Blue Light Improves Photosynthetic Performance and Biomass Partitioning toward Harvestable Organs in Saffron (Crocus sativus L.)
Abstract
:1. Introduction
2. Materials and Methods
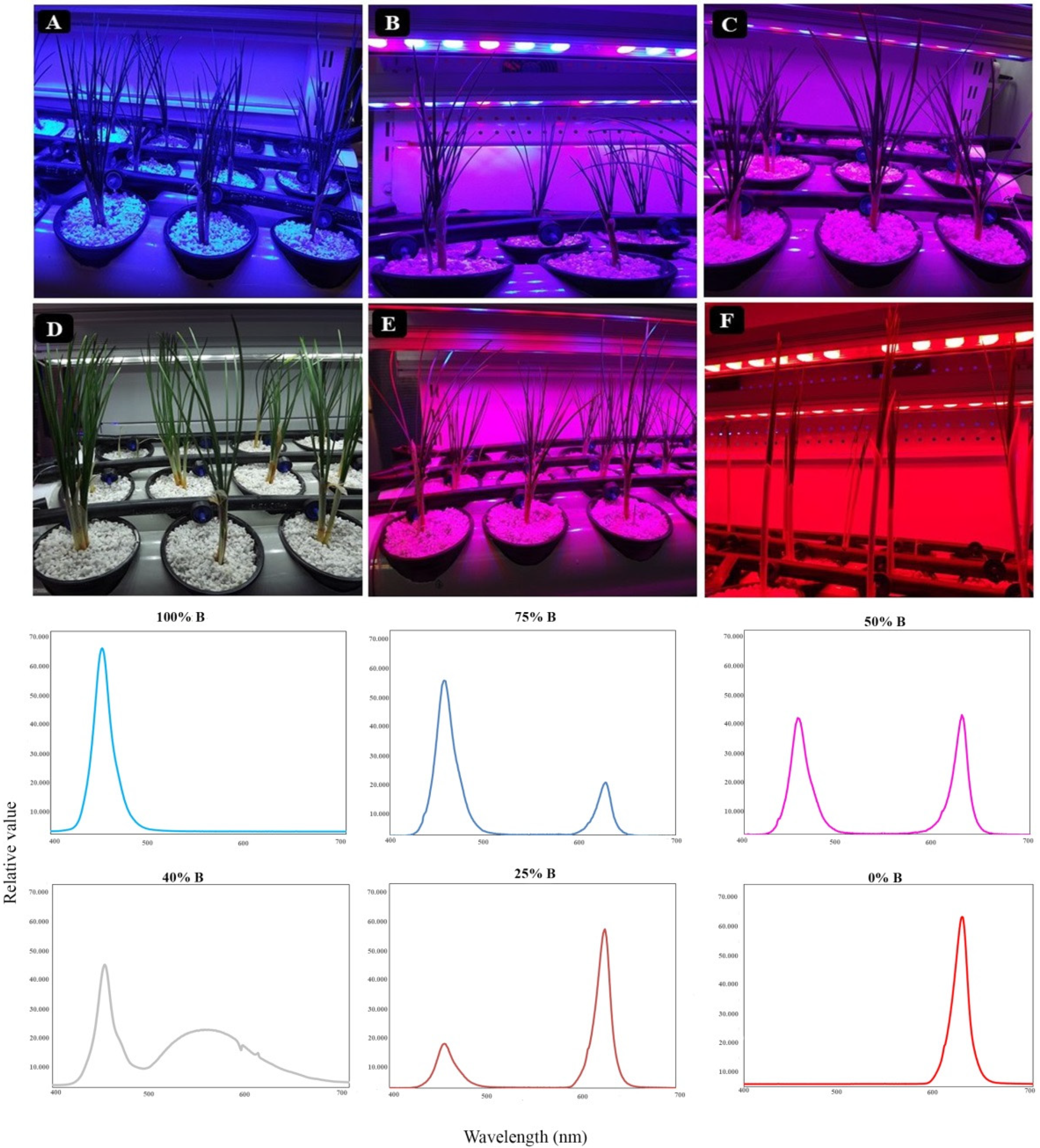
2.1. Plant Materials and Growth Conditions
2.2. Morphological and Growth Measurements
2.3. Analysis of Chl Fluorescence and OJIP Test Measurements
2.4. Pigments Measurements
2.5. Determination of Antioxidant Capacity
2.6. Measurement of Antioxidant Enzymes Activity
2.7. Determination of Carbohydrates in Leaves and Corms
2.8. Statistical Analysis
3. Results
3.1. Plant Morphology and Architecture
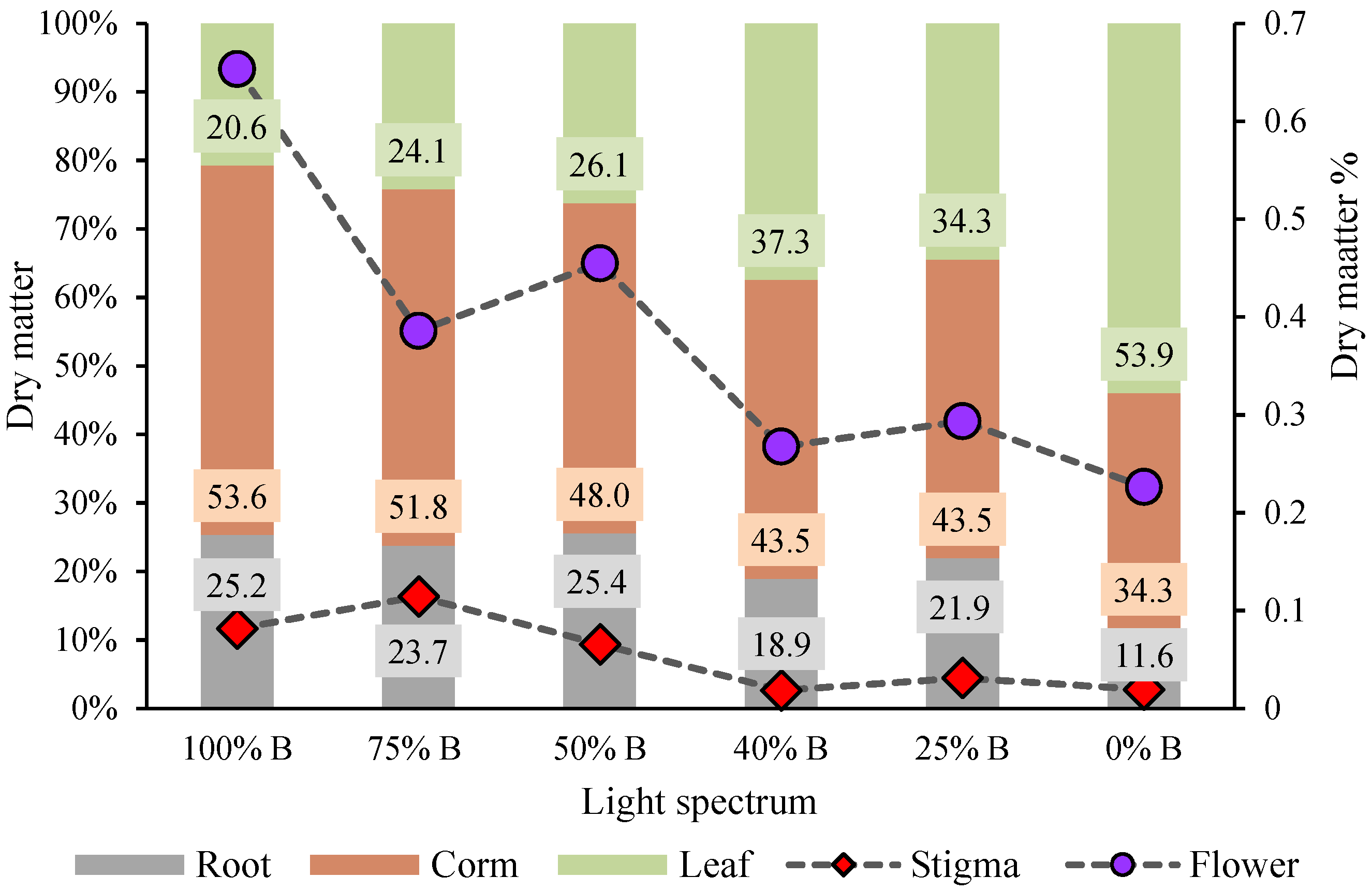
3.2. Carbohydrate Contents and Biomass Partitioning
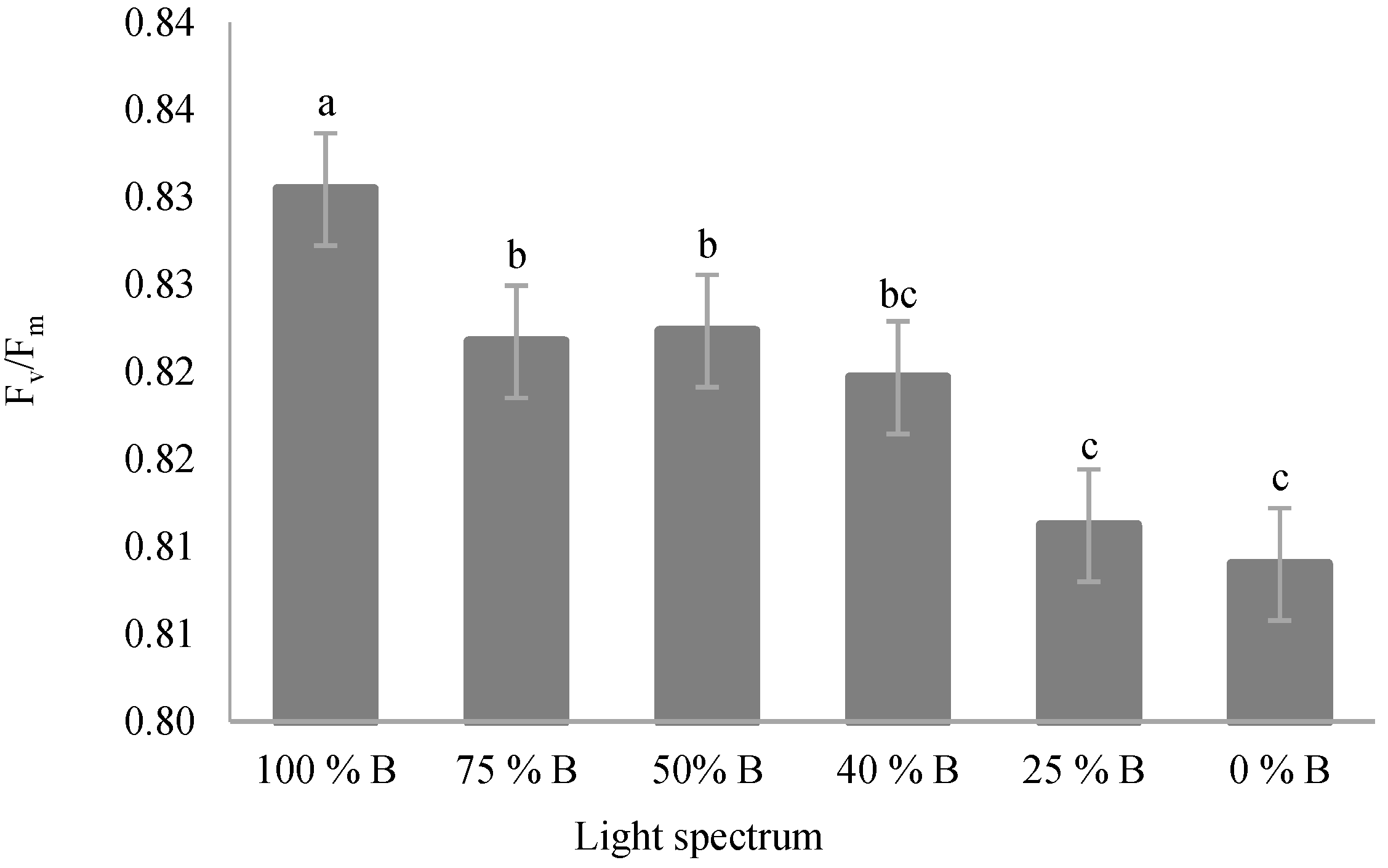
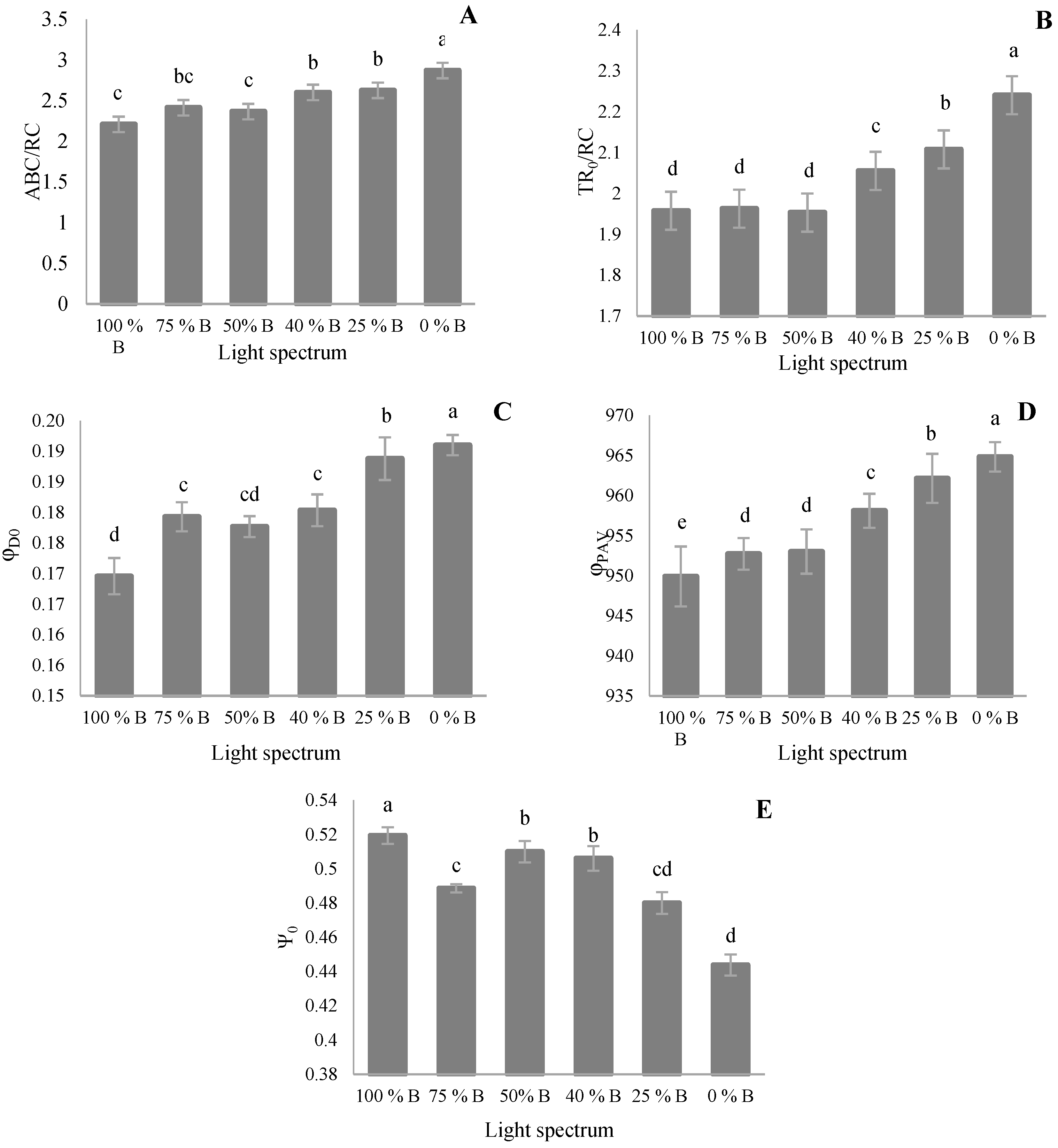
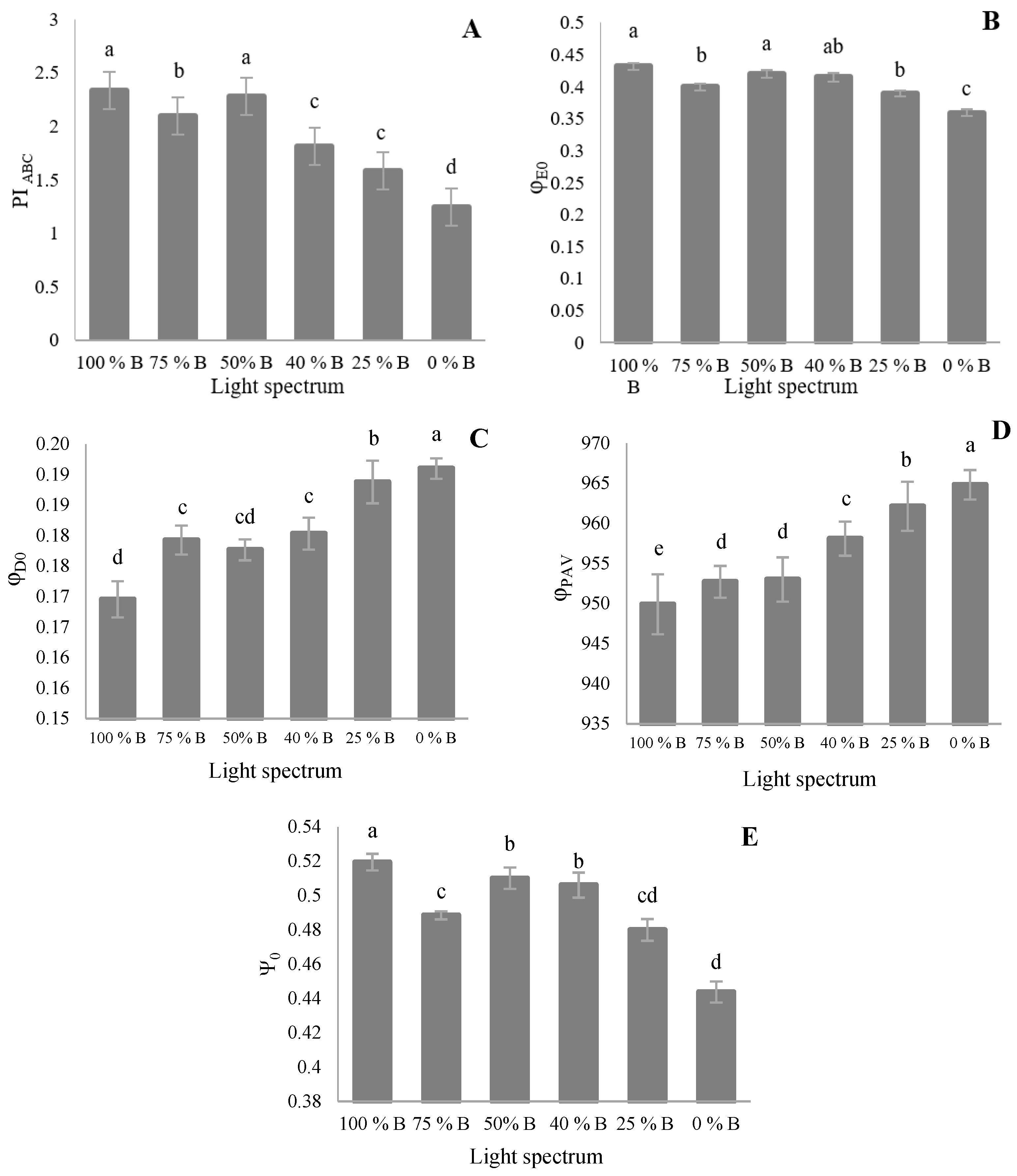
3.3. Photosynthetic Parameters
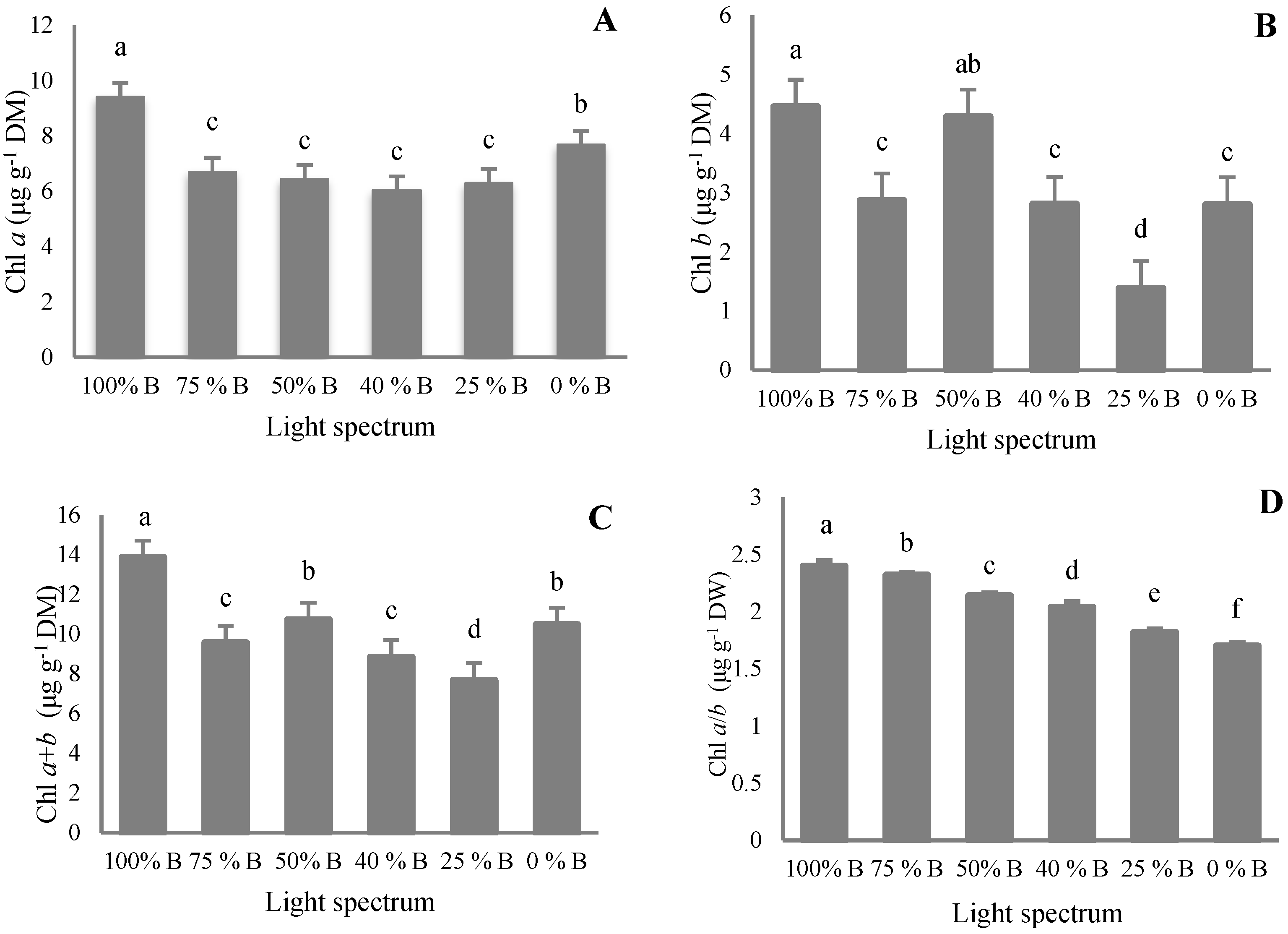
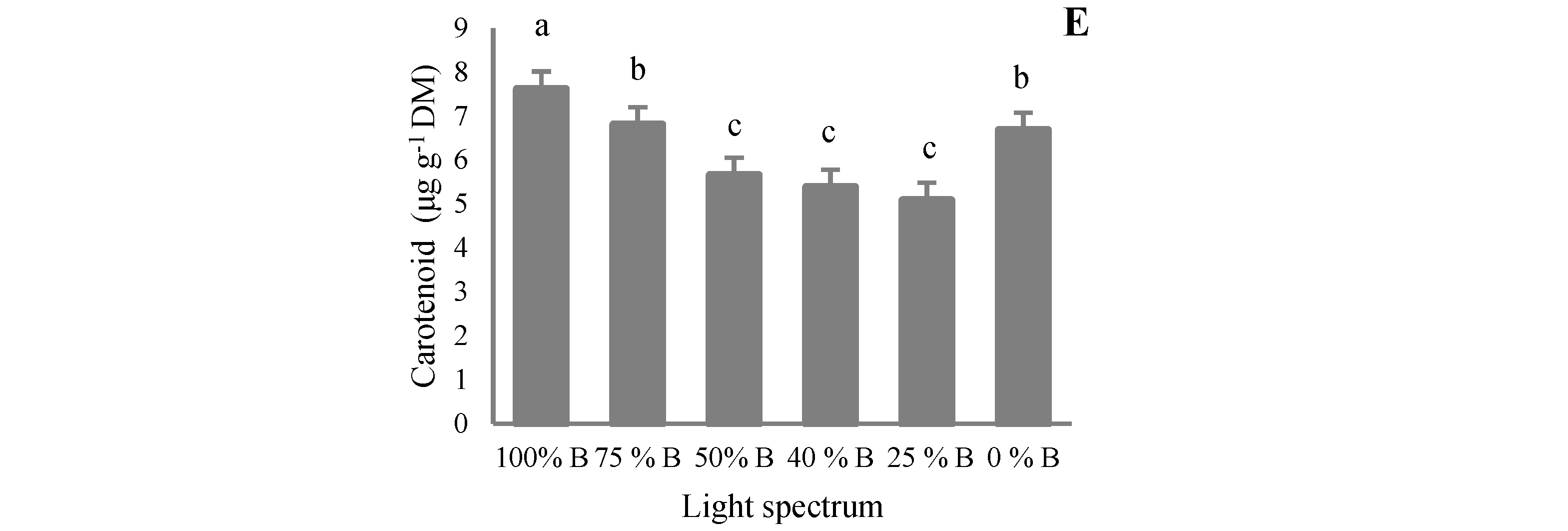
3.4. Pigment Content
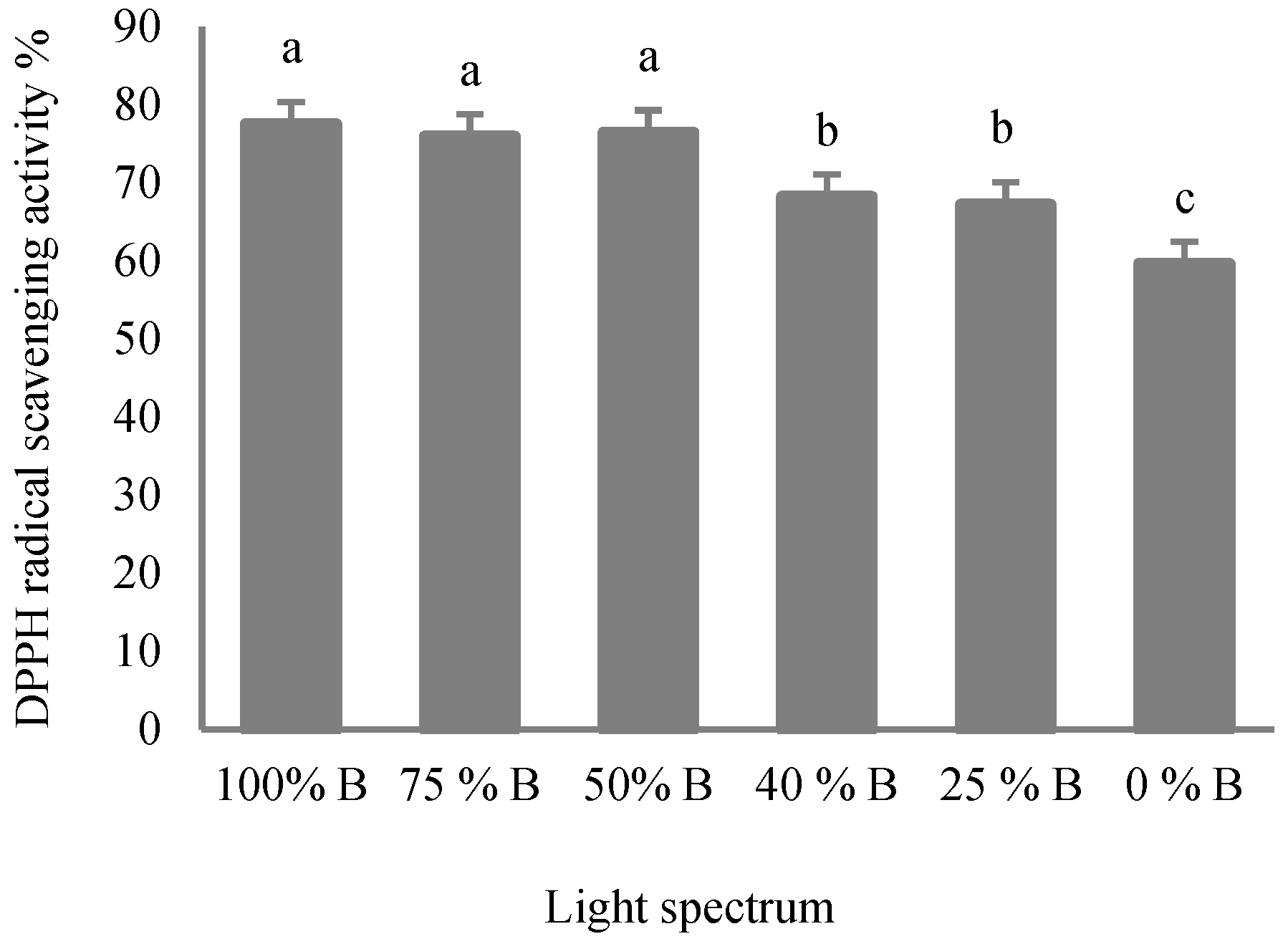
3.5. Antioxidant Capacity
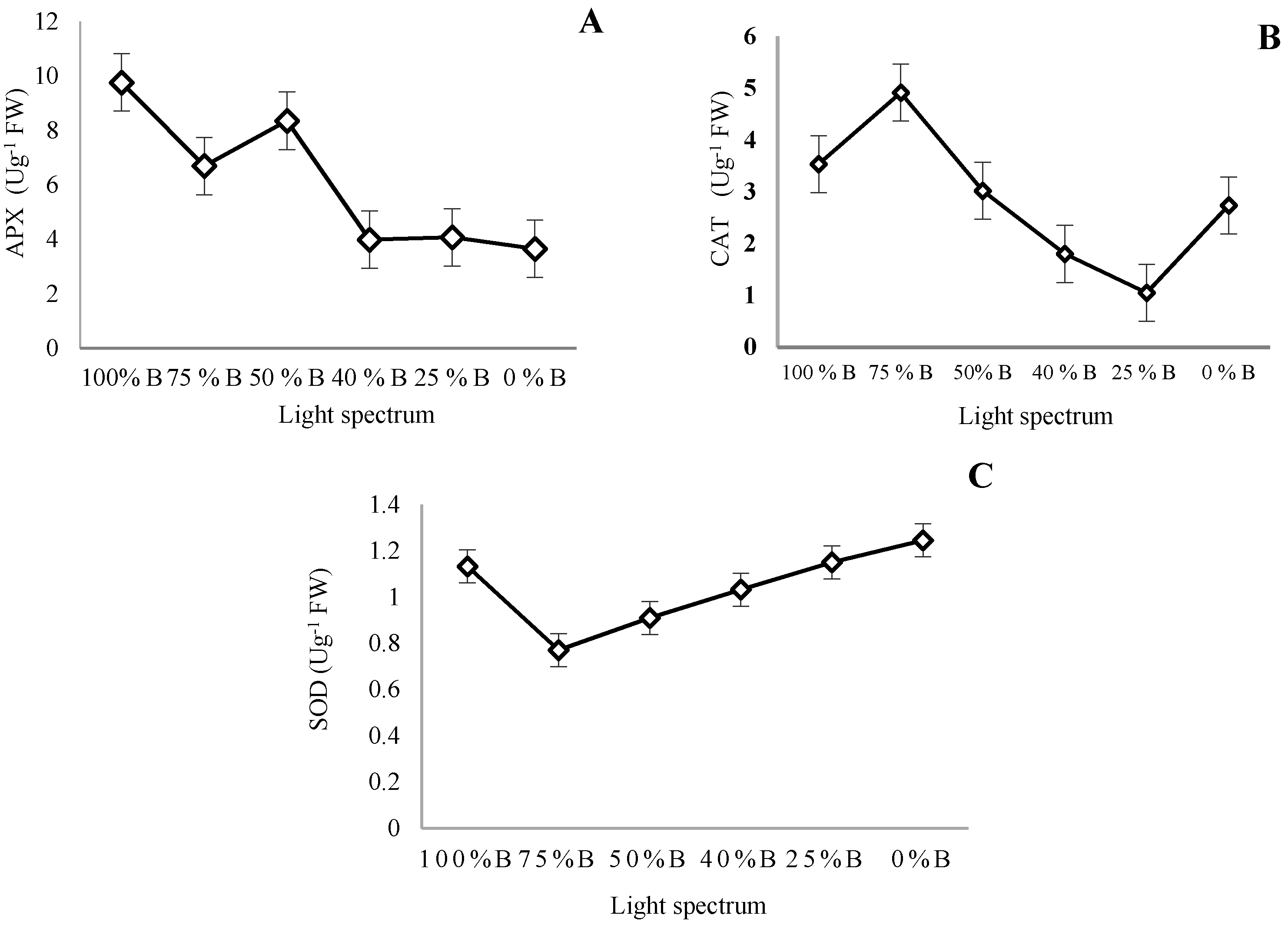
3.6. Antioxidant Enzymes Activity
4. Discussion
4.1. Growth and Morphology of Saffron Plants Were Influenced by Light Quality
4.2. B Light Directed Carbohydrates and Biomass towards the Underground Parts of Saffron
4.3. Photosynthetic Functionality of Saffron Plants Down-Regulated by R Light
4.4. Pigment Accumulation in Saffron Occurred by B Light Exposure
4.5. Antioxidant Enzymes and Oxidative Damage Are Influenced by Light Quality in Saffron
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernández, J.A. Genetic resources of saffron and allies (Crocus spp.). Acta Hortic. 2007, 739, 167–185. [Google Scholar] [CrossRef]
- Molina, R.V.; Renau-Morata, B.; Nebauer, S.G.; García-Luis, A.; Guardiola, J.L. Greenhouse saffron culture-temperature effects on flower emergence and vegetative growth of the plants. Acta Hortic. 2010, 850, 91–94. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G.; Takagaki, M. Plant Factory; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128166918. [Google Scholar]
- Kozai, T. Resource use efficiency of closed plant production system with artificial light: Concept, estimation and application to plant factory. Proc. Jpn. Acad. Ser. B 2013, 89, 447–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eldridge, B.M.; Manzoni, L.R.; Graham, C.A.; Rodgers, B.; Farmer, J.R.; Dodd, A.N. Getting to the roots of aeroponic indoor farming. New Phytol. 2020, 228, 1183–1192. [Google Scholar] [CrossRef]
- Souret, F.F.; Weathers, P.J. The growth of saffron (Crocussativus L.) in aeroponics and hydroponics. J. Herbs. Spices Med. Plants 2000, 7, 25–35. [Google Scholar] [CrossRef]
- Maggio, A.; Raimondi, G.; Martino, A.; De Pascale, S. Soilless cultivation of saffron in Mediterranean environment. In Proceedings of the III International Symposium on models for plant growth, environmental control and farm management in protected cultivation, Wageningen, The Netherlands, 29 October–2 November 2006; Volume 718, pp. 515–521. [Google Scholar]
- Çavuşoǧlu, A.; Erkel, E.I.; Sülüşoǧlu, M. Saffron (Crocus sativus. L.) studies with two mother corm dimensions on yield and harvest period under greenhouse condition. Am. J. Sustain. Agric. 2009, 3, 126–129. [Google Scholar]
- Renau-Morata, B.; Nebauer, S.G.; Sánchez, M.; Molina, R.V. Effect of corm size, water stress and cultivation conditions on photosynthesis and biomass partitioning during the vegetative growth of saffron (Crocus sativus L.). Ind. Crop. Prod. 2012, 39, 40–46. [Google Scholar] [CrossRef]
- Poggi, L.M.; Portela, J.A.; Pontın, M.A.; Moratalla-López, N.; Alonso, G.L. New proposal for handling of saffron flowering (Crocus sativus L.) under environmentally controlled conditions. Acta Hortic. 2017, 1184, 245–252. [Google Scholar] [CrossRef]
- Molina, R.V.; Valero, M.; Navarro, Y.; García-Luis, A.; Guardiola, J.L. The effect of time of corm lifting and duration of incubation at inductive temperature on flowering in the saffron plant (Crocus sativus L.). Sci. Hortic. 2004, 103, 79–91. [Google Scholar] [CrossRef]
- Molina, R.V.; Valero, M.; Navarro, Y.; Guardiola, J.L.; García-Luis, A. Temperature effects on flower formation in saffron (Crocus sativus L.). Sci. Hortic. 2005, 103, 361–379. [Google Scholar] [CrossRef]
- Molina, R.V.; Valero, M.; Navarro, Y.; García-Luis, A.; Guardiola, J.L. Low temperature storage of corms extends the flowering season of saffron (Crocus sativus L.). J. Hortic. Sci. Biotechnol. 2005, 80, 319–326. [Google Scholar] [CrossRef]
- Klem, K.; Gargallo-Garriga, A.; Rattanapichai, W.; Oravec, M.; Holub, P.; Veselá, B.; Sardans, J.; Peñuelas, J.; Urban, O. Distinct morphological, physiological, and biochemical responses to light quality in barley leaves and roots. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J.G. Effects of light quality on growth and phytonutrient accumulation of herbs under controlled environments. Horticulturae 2017, 3, 36. [Google Scholar] [CrossRef] [Green Version]
- Huber, M.; Nieuwendijk, N.M.; Pantazopoulou, C.K.; Pierik, R. Light signalling shapes plant–plant interactions in dense canopies. Plant Cell Environ. 2021, 44, 1014–1029. [Google Scholar] [CrossRef]
- Fan, X.X.; Zang, J.; Xu, Z.G.; Guo, S.R.; Jiao, X.L.; Liu, X.Y.; Gao, Y. Effects of different light quality on growth, chlorophyll concentration and chlorophyll biosynthesis precursors of non-heading Chinese cabbage (Brassica campestris L.). Acta Physiol. Plant. 2013, 35, 2721–2726. [Google Scholar] [CrossRef]
- Amiri, A.; Kafi, M.; Kalate-Jari, S.; Matinizadeh, M. Tulip response to different light sources. J. Anim. Plant Sci. 2018, 28, 539–545. [Google Scholar]
- Aalifar, M.; Aliniaeifard, S.; Arab, M.; Zare Mehrjerdi, M.; Dianati Daylami, S.; Serek, M.; Woltering, E.; Li, T. Blue light improves vase life of carnation cut flowers through its effect on the antioxidant defense system. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanzadeh, P.; Aliniaeifard, S.; Esmaeili, M.; Mashal, M.; Azadegan, B.; Seif, M. Dependency of growth, water use efficiency, chlorophyll fluorescence, and stomatal characteristics of lettuce plants to light intensity. J. Plant Growth Regul. 2020. [Google Scholar] [CrossRef]
- Seif, M.; Aliniaeifard, S.; Arab, M.; Mehrjerdi, M.Z.; Shomali, A.; Fanourakis, D.; Li, T.; Woltering, E. Monochromatic red light during plant growth decreases the size and improves the functionality of stomata in chrysanthemum. Funct. Plant Biol. 2021, 48, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil: University of California. Circ. Calif. Agric. Exp. Stn. 1938, 347, 32. [Google Scholar]
- Miyagawa, S.; Itoh, H.; Uno, Y.; Kuroki, S.; Miyaji, D.; Kitaak, H.; Ueda, Y.; Kobayashi, M.; Kaji, K. Effect of plant shoot number and air temperature on saffron (Crocus sativus L.) corm production and total crocin content. Shokubutsu Kankyo Kogaku 2015, 27, 204–212. [Google Scholar] [CrossRef] [Green Version]
- Kajikawa, N.; Uno, Y.; Kuroki, S.; Miyagawa, S.; Yamashita, Y.; Hamaguchi, Y.; Ueda, Y.; Kobayashi, M.; Kaji, K.; Itoh, H. Effect of far-red light on saffron (crocus sativus L.) growth and crocin yields. Environ. Control Biol. 2018, 56, 51–57. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Díaz-Vivancos, P.; Clemente-Moreno, M.J.; Rubio, M.; Olmos, E.; García, J.A.; Martínez-Gómez, P.; Hernández, J.A. Alteration in the chloroplastic metabolism leads to ROS accumulation in pea plants in response to plum pox virus. J. Exp. Bot. 2008, 59, 2147–2160. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Van Doorn, W.G. Water relations of cut flowers: An update. Hortic. Rev. 2012, 40, 55–106. [Google Scholar] [CrossRef]
- McCready, R.M.; Guggolz, J.; Silviera, V.; Owens, H.S. Determination of starch and amylose in vegetables. Anal. Chem. 1950, 22, 1156–1158. [Google Scholar] [CrossRef]
- Naznin, M.T.; Lefsrud, M.; Gravel, V.; Azad, M.O.K. Blue light added with red LEDs enhance growth characteristics, pigments content, and antioxidant capacity in lettuce, spinach, kale, basil, and sweet pepper in a controlled environment. Plants 2019, 8, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Liu, H.; Klejnot, J.; Lin, C. The cryptochrome blue light receptors. Arab. Book 2010, 8, e0135. [Google Scholar] [CrossRef] [Green Version]
- Tarakanov, I.; Yakovleva, O.; Konovalova, I.; Paliutina, G.; Anisimov, A. Light-emitting diodes: On the way to combinatorial lighting technologies for basic research and crop production. Acta Hortic. 2012, 956, 171–178. [Google Scholar] [CrossRef]
- Dierck, R.; Dhooghe, E.; Van Huylenbroeck, J.; Van Der Straeten, D.; De Keyser, E. Light quality regulates plant architecture in different genotypes of Chrysanthemum morifolium Ramat. Sci. Hortic. 2017, 218, 177–186. [Google Scholar] [CrossRef]
- Ouzounis, T.; Fretté, X.; Rosenqvist, E.; Ottosen, C.O. Spectral effects of supplementary lighting on the secondary metabolites in roses, chrysanthemums, and campanulas. J. Plant Physiol. 2014, 171, 1491–1499. [Google Scholar] [CrossRef]
- Yang, F.O.; Mao, J.F.; Wang, J.; Zhang, S.; Li, Y. Transcriptome analysis reveals that red and blue light regulate growth and phytohormone metabolism in Norway spruce [Picea abies (L.) Karst.]. PLoS ONE 2015, 10, e0127896. [Google Scholar] [CrossRef] [Green Version]
- Hernández, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Aksenova, N.P.; Konstantinova, T.N.; Sergeeva, L.I.; Macháčková, I.; Golyanovskaya, S.A. Morphogenesis of potato plants in vitro. I. Effect of light quality and hormones. J. Plant Growth Regul. 1994, 13, 143–146. [Google Scholar] [CrossRef]
- Negbi, M.; Dagan, B.; Dror, A.; Basker, D. Growth, flowering, vegetative reproduction, and dormancy in the saffron crocus (Crocus sativus L.). Isr. J. Bot. 1989, 38, 95–113. [Google Scholar] [CrossRef]
- de Juan, J.A.; Moya, A.; Lopez, S.; Botella, O.; Lopez, H.; Munoz, R. Influence of the corm size and the density of plantation in the yield and the quality of the production of corms of Crocus sativus L. ITEA Prod. Veg. 2003, 99, 169–180. [Google Scholar]
- Lian, M.L.; Murthy, H.N.; Paek, K.Y. Effects of light emitting diodes (LEDs) on the In Vitro induction and growth of bulblets of Lilium oriental hybrid “Pesaro. Sci. Hortic. 2002, 94, 365–370. [Google Scholar] [CrossRef]
- Goins, G.D.; Yorio, N.C.; Sanwo, M.M.; Brown, C.S. Photomorphogenesis, photosynthesis, and seed yield of wheat plants grown under red light-emitting diodes (LEDs) with and without supplemental blue lighting. J. Exp. Bot. 1997, 48, 1407–1413. [Google Scholar] [CrossRef] [Green Version]
- Samuoliene, G.; Brazaityte, A.; Duchovskis, P.; Viršile, A.; Jankauskiene, J.; Sirtautas, R.; Novičkovas, A.; Sakalauskiene, S.; Sakalauskaite, J. Cultivation of vegetable transplants using solid-state lamps for the short-wavelength supplementary lighting in greenhouses. Acta Hortic. 2012, 952, 885–892. [Google Scholar] [CrossRef]
- Sæbø, A.; Krekling, T.; Appelgren, M. Light quality affects photosynthesis and leaf anatomy of birch plantlets in vitro. Plant Cell Tissue Organ Cult. 1995, 41, 177–185. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.K.; Gantait, S.; Jeong, B.R.; Hwang, S.J. Enhanced growth and cardenolides production in Digitalis purpurea under the influence of different LED exposures in the plant factory. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estaji, A.; Kalaji, H.M.; Karimi, H.R.; Roosta, H.R.; Moosavi-Nezhad, S.M. How glycine betaine induces tolerance of cucumber plants to salinity stress? Photosynthetica 2019, 57, 753–761. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.E.; Mao, H.T.; Wu, N.; Din, A.M.U.; Khan, A.; Zhang, H.Y.; Yuan, S. Salicylic acid protects photosystem ii by alleviating photoinhibition in arabidopsis thaliana under high light. Int. J. Mol. Sci. 2020, 21, 1229. [Google Scholar] [CrossRef] [Green Version]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Miao, Y.; Chen, Q.; Qu, M.; Gao, L.; Hou, L. Blue light alleviates ‘red light syndrome’ by regulating chloroplast ultrastructure, photosynthetic traits and nutrient accumulation in cucumber plants. Sci. Hortic. 2019, 257. [Google Scholar] [CrossRef]
- Aliniaeifard, S.; Seif, M.; Arab, M.; Zare Mehrjerdi, M.; Li, T.; Lastochkina, O. Growth and photosynthetic performance of Calendula officinalis under monochromatic red light. Int. J. Hortic. Sci. Technol. 2018, 5, 123–132. [Google Scholar] [CrossRef]
- Hosseini, A.; Zare Mehrjerdi, M.; Aliniaeifard, S.; Seif, M. Photosynthetic and growth responses of green and purple basil plants under different spectral compositions. Physiol. Mol. Biol. Plants 2019, 25, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Falqueto, A.R.; da Silva Júnior, R.A.; Gomes, M.T.G.; Martins, J.P.R.; Silva, D.M.; Partelli, F.L. Effects of drought stress on chlorophyll a fluorescence in two rubber tree clones. Sci. Hortic. 2017, 224, 238–243. [Google Scholar] [CrossRef]
- Lu, C.; Vonshak, A. Photoinhibition in outdoor Spirulina platensis cultures assessed by polyphasic chlorophyll fluorescence transients. J. Appl. Phycol. 1999, 11, 355–359. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanisms, Regulation and Adaptation; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Parvanova, D.; Popova, A.; Zaharieva, I.; Lambrev, P.; Konstantinova, T.; Taneva, S.; Atanassov, A.; Goltsev, V.; Djilianov, D. Low temperature tolerance of tobacco plants transformed to accumulate proline, fructans, or glycine betaine. Variable chlorophyll fluorescence evidence. Photosynthetica 2004, 42, 179–185. [Google Scholar] [CrossRef]
- Bayat, L.; Arab, M.; Aliniaeifard, S.; Seif, M.; Lastochkina, O.; Li, T. Effects of growth under different light spectra on the subsequent high light tolerance in rose plants. AoB Plants 2018. [Google Scholar] [CrossRef]
- Martinazzo, E.G.; Ramm, A.; Bacarin, M.A. The chlorophyll a fluorescence as an indicator of the temperature stress in the leaves of Prunus persica. Braz. J. Plant Physiol. 2012, 24, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Mathur, S.; Mehta, P.; Jajoo, A. Effects of dual stress (high salt and high temperature) on the photochemical efficiency of wheat leaves (Triticum aestivum). Physiol. Mol. Biol. Plants 2013, 19, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Bukhov, N.G.; Drozdova, I.S.; Bondar, V.V.; Mokronosov, A.T. Blue, red and blue plus red light control of chlorophyll content and CO2 gas exchange in barley leaves: Quantitative description of the effects of light quality and fluence rate. Physiol. Plant. 1992, 85, 632–638. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Zhong, S. Interplay between light and plant hormones in the control of arabidopsis seedling chlorophyll biosynthesis. Front. Plant Sci. 2017, 8, 1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizuno, T.; Amaki, W.; Watanabe, H. Effects of monochromatic light irradiation by LED on the growth and anthocyanin contents in leaves of cabbage seedlings. Acta Hortic. 2011, 907, 179–184. [Google Scholar] [CrossRef]
- Li, H.; Xu, Z.; Liu, X.; Han, X. Effects of different light sources on the growth of non-heading Chinese cabbage (Brassica campestris L.). J. Agric. Sci. 2012, 4, 262. [Google Scholar] [CrossRef] [Green Version]
- Snowden, M.C.; Cope, K.R.; Bugbee, B. Sensitivity of seven diverse species to blue and green light: Interactions with photon flux. PLoS ONE 2016, 11, e0163121. [Google Scholar] [CrossRef]
- Aalifar, M.; Aliniaeifard, S.; Arab, M.; Mehrjerdi, M.Z.; Serek, M. Blue light postpones senescence of carnation flowers through regulation of ethylene and abscisic acid pathway-related genes. Plant Physiol. Biochem. 2020, 151, 103–112. [Google Scholar] [CrossRef]
- Yu, W.; Liu, Y.; Song, L.; Jacobs, D.F.; Du, X.; Ying, Y.; Shao, Q.; Wu, J. Effect of differential light quality on morphology, photosynthesis, and antioxidant enzyme activity in Camptotheca acuminata seedlings. J. Plant Growth Regul. 2017, 36, 148–160. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Kim, K.M.; Kook, H.S.; Jang, Y.J.; Lee, W.H.; Kamala-Kannan, S.; Chae, J.C.; Lee, K.J. The Effect of blue-light-emitting diodes on antioxidant properties and resistance to Botrytis cinerea in tomato. J. Plant Pathol. Microbiol. 2013, 4, 203. [Google Scholar] [CrossRef] [Green Version]
- Bartoli, C.G.; Yu, J.; Gómez, F.; Fernández, L.; McIntosh, L.; Foyer, C.H. Inter-relationships between light and respiration in the control of ascorbic acid synthesis and accumulation in Arabidopsis thaliana leaves. J. Exp. Bot. 2006, 57, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Zhang, L.; Kato, M.; Yamawaki, K.; Kiriiwa, Y.; Yahata, M.; Ikoma, Y.; Matsumoto, H. Effect of blue and red LED light irradiation on β-cryptoxanthin accumulation in the flavedo of citrus fruits. J. Agric. Food Chem. 2012, 60, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, G.; Yamawaki, K.; Ikoma, Y.; Matsumoto, H.; Yoshioka, T.; Ohta, S.; Kato, M. Regulation of ascorbic acid metabolism by blue LED light irradiation in citrus juice sacs. Plant Sci. 2015, 233, 134–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojciechowska, R.; Dugosz-Grochowska, O.; Koton, A.; Zupnik, M. Effects of LED supplemental lighting on yield and some quality parameters of lamb’s lettuce grown in two winter cycles. Sci. Hortic. 2015, 187, 80–86. [Google Scholar] [CrossRef]
- Simlat, M.; Ślęzak, P.; Moś, M.; Warchoł, M.; Skrzypek, E.; Ptak, A. The effect of light quality on seed germination, seedling growth and selected biochemical properties of Stevia rebaudiana Bertoni. Sci. Hortic. 2016, 211, 295–304. [Google Scholar] [CrossRef]


| Basic Parameters | ||
| F0 | Minimum fluorescence, when all PSII reaction centers (RCs) are open (O-step of OJIP transient) | F50µs |
| FJ | Fluorescence intensity at the J-step (2 ms) of OJIP | F2ms |
| FI | Fluorescence intensity at the I-step (30 ms) of OJIP | F30ms |
| Fluorescence Parameters | ||
| Fm | Maximum fluorescence, when all PSII RCs are closed (P-step of OJIP transient) | F1s |
| Fv | Variable fluorescence of the dark-adapted leaf | Fm − F0 |
| Calculated Parameters | ||
| VJ | Relative variable fluorescence at time 2 ms (J-step) after the start of the actinic light pulse | (FJ − F0)/(Fm − F0) |
| VI | Relative variable fluorescence at time 30 ms (I-step) after the start of the actinic light pulse | (F30ms − F0)/(Fm − F0) |
| Fv/Fm | The maximal quantum yield of PSII photochemistry | 1 − (F0/Fm) = (Fm − F0)/Fm= φP0 |
| Quantum Yields and Efficiencies/Probabilities | ||
| φE0 | The quantum yield of electron transport | [1-(F0/Fm)](1 − VJ) |
| φD0 | Quantum yield of energy dissipation | F0/Fm |
| φPAV | Average (from time 0 to tFM) quantum yield for primary photochemistry | φP0 (1 − VJ) = φP0 (SM/tFM) |
| TR0/ABS | Specific energy fluxes (per QA reducing PSII RC) | |
| ABS/RC | The specific energy fluxes per RC for energy absorption | M0 (1/VJ)(1/φP0) |
| TR0/RC | Trapped energy flux (leading to QA reduction) per RC | M0 (1/VJ) |
| ET0/RC | Electron transport flux (further than QA−) per RC | M0 (1/VJ)(1 − VJ) |
| DI0/RC | Dissipated energy flux | (ABS/RC) − (TR0 /RC) |
| Performance Indexes (Products of Terms Expressing Partial Potentials at Steps of Energy Bifurcations) | ||
| PIABS | Performance index for the photochemical activity | [(γRC/1 − γRC)(φP0 /1 − φP0)(ψE0/1 − ψE0)] |
| Treatment | No. of Flower Per Corm | Flower FW (mg) | Flower DW (mg) | Stigma FW (mg) | Stigma DW (mg) |
|---|---|---|---|---|---|
| 100% B | 2.3 a | 622.2 a | 112.7 a | 54.03 a | 16.57 a |
| 75% B | 0.98 b | 408.3 bc | 48.43 bc | 44.03 b | 9.25 b |
| 50% B | 1.3 ab | 494.4 b | 75.44 b | 52.03 ab | 13.33 ab |
| 40% B | 0.66 c | 277.9 c | 45.03 bc | 35.86 c | 5.83 c |
| 25% B | 0.77 c | 275 c | 42.43 bc | 24.54 c | 4.03 c |
| 0% B | 0.66 c | 222 c | 32.03 c | 26.03 c | 3.83 c |
| p | <0.0001 | <0.0001 | 0.0027 | <0.0001 | <0.0001 |
| CV % | 20 | 18.6 | 15 | 11.4 | 15.5 |
| Treatment | No. of Leaves | Leaf FW (mg) | Leaf DW (mg) | Leaf Length (cm) | Root FW (mg) | Root DW (mg) | Root Length (cm) |
|---|---|---|---|---|---|---|---|
| 100% B | 15.27 d | 4.33 c | 0.73 b | 46.4 d | 4.93 a | 0.44 a | 3.47 a |
| 75% B | 15.53 d | 4 c | 0.67 b | 45 d | 3.93 b | 0.22 c | 3.03 ab |
| 50% B | 16.6 c | 5.16 b | 0.5 c | 50 c | 5.03 a | 0.49 a | 3.33 a |
| 40% B | 17.3 b | 6.5 a | 1.66 a | 53.03 b | 3.5 bc | 0.27 bc | 2.03 b |
| 25% B | 20.77 a | 5.5 ab | 0.61 b | 50.9 c | 3.98 b | 0.36 b | 2.9 b |
| 0% B | 18.66 ab | 6.33 a | 1.03 a | 59.77 a | 1.83 c | 0.14 c | 1.77 b |
| p | <0.0001 | <0.0001 | 0.0003 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| CV % | 20.17 | 17.56 | 25 | 16.4 | 18.43 | 25 | 15.5 |
| Treatment | No. of Daughter Corm Per Mother Corm | Biggest Daughter Corm (g) | Daughter Corm Diameter | Daughter Corm FW (g) | Daughter Corm DW (g) |
|---|---|---|---|---|---|
| 100% B | 2.06 c | 6.47 a | 23.39 a | 10.5 a | 5.57 a |
| 75% B | 3 b | 3.03 b | 19.43 b | 6 b | 2.67 c |
| 50% B | 2.88 ab | 5.33 a | 22.38 a | 9.95 a | 4.25 b |
| 40% B | 3 b | 3.07 b | 19 b | 6.03 b | 2.46 c |
| 25% B | 3.3 b | 2.9 bc | 17.86 bc | 5.06 b | 2.16 c |
| 0% B | 4 a | 1.97 c | 15.66 c | 4.03 c | 0.83 d |
| p | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| CV % | 11.3 | 20 | 6.2 | 16.7 | 15.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moradi, S.; Kafi, M.; Aliniaeifard, S.; Salami, S.A.; Shokrpour, M.; Pedersen, C.; Moosavi-Nezhad, M.; Wróbel, J.; Kalaji, H.M. Blue Light Improves Photosynthetic Performance and Biomass Partitioning toward Harvestable Organs in Saffron (Crocus sativus L.). Cells 2021, 10, 1994. https://doi.org/10.3390/cells10081994
Moradi S, Kafi M, Aliniaeifard S, Salami SA, Shokrpour M, Pedersen C, Moosavi-Nezhad M, Wróbel J, Kalaji HM. Blue Light Improves Photosynthetic Performance and Biomass Partitioning toward Harvestable Organs in Saffron (Crocus sativus L.). Cells. 2021; 10(8):1994. https://doi.org/10.3390/cells10081994
Chicago/Turabian StyleMoradi, Shirin, Mohsen Kafi, Sasan Aliniaeifard, Seyed Alireza Salami, Majid Shokrpour, Carsten Pedersen, Moein Moosavi-Nezhad, Jacek Wróbel, and Hazem M. Kalaji. 2021. "Blue Light Improves Photosynthetic Performance and Biomass Partitioning toward Harvestable Organs in Saffron (Crocus sativus L.)" Cells 10, no. 8: 1994. https://doi.org/10.3390/cells10081994
APA StyleMoradi, S., Kafi, M., Aliniaeifard, S., Salami, S. A., Shokrpour, M., Pedersen, C., Moosavi-Nezhad, M., Wróbel, J., & Kalaji, H. M. (2021). Blue Light Improves Photosynthetic Performance and Biomass Partitioning toward Harvestable Organs in Saffron (Crocus sativus L.). Cells, 10(8), 1994. https://doi.org/10.3390/cells10081994









