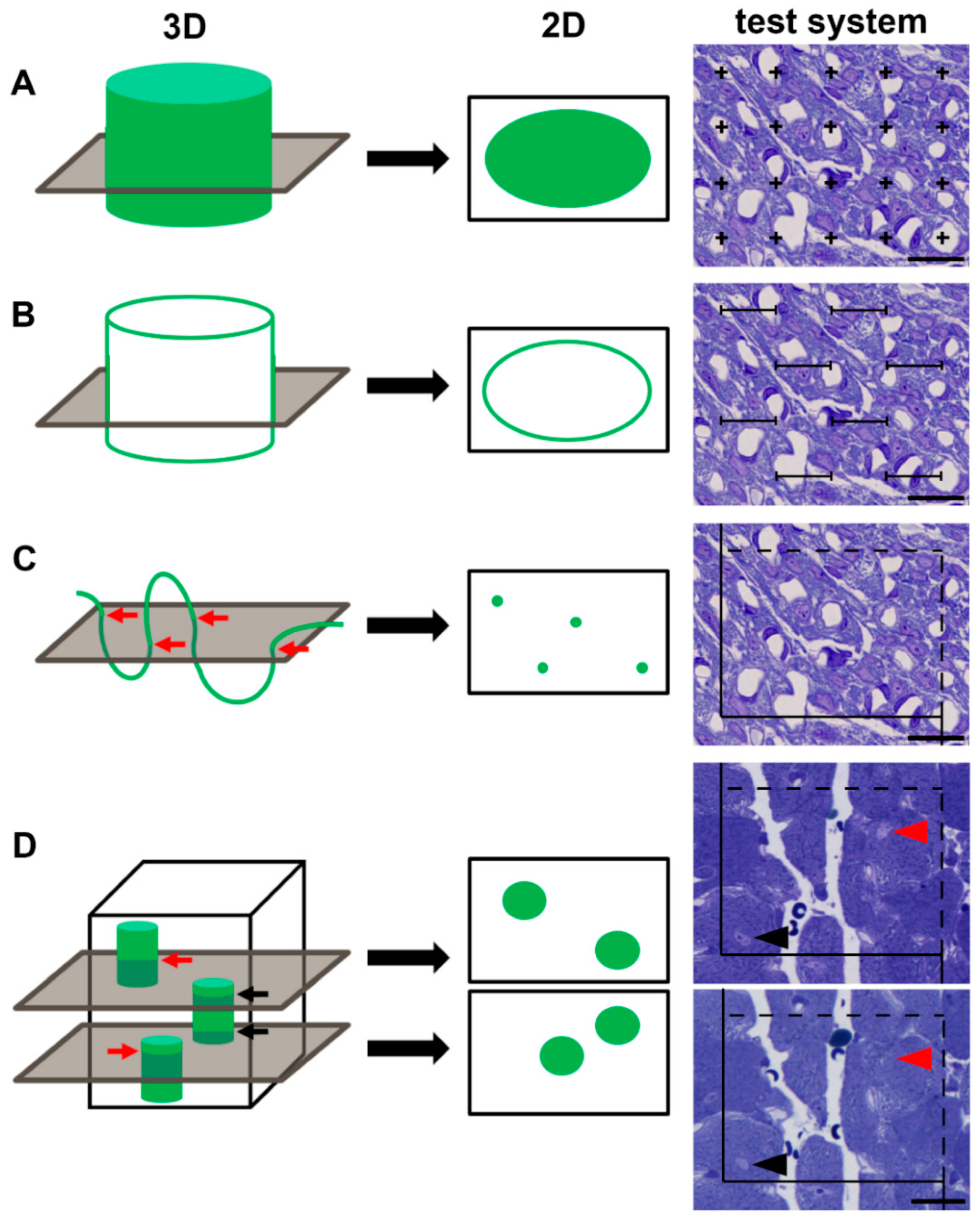
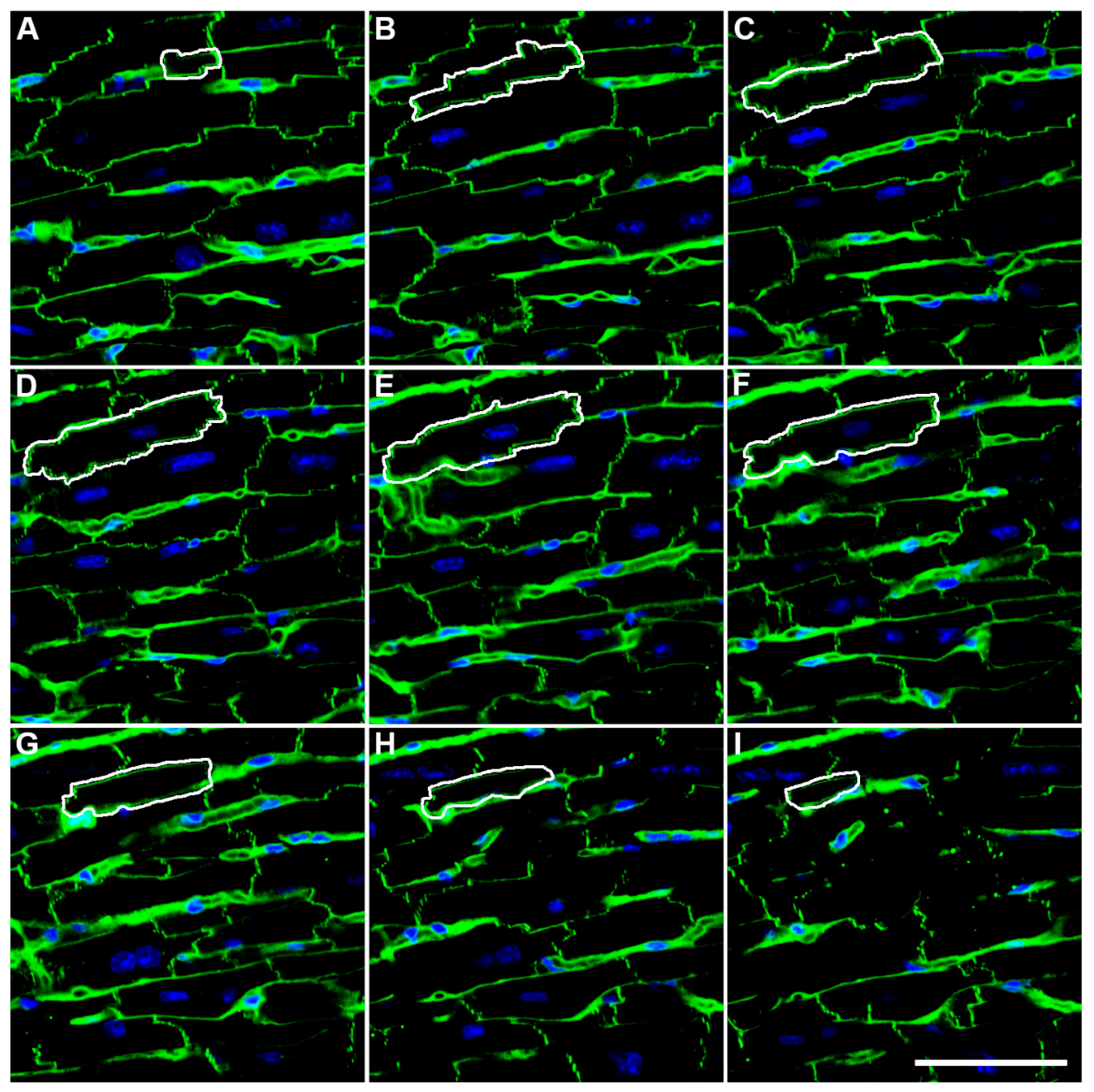
2. Methodological Advances
3. Developmental Physiological Growth and Cardiomyocyte Number
4. Intrauterine Growth Restriction and Cardiomyocyte Number
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mayhew, T.M.; Huppertz, B.; Kaufmann, P.; Kingdom, J.C.P. The ‘reference trap’ revisited: Examples of the dangers in using ratios to describe fetoplacental angiogenesis and trophoblast turnover. Placenta 2003, 24, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nyengaard, J.R. Stereological methods and their application in kidney research. J. Am. Soc. Nephrol. 1999, 10, 1100–1123. [Google Scholar] [CrossRef] [PubMed]
- Hsia, C.C.; Hyde, D.M.; Ochs, M.; Weibel, E.R.; ATS/ERS Joint Task Force on Quantitative Assessment of Lung Structure. An official research policy statement of the American Thoracic Society/European Respiratory Society: Standards for quantitative assessment of lung structure. Am. J. Respir. Crit. Care Med. 2010, 181, 394–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochs, M.; Mühlfeld, C. Quantitative microscopy of the lung: A problem-based approach. Part 1: Basic principles of lung stereology. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 305, L15–L22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mühlfeld, C.; Ochs, M. Quantitative microscopy of the lung: A problem-based approach. Part 2: Stereological parameters and study designs in various diseases of the respiratory tract. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 305, L205–L221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandarim-de-Lacerda, C.A.; del Sol, M. Tips for studies with quantitative morphology (morphometry and stereology). Int. J. Morphol. 2017, 35, 1482–1494. [Google Scholar] [CrossRef] [Green Version]
- Mühlfeld, C.; Nyengaard, J.R.; Mayhew, T.M. A review of state-of-the-art stereology for better quantitative 3D morphology in cardiac research. Cardiovasc. Pathol. 2010, 19, 65–82. [Google Scholar] [CrossRef]
- Mühlfeld, C. Quantitative morphology of the vascularisation of organs: A stereological approach illustrated using the cardiac circulation. Ann. Anat. 2014, 196, 12–19. [Google Scholar] [CrossRef]
- Sterio, D.C. The unbiased estimation of number and sizes of arbitrary particles using the disector. J. Microsc. 1984, 134, 127–136. [Google Scholar] [CrossRef]
- Brüel, A.; Nyengaard, J.R. Design-based stereological estimation of the total number of cardiac myocytes in histological sections. Basic Res. Cardiol. 2005, 100, 311–319. [Google Scholar] [CrossRef]
- Schipke, J.; Banmann, E.; Nikam, S.; Voswinckel, R.; Kohlstedt, K.; Loot, A.E.; Fleming, I.; Mühlfeld, C. The number of cardiac myocytes in the hypertrophic and hypotrophic left ventricle of the obese and calorie-restricted mouse heart. J. Anat. 2014, 225, 539–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corstius, H.B.; Zimanyi, M.A.; Maka, N.; Herath, T.; Thomas, W.; van der Laarse, A.; Wreford, N.G.; Black, M.J. Effect of intrauterine growth restriction on the number of cardiomyocytes in rat hearts. Pediatr. Res. 2005, 57, 796–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampaio-Pinto, V.; Silva, E.D.; Laundos, T.L.; Costa Martins, P.D.; Pinto-do-Ó, P.; Nascimento, D.S. Stereological estimation of cardiomyocyte number and proliferation. Methods 2021, 190, 55–62. [Google Scholar] [CrossRef]
- Tan, L.; Bogush, N.; Naqvi, E.; Calvert, J.W.; Graham, R.M.; Taylor, W.R.; Naqvi, N.; Husain, A. Thyroid hormone plus dual-specificity phosphatase-5 siRNA increases the number of cardiac muscle cells and improves left ventricular contractile function in chronic doxorubicin-injured hearts. Theranostics 2021, 11, 4790–4808. [Google Scholar] [CrossRef]
- Jansing, N.L.; Patel, N.; McClendon, J.; Redente, E.F.; Henson, P.M.; Tuder, R.M.; Hyde, D.M.; Nyengaard, J.R.; Zemans, R.L. Flow Cytometry Underestimates and Planimetry Overestimates Alveolar Epithelial Type 2 Cell Expansion after Lung Injury. Am. J. Respir. Crit. Care Med. 2018, 198, 390–392. [Google Scholar] [CrossRef] [Green Version]
- Dzhuraev, G.; Rodríguez-Castillo, J.A.; Ruiz-Camp, J.; Salwig, I.; Szibor, M.; Vadász, I.; Herold, S.; Braun, T.; Ahlbrecht, K.; Atzberger, A.; et al. Estimation of absolute number of alveolar epithelial type 2 cells in mouse lungs: A comparison between stereology and flow cytometry. J. Microsc. 2019, 275, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Mühlfeld, C.; Papadakis, T.; Krasteva, G.; Nyengaard, J.R.; Hahn, U.; Kummer, W. An unbiased stereological method for efficiently quantifying the innervation of the heart and other organs based on total length estimations. J. Appl. Physiol. 2010, 108, 1402–1409. [Google Scholar] [CrossRef]
- Schipke, J.; Mayhew, T.M.; Mühlfeld, C. Allometry of left ventricular myocardial innervation. J. Anat. 2014, 224, 518–526. [Google Scholar] [CrossRef] [Green Version]
- Hadi, A.M.; Mouchaers, K.T.; Schalij, I.; Grunberg, K.; Meijer, G.A.; Vonk-Noordegraaf, A.; van der Laarse, W.J.; Beliën, J.A. Rapid quantification of myocardial fibrosis: A new macro-based automated analysis. Cell. Oncol. 2011, 34, 343–354. [Google Scholar] [CrossRef] [Green Version]
- Daunoravicius, D.; Besusparis, J.; Zurauskas, E.; Laurinaviciene, A.; Bironaite, D.; Pankuweit, S.; Plancoulaine, B.; Herlin, P.; Bogomolovas, J.; Grabauskiene, V.; et al. Quantification of myocardial fibrosis by digital image analysis and interactive stereology. Diagn. Pathol. 2014, 9, 114. [Google Scholar] [CrossRef] [Green Version]
- Schipke, J.; Brandenberger, C.; Rajces, A.; Manninger, M.; Alogna, A.; Post, H.; Mühlfeld, C. Assessment of cardiac fibrosis: A morphometric method comparison for collagen quantification. J. Appl. Physiol. 2017, 122, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.P.; Ochs, M. Alterations of mouse lung tissue dimensions during processing for morphometry: A comparison of methods. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 306, L341–L350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mollova, M.; Bersell, K.; Walsh, S.; Savla, J.; Das, L.T.; Park, S.Y.; Silberstein, L.E.; Dos Remedios, C.G.; Graham, D.; Colan, S.; et al. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc. Natl. Acad. Sci. USA 2013, 110, 1446–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergmann, O.; Zdunek, S.; Felker, A.; Salehpour, M.; Alkass, K.; Bernard, S.; Sjostrom, S.L.; Szewczykowska, M.; Jackowska, T.; Dos Remedios, C.; et al. Dynamics of cell generation and turnover in the human heart. Cell 2015, 161, 1566–1575. [Google Scholar] [CrossRef] [Green Version]
- Alkass, K.; Panula, J.; Westman, M.; Wu, T.D.; Guerquin-Kern, J.L.; Bergmann, O. No evidence for cardiomyocyte number expansion in preadolescent mice. Cell 2015, 163, 1026–1036. [Google Scholar] [CrossRef] [Green Version]
- Puente, B.N.; Kimura, W.; Muralidhar, S.A.; Moon, J.; Amatruda, J.F.; Phelps, K.L.; Grinsfelder, D.; Rothermel, B.A.; Chen, R.; Garcia, J.A.; et al. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell 2014, 157, 565–579. [Google Scholar] [CrossRef] [Green Version]
- Soonpaa, M.H.; Kim, K.K.; Pajak, L.; Franklin, M.; Field, L.J. Cardiomyocyte DNA synthesis and binucleation during murine development. Am. J. Physiol. 1996, 271, H2183–H2189. [Google Scholar] [CrossRef]
- Walsh, S.; Pontén, A.; Fleischmann, B.K.; Jovinge, S. Cardiomyocyte cell cycle control and growth estimation in vivo—An analysis based on cardiomyocyte nuclei. Cardiovasc. Res. 2010, 86, 365–373. [Google Scholar] [CrossRef]
- Naqvi, N.; Li, M.; Calvert, J.W.; Tejada, T.; Lambert, J.P.; Wu, J.; Kesteven, S.H.; Holman, S.R.; Matsuda, T.; Lovelock, J.D.; et al. A proliferative burst during preadolescence establishes the final cardiomyocyte number. Cell 2014, 157, 795–807. [Google Scholar] [CrossRef] [Green Version]
- Asif, Y.; Wlodek, M.E.; Black, M.J.; Russell, A.P.; Soeding, P.F.; Wadley, G.D. Sustained cardiac programming by short-term juvenile exercise training in male rats. J. Physiol. 2018, 596, 163–180. [Google Scholar] [CrossRef]
- Brüel, A.; Christoffersen, T.E.; Nyengaard, J.R. Growth hormone increases the proliferation of existing cardiac myocytes and the total number of cardiac myocytes in the rat heart. Cardiovasc. Res. 2007, 76, 400–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisele, J.C.; Schaefer, I.M.; Nyengaard, J.R.; Post, H.; Liebetanz, D.; Brüel, A.; Mühlfeld, C. Effect of voluntary exercise on number and volume of cardiomyocytes and their mitochondria in the mouse left ventricle. Basic Res. Cardiol. 2008, 103, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Derks, W.; Bergmann, O. Polyploidy in Cardiomyocytes: Roadblock to Heart Regeneration? Circ. Res. 2020, 126, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Patterson, M.; Barske, L.; Van Handel, B.; Rau, C.D.; Gan, P.; Sharma, A.; Parikh, S.; Denholtz, M.; Huang, Y.; Yamaguchi, Y.; et al. Frequency of mononuclear diploid cardiomyocytes underlies natural variation in heart regeneration. Nat. Genet. 2017, 49, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Gentric, G.; Desdouets, C. Polyploidization in liver tissue. Am. J. Pathol. 2014, 184, 322–331. [Google Scholar] [CrossRef]
- Miyaoka, Y.; Ebato, K.; Kato, H.; Arakawa, S.; Shimizu, S.; Miyajima, A. Hypertrophy and unconventional cell division of hepatocytes underlie liver regeneration. Curr. Biol. 2012, 22, 1166–1175. [Google Scholar] [CrossRef] [Green Version]
- Windmueller, R.; Leach, J.P.; Babu, A.; Zhou, S.; Morley, M.P.; Wakabayashi, A.; Petrenko, N.B.; Viatour, P.; Morrisey, E.E. Direct Comparison of Mononucleated and Binucleated Cardiomyocytes Reveals Molecular Mechanisms Underlying Distinct Proliferative Competencies. Cell Rep. 2020, 30, 3105–3116.e4. [Google Scholar] [CrossRef] [Green Version]
- Armengaud, J.B.; Yzydorczyk, C.; Siddeek, B.; Peyter, A.C.; Simeoni, U. Intrauterine growth restriction: Clinical consequences on health and disease at adulthood. Reprod. Toxicol. 2021, 99, 168–176. [Google Scholar] [CrossRef]
- Barker, D.J.; Osmond, C.; Golding, J.; Kuh, D.; Wadsworth, M.E. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ 1989, 298, 564–567. [Google Scholar] [CrossRef] [Green Version]
- Barker, D.J.; Gelow, J.; Thornburg, K.; Osmond, C.; Kajantie, E.; Eriksson, J.G. The early origins of chronic heart failure: Impaired placental growth and initiation of insulin resistance in childhood. Eur. J. Heart Fail 2010, 12, 819–825. [Google Scholar] [CrossRef] [Green Version]
- Botting, K.J.; Loke, X.Y.; Zhang, S.; Andersen, J.B.; Nyengaard, J.R.; Morrison, J.L. IUGR decreases cardiomyocyte endowment and alters cardiac metabolism in a sex- and cause-of-IUGR-specific manner. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R48–R67. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Zimanyi, M.A.; Black, M.J. Effect of maternal protein restriction during pregnancy and lactation on the number of cardiomyocytes in the postproliferative weanling rat heart. Anat. Rec. 2010, 293, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Botting, K.J.; McMillen, I.C.; Forbes, H.; Nyengaard, J.R.; Morrison, J.L. Chronic hypoxemia in late gestation decreases cardiomyocyte number but does not change expression of hypoxia-responsive genes. J. Am. Heart Assoc. 2014, 3, e000531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schipke, J.; Gonzalez-Tendero, A.; Cornejo, L.; Willführ, A.; Bijnens, B.; Crispi, F.; Mühlfeld, C.; Gratacós, E. Experimentally induced intrauterine growth restriction in rabbits leads to differential remodelling of left versus right ventricular myocardial microstructure. Histochem. Cell Biol. 2017, 148, 557–567. [Google Scholar] [CrossRef]
- Vranas, S.; Heinemann, G.K.; Liu, H.; De Blasio, M.J.; Owens, J.A.; Gatford, K.L.; Black, M.J. Small size at birth predicts decreased cardiomyocyte number in the adult bovine heart. J. Dev. Orig. Health Dis. 2017, 8, 618–625. [Google Scholar] [CrossRef]
- Gundersen, H.J. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William, R. Thompson. J. Microsc. 1986, 143, 3–45. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mühlfeld, C.; Schipke, J. Methodological Progress of Stereology in Cardiac Research and Its Application to Normal and Pathological Heart Development. Cells 2022, 11, 2032. https://doi.org/10.3390/cells11132032
Mühlfeld C, Schipke J. Methodological Progress of Stereology in Cardiac Research and Its Application to Normal and Pathological Heart Development. Cells. 2022; 11(13):2032. https://doi.org/10.3390/cells11132032
Chicago/Turabian StyleMühlfeld, Christian, and Julia Schipke. 2022. "Methodological Progress of Stereology in Cardiac Research and Its Application to Normal and Pathological Heart Development" Cells 11, no. 13: 2032. https://doi.org/10.3390/cells11132032
APA StyleMühlfeld, C., & Schipke, J. (2022). Methodological Progress of Stereology in Cardiac Research and Its Application to Normal and Pathological Heart Development. Cells, 11(13), 2032. https://doi.org/10.3390/cells11132032





