Calreticulin and the Heart
Abstract
:1. Introduction
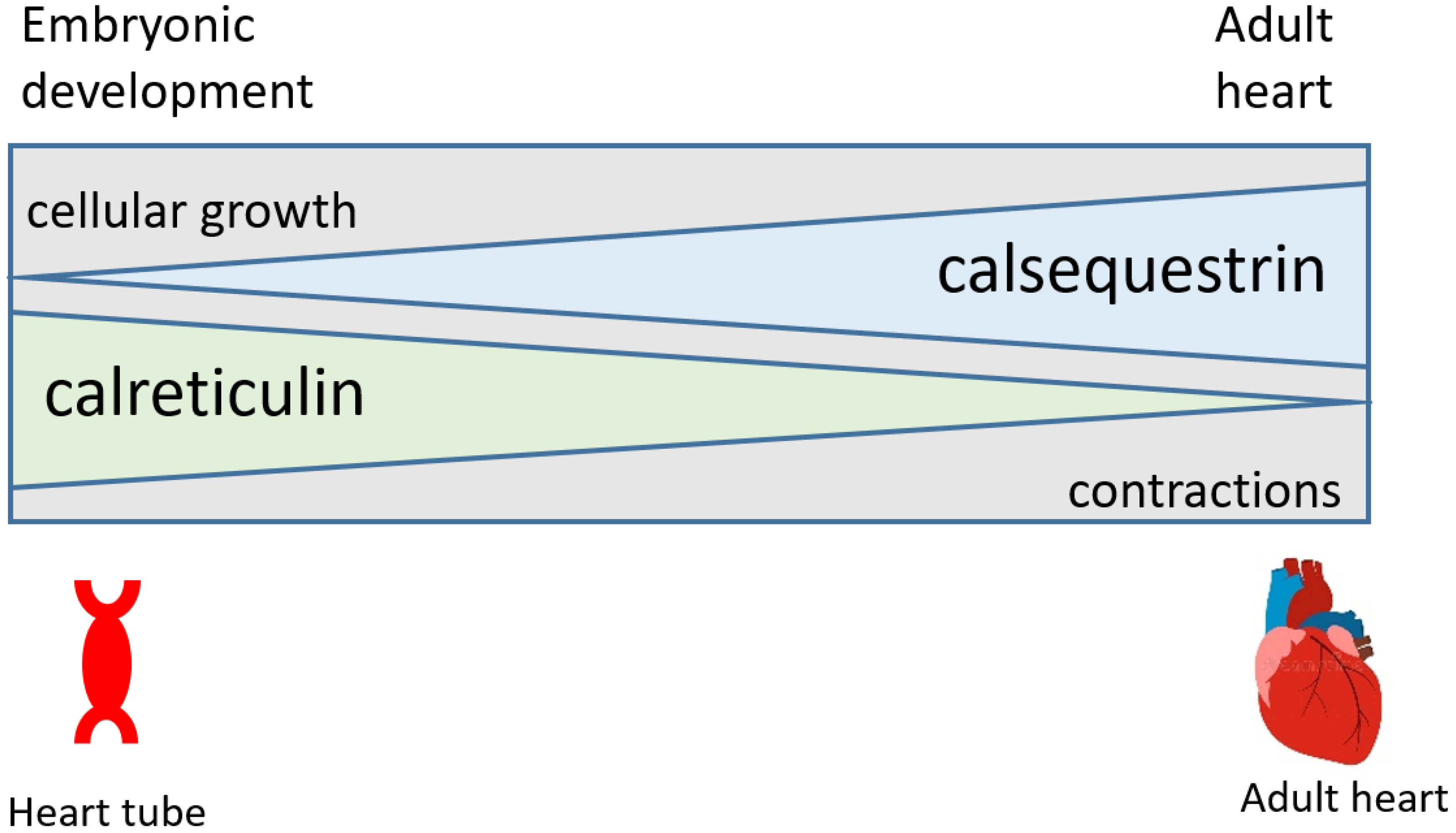
2. Calreticulin and Heart Development
3. Calreticulin in the Adult Heart
4. Conclusions and Future Challenges
- ER Ca2+ capacity/homeostasis is essential for cardiac development and leads to heart disease when dysregulated.
- Calreticulin supports Ca2+-dependent signaling events that are critical to cardiomyocyte differentiation and cardiogenesis.
- Calreticulin is a major Ca2+ binding protein in the ER/SR of the developing heart and is downregulated after birth when calsequestrin, a muscle-specific Ca2+ binding/storage protein, is upregulated to supply Ca2+ that supports E-C coupling.
- The increased expression of calreticulin and an increased ER/SR Ca2+ capacity produce cardiomyocytes with enhanced efficiency, triggering detrimental mechanical stretching of cardiac fibroblasts and leading to cardiac pathology.
- The calreticulin-dependent Ca2+ pool in adult cardiomyocytes must be controlled as any increase or decrease in calreticulin results in heart pathology.
- Resolving the specific functions of ER versus SR in muscle cells remains a challenge.
- The role of calreticulin mutants needs to be explored for a better understanding of their role in cardiac pathophysiology.
- Do other ER-associated chaperones play a role in cardiomyocyte Ca2+ homeostasis?
- Further understanding of the role of ER/SR lumenal Ca2+ homeostasis will allow for the development of more targeted approaches to combat heart disease.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fabiato, A.; Fabiato, F. Calcium and cardiac excitation-contraction coupling. Annu. Rev. Physiol. 1979, 41, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Benitah, J.P.; Perrier, R.; Mercadier, J.J.; Pereira, L.; Gomez, A.M. RyR2 and Calcium Release in Heart Failure. Front. Physiol. 2021, 12, 734210. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M.; Despa, S. Cardiac myocytes Ca2+ and Na+ regulation in normal and failing hearts. J. Pharmacol. Sci. 2006, 100, 315–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalak, M.; Opas, M. Endoplasmic and sarcoplasmic reticulum in the heart. Trends Cell. Biol. 2009, 19, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Corbett, E.F.; Mesaeli, N.; Nakamura, K.; Opas, M. Calreticulin: One protein, one gene, many functions. Biochem. J. 1999, 344, 281–292. [Google Scholar] [CrossRef]
- High, S.; Lecomte, F.J.; Russell, S.J.; Abell, B.M.; Oliver, J.D. Glycoprotein folding in the endoplasmic reticulum: A tale of three chaperones? FEBS Lett. 2000, 476, 38–41. [Google Scholar] [CrossRef]
- Zapun, A.; Jakob, C.A.; Thomas, D.Y.; Bergeron, J.J. Protein folding in a specialized compartment: The endoplasmic reticulum. Struct. Fold. Des. 1999, 7, R173–R182. [Google Scholar] [CrossRef] [Green Version]
- Bergeron, J.J.M.; Brenner, M.B.; Thomas, D.Y.; Williams, D.B. Calnexin: A membrane-bound chaperone of the endoplasmic reticulum. Trends Biochem. Sci. 1994, 19, 124–128. [Google Scholar] [CrossRef]
- Saito, Y.; Ihara, Y.; Leach, M.R.; Cohen-Doyle, M.F.; Williams, D.B. Calreticulin functions in vitro as a molecular chaperone for both glycosylated and non-glycosylated proteins. EMBO J. 1999, 18, 6718–6729. [Google Scholar] [CrossRef] [Green Version]
- Ellgaard, L.; Frickel, E.M. Calnexin, calreticulin, and ERp57: Teammates in glycoprotein folding. Cell Biochem. Biophys. 2003, 39, 223–247. [Google Scholar] [CrossRef]
- Morton, S.U.; Quiat, D.; Seidman, J.G.; Seidman, C.E. Genomic frontiers in congenital heart disease. Nat. Rev. Cardiol. 2022, 19, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Mesaeli, N.; Nakamura, K.; Zvaritch, E.; Dickie, P.; Dziak, E.; Krause, K.H.; Opas, M.; MacLennan, D.H.; Michalak, M. Calreticulin is essential for cardiac development. J. Cell Biol. 1999, 144, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Puceat, M.; Perez-Terzic, C.; Mery, A.; Nakamura, K.; Michalak, M.; Krause, K.-H.; Jaconi, M.E. Calreticulin reveals a critical Ca2+ checkpoint in cardiac myofibrillogenesis. J. Cell Biol. 2002, 158, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Nakamura, K.; Lynch, J.; Opas, M.; Olson, E.N.; Agellona, L.B.; Michalak, M. Cardiac specific expression of calcineurin reverses embryonic lethality in calreticulin-deficient mouse. J. Biol. Chem. 2002, 277, 50776–50779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, J.M.; Chilibeck, K.; Qui, Y.; Michalak, M. Assembling pieces of the cardiac puzzle; calreticulin and calcium-dependent pathways in cardiac development, health, and disease. Trends Cardiovasc. Med. 2006, 16, 65–69. [Google Scholar] [CrossRef]
- Sokol, S.Y. Maintaining embryonic stem cell pluripotency with Wnt signaling. Development 2011, 138, 4341–4350. [Google Scholar] [CrossRef] [Green Version]
- Groenendyk, J.; Michalak, M. Disrupted WNT signaling in mouse embryonic stem cells in the absence of calreticulin. Stem Cell Rev. 2014, 10, 191–206. [Google Scholar] [CrossRef]
- Groenendyk, J.; Michalak, M. A genome-wide siRNA screen identifies novel phospho-enzymes affecting Wnt/beta-catenin signaling in mouse embryonic stem cells. Stem Cell Rev. 2011, 7, 910–926. [Google Scholar] [CrossRef]
- Groenendyk, J.; Fan, X.; Peng, Z.; Kurgan, L.; Michalak, M. Endoplasmic reticulum and the microRNA environment in the cardiovascular system. Can. J. Physiol. Pharmacol. 2019, 97, 515–527. [Google Scholar] [CrossRef]
- Olson, E.N. MicroRNAs as therapeutic targets and biomarkers of cardiovascular disease. Sci. Transl. Med. 2014, 6, 239ps233. [Google Scholar] [CrossRef] [Green Version]
- Small, E.M.; Frost, R.J.; Olson, E.N. MicroRNAs add a new dimension to cardiovascular disease. Circulation 2010, 121, 1022–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahimi, K.; Fuchtbauer, A.C.; Fathi, F.; Mowla, S.J.; Fuchtbauer, E.M. Expression of the miR-302/367 microRNA cluster is regulated by a conserved long non-coding host-gene. Sci. Rep. 2021, 11, 11115. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Jiang, L.; Hu, Q.; Li, Y. MiR-302e attenuates allergic inflammation in vitro model by targeting RelA. Biosci. Rep. 2018, 38, BSR20180025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassler, J.; Cao, S.S.; Kaufman, R.J. IRE1, a double-edged sword in pre-miRNA slicing and cell death. Dev. Cell 2012, 23, 921–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groenendyk, J.; Fan, X.; Peng, Z.; Ilnytskyy, Y.; Kurgan, L.; Michalak, M. Genome-wide analysis of thapsigargin-induced microRNAs and their targets in NIH3T3 cells. Genom. Data 2014, 2, 325–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macer, D.R.; Koch, G.L. Identification of a set of calcium-binding proteins in reticuloplasm, the luminal content of the endoplasmic reticulum. J. Cell Sci. 1988, 91, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Wanderling, S.; Simen, B.B.; Ostrovsky, O.; Ahmed, N.T.; Vogen, S.M.; Gidalevitz, T.; Argon, Y. GRP94 is essential for mesoderm induction and muscle development because it regulates insulin-like growth factor secretion. Mol. Biol. Cell 2007, 18, 3764–3775. [Google Scholar] [CrossRef]
- Prins, D.; Gonzalez Arias, C.; Klampfl, T.; Grinfeld, J.; Green, A.R. Mutant Calreticulin in the Myeloproliferative Neoplasms. Hemasphere 2020, 4, e333. [Google Scholar] [CrossRef]
- Balligand, T.; Achouri, Y.; Pecquet, C.; Gaudray, G.; Colau, D.; Hug, E.; Rahmani, Y.; Stroobant, V.; Plo, I.; Vainchenker, W.; et al. Knock-in of murine Calr del52 induces essential thrombocythemia with slow-rising dominance in mice and reveals key role of Calr exon 9 in cardiac development. Leukemia 2020, 34, 510–521. [Google Scholar] [CrossRef]
- Martinho-Dias, D.; Leite-Moreira, A.; Castro-Chaves, P. Calreticulin in the Heart: From Embryological Development to Cardiac Pathology. Curr. Mol. Med. 2016, 16, 12–22. [Google Scholar] [CrossRef]
- Okada, K.; Minamino, T.; Tsukamoto, Y.; Liao, Y.; Tsukamoto, O.; Takashima, S.; Hirata, A.; Fujita, M.; Nagamachi, Y.; Nakatani, T.; et al. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: Possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation 2004, 110, 705–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.; Dai, W.; Zhang, L.; Juhaeri, J.; Wang, Y.; Caubel, P. Risk of Cardiovascular Events, Stroke, Congestive Heart Failure, Interstitial Lung Disease, and Acute Liver Injury: Dronedarone versus Amiodarone and Other Antiarrhythmics. J. Atr. Fibrillation 2013, 6, 890. [Google Scholar] [CrossRef] [PubMed]
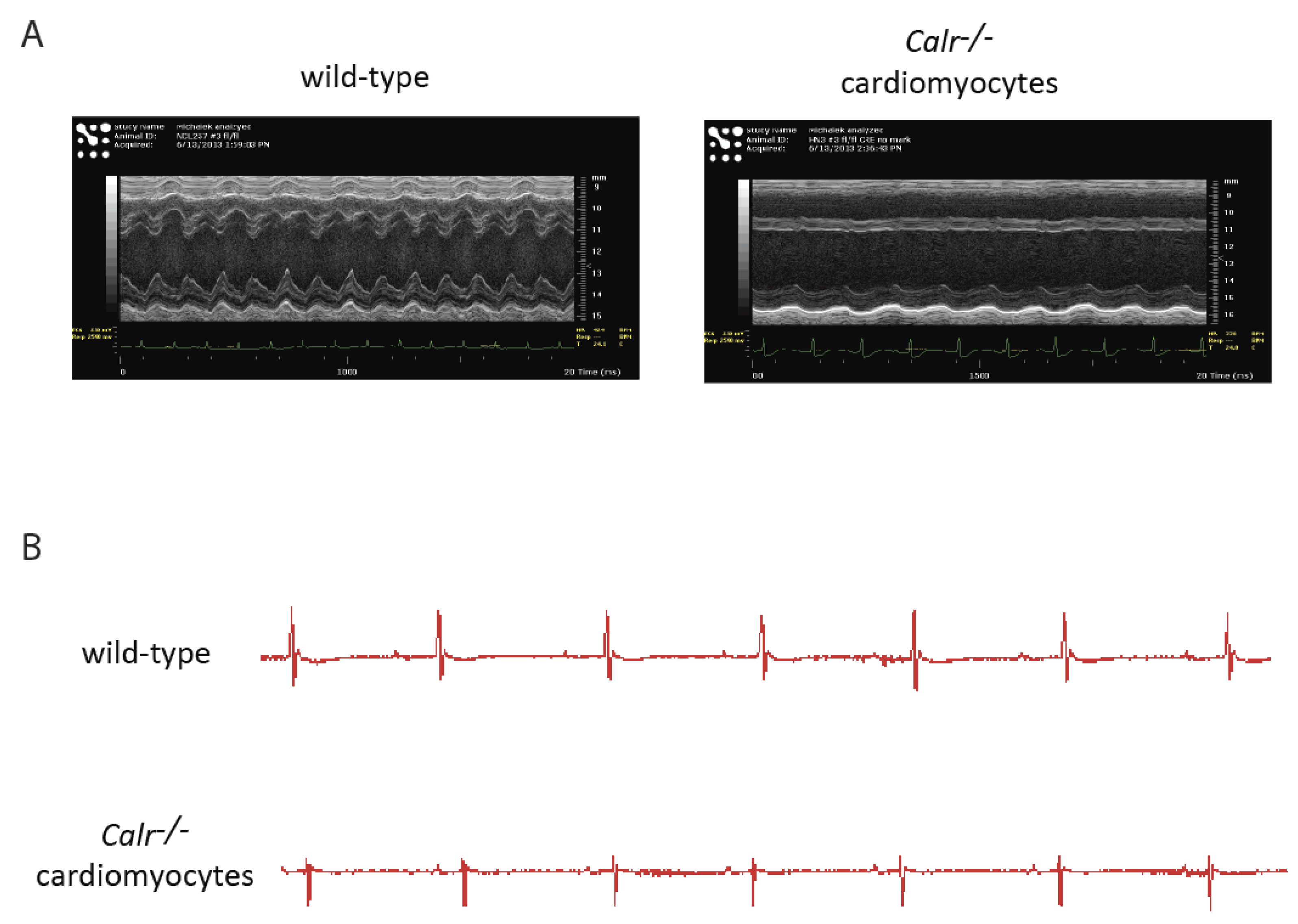
- Lee, D.; Oka, T.; Hunter, B.; Robinson, A.; Papp, S.; Nakamura, K.; Srisakuldee, W.; Nickel, B.E.; Light, P.E.; Dyck, J.R.; et al. Calreticulin induces dilated cardiomyopathy. PLoS ONE 2013, 8, e56387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groenendyk, J.; Lee, D.; Jung, J.; Dyck, J.R.; Lopaschuk, G.D.; Agellon, L.B.; Michalak, M. Inhibition of the Unfolded Protein Response Mechanism Prevents Cardiac Fibrosis. PLoS ONE 2016, 11, e0159682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, K.; Robertson, M.; Liu, G.; Dickie, P.; Nakamura, K.; Guo, J.Q.; Duff, H.J.; Opas, M.; Kavanagh, K.; Michalak, M. Complete heart block and sudden death in mice overexpressing calreticulin. J. Clin. Invest. 2001, 107, 1245–1253. [Google Scholar] [CrossRef] [Green Version]
- Groenendyk, J.; Wang, Q.; Wagg, C.; Lee, D.; Robinson, A.; Barr, A.; Light, P.E.; Lopaschuk, G.D.; Agellon, L.B.; Michalak, M. Selective enhancement of cardiomyocyte efficiency results in a pernicious heart condition. PLoS ONE 2020, 15, e0236457. [Google Scholar] [CrossRef]
- Vega, H.; Agellon, L.B.; Michalak, M. The rise of proteostasis promoters. IUBMB Life 2016, 68, 943–954. [Google Scholar] [CrossRef]
- Rani, S.; Sreenivasaiah, P.K.; Kim, J.O.; Lee, M.Y.; Kang, W.S.; Kim, Y.S.; Ahn, Y.; Park, W.J.; Cho, C.; Kim, D.H. Tauroursodeoxycholic acid (TUDCA) attenuates pressure overload-induced cardiac remodeling by reducing endoplasmic reticulum stress. PLoS ONE 2017, 12, e0176071. [Google Scholar] [CrossRef] [Green Version]
- Taegtmeyer, H.; Sen, S.; Vela, D. Return to the fetal gene program: A suggested metabolic link to gene expression in the heart. Ann. N. Y. Acad. Sci. 2010, 1188, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Rajabi, M.; Kassiotis, C.; Razeghi, P.; Taegtmeyer, H. Return to the fetal gene program protects the stressed heart: A strong hypothesis. Heart Fail. Rev. 2007, 12, 331–343. [Google Scholar] [CrossRef]
- Guimaraes-Camboa, N.; Stowe, J.; Aneas, I.; Sakabe, N.; Cattaneo, P.; Henderson, L.; Kilberg, M.S.; Johnson, R.S.; Chen, J.; McCulloch, A.D.; et al. HIF1alpha Represses Cell Stress Pathways to Allow Proliferation of Hypoxic Fetal Cardiomyocytes. Dev. Cell 2015, 33, 507–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Xie, P.; Hao, N.; Zhang, M.; Liu, Y.; Liu, P.; Semenza, G.L.; He, J.; Zhang, H. HIF-1-regulated expression of calreticulin promotes breast tumorigenesis and progression through Wnt/beta-catenin pathway activation. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Haze, K.; Yanagi, H.; Yura, T.; Mori, K. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J. Biol. Chem. 1998, 273, 33741–33749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waser, M.; Mesaeli, N.; Spencer, C.; Michalak, M. Regulation of calreticulin gene expression by calcium. J. Cell Biol. 1997, 138, 547–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belmont, P.J.; Tadimalla, A.; Chen, W.J.; Martindale, J.J.; Thuerauf, D.J.; Marcinko, M.; Gude, N.; Sussman, M.A.; Glembotski, C.C. Coordination of growth and endoplasmic reticulum stress signaling by regulator of calcineurin 1 (RCAN1), a novel ATF6-inducible gene. J. Biol. Chem. 2008, 283, 14012–14021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokuhiro, K.; Satouh, Y.; Nozawa, K.; Isotani, A.; Fujihara, Y.; Hirashima, Y.; Matsumura, H.; Takumi, K.; Miyano, T.; Okabe, M.; et al. Calreticulin is required for development of the cumulus oocyte complex and female fertility. Sci. Rep. 2015, 5, 14254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heine, H.L.; Leong, H.S.; Rossi, F.M.; McManus, B.M.; Podor, T.J. Strategies of conditional gene expression in myocardium: An overview. Methods Mol. Med. 2005, 112, 109–154. [Google Scholar] [CrossRef]
- Lankipalli, R.S.; Zhu, T.; Guo, D.; Yan, G.X. Mechanisms underlying arrhythmogenesis in long QT syndrome. J. Electrocardiol. 2005, 38, 69–73. [Google Scholar] [CrossRef]
- Landstrom, A.P.; Dobrev, D.; Wehrens, X.H.T. Calcium Signaling and Cardiac Arrhythmias. Circ. Res. 2017, 120, 1969–1993. [Google Scholar] [CrossRef]
- Schimpf, R.; Borggrefe, M.; Wolpert, C. Clinical and molecular genetics of the short QT syndrome. Curr. Opin. Cardiol. 2008, 23, 192–198. [Google Scholar] [CrossRef]
| Molecular and Cellular Functions | Number of Molecules |
| Cellular Growth and Proliferation | 883 |
| Cellular Development | 763 |
| Cellular Movement | 584 |
| Top Canonical Pathways | Ratio |
| Wnt Signaling | 40/63 |
| TGF-β Signaling | 52/86 |
| Cardiac Hypertrophy Signaling | 106/259 |
| Wild-Type | Calr−/− | Calr OE | |
|---|---|---|---|
| Body weight (g) | 20.725 ± 0.245 | 16.285 ± 0.595 | 20.71 ± 0.581 |
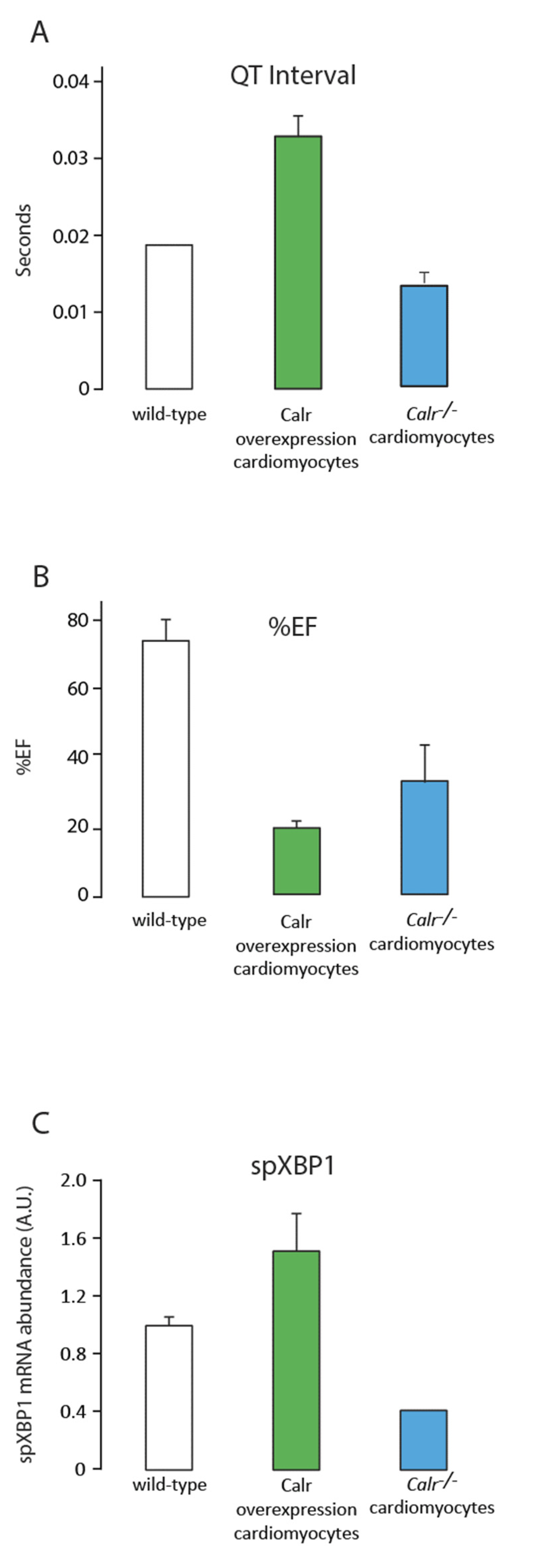
| % EF | 75.485 ± 7.765 | 22.775 ± 11.875 | 15.40556 ± 4.430 |
| % FS | 44.035 ± 7.125 | 10.5 ± 5.710 | 10.62471 ± 2.200 |
| LV Mass (g) | 73.875 ± 0.615 | 66.22 ± 8.650 | 85.10569 ± 4.049 |
| Wild-Type | Calr−/− | Calr OE | ||
|---|---|---|---|---|
| RR Interval | (s) | 0.152 ± 0.013 | 0.159 ± 0.034 | 0.132 ± 0.011 |
| Heart Rate | (BPM) | 403.173 ± 32.553 | 388.470 ± 70.599 | 486.023 ± 50.192 |
| PR Interval | (s) | 0.036 ± 0.004 | 0.038 ± 0.003 | 0.031 ± 0.005 |
| P Duration | (s) | 0.017 ± 0.004 | 0.019 ± 0.001 | 0.013 ± 0.003 |
| QRS Interval | (s) | 0.009 ± 0.001 | 0.009 ± 0.002 | 0.013 ± 0.001 |
| QT Interval | (s) | 0.020 ± 0.002 | 0.017 ± 0.002 | 0.031 ± 0.004 |
| JT Interval | (s) | 0.010 ± 0.004 | 0.008 ± 0.001 | 0.016 ± 0.005 |
| P Amplitude | (mV) | 0.068 ± 0.033 | 0.085 ± 0.010 | 0.060 ± 0.032 |
| ST Height | (mV) | 0.063 ± 0.035 | 0.046 ± 0.025 | −0.170 ± 0.147 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Groenendyk, J.; Wang, W.-A.; Robinson, A.; Michalak, M. Calreticulin and the Heart. Cells 2022, 11, 1722. https://doi.org/10.3390/cells11111722
Groenendyk J, Wang W-A, Robinson A, Michalak M. Calreticulin and the Heart. Cells. 2022; 11(11):1722. https://doi.org/10.3390/cells11111722
Chicago/Turabian StyleGroenendyk, Jody, Wen-An Wang, Alison Robinson, and Marek Michalak. 2022. "Calreticulin and the Heart" Cells 11, no. 11: 1722. https://doi.org/10.3390/cells11111722






