In Vitro Examinations of Cell Death Induction and the Immune Phenotype of Cancer Cells Following Radiative-Based Hyperthermia with 915 MHz in Combination with Radiotherapy
Abstract
:1. Introduction
2. Materials and Methods
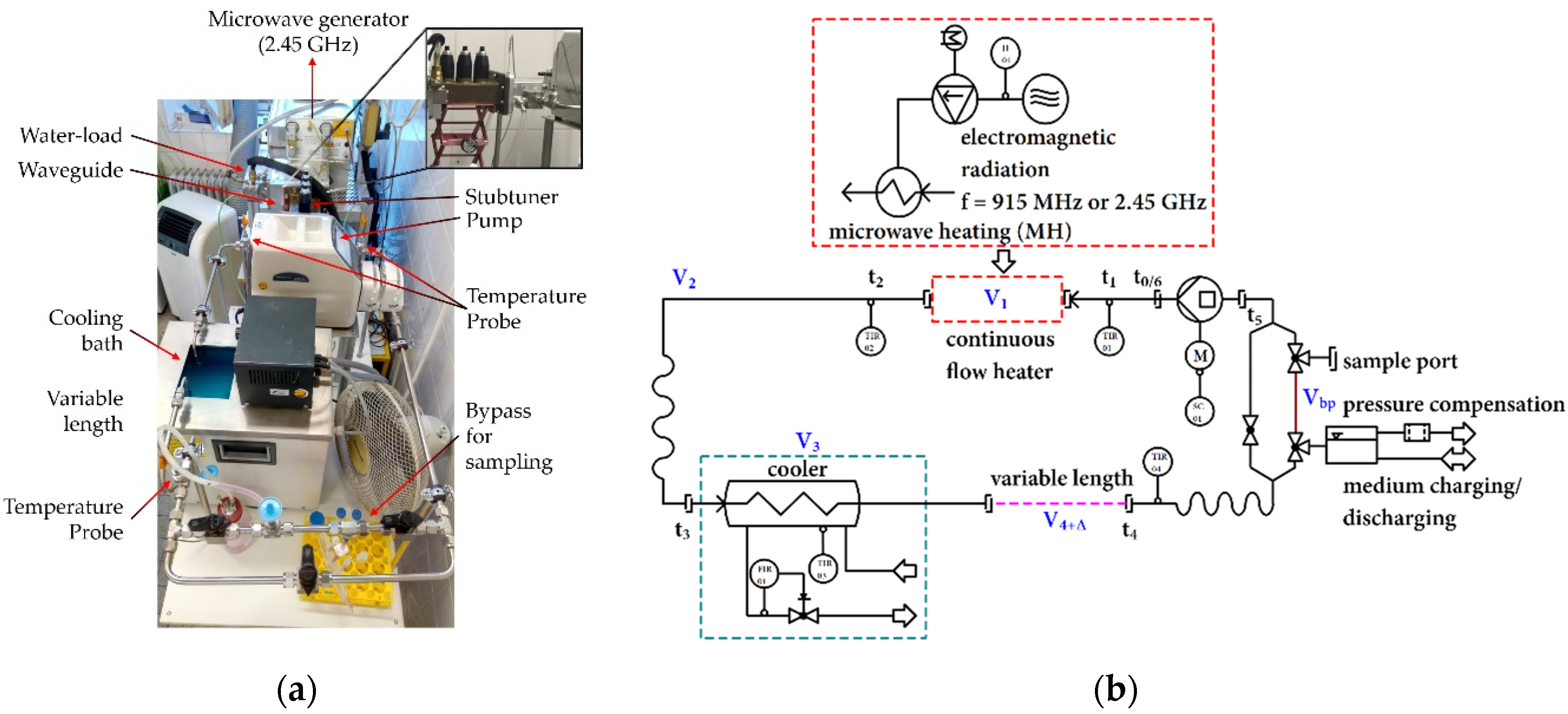
2.1. Closed-Loop System
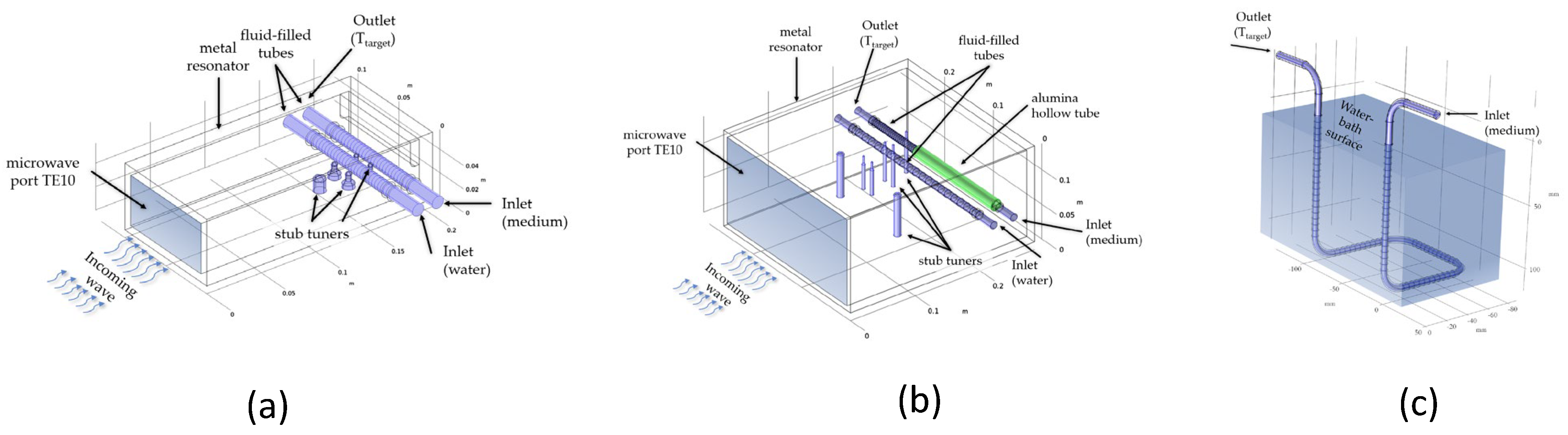
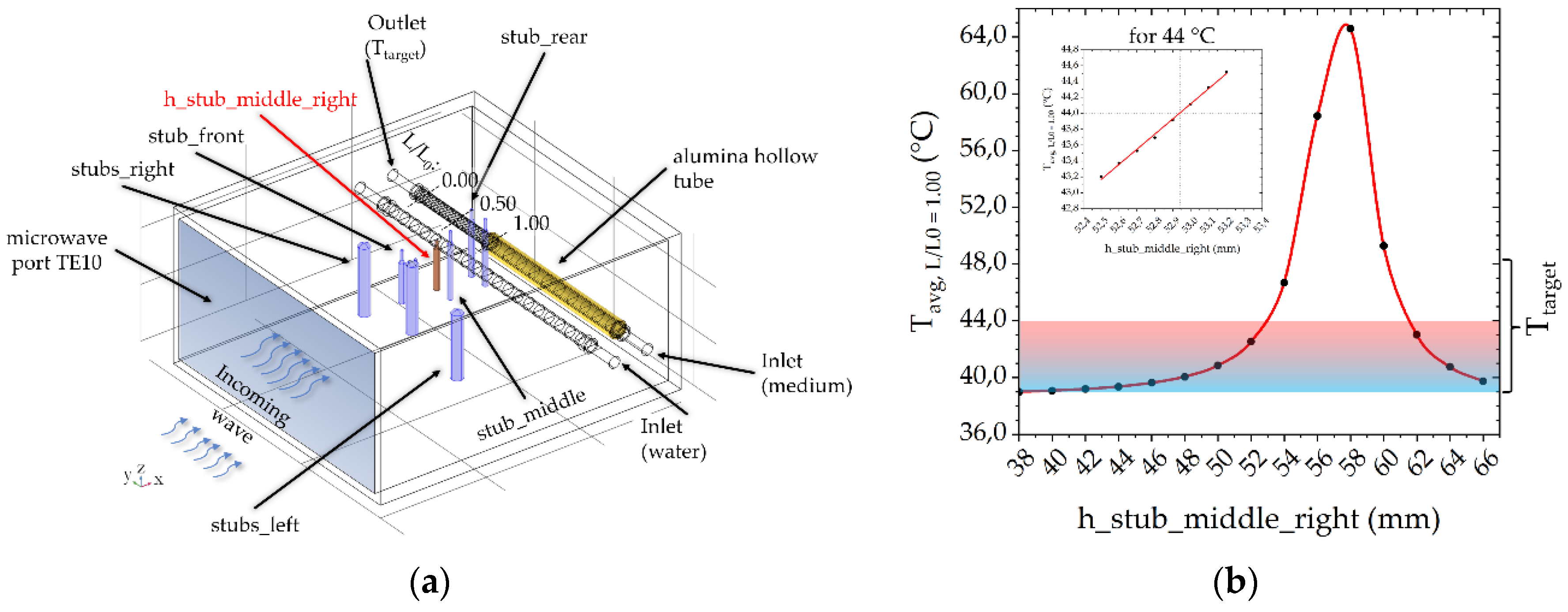
2.2. Numerical Simulations of the Heating Units
2.3. Cultivation and Inactivation of Tumor Cells
2.4. Flow Cytometry Analysis for the Detection of Cell Death Forms and Immune Checkpoint Molecule Expression
2.5. Single Cell and Molecular Dynamics Simulation of DNA-Components
3. Results
3.1. Self-Developed 915 MHz Hyperthermia Heating System Allows Reproducible Treatment without Excessive Hot-Spots
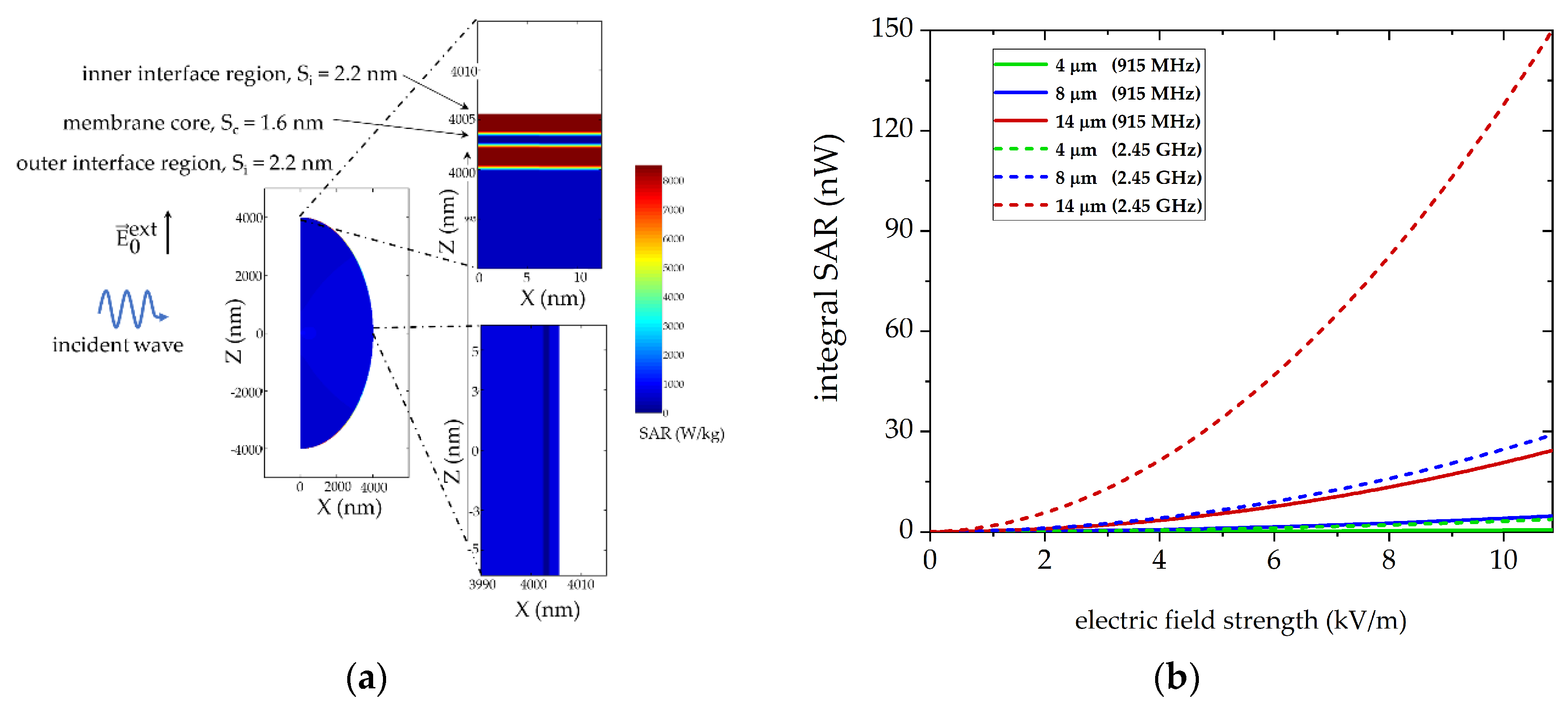
3.2. Numerical Simulations to Demonstrate Comparable Heating Conditions in Both Microwave Cavities
3.3. Cell Death Induction by Radiative-Based Hyperthmia and Combinatory Treatment with Radiotherapy in B16-Melanoma and MCF-7 and MDA-MB-231 Breast Cancer Cells
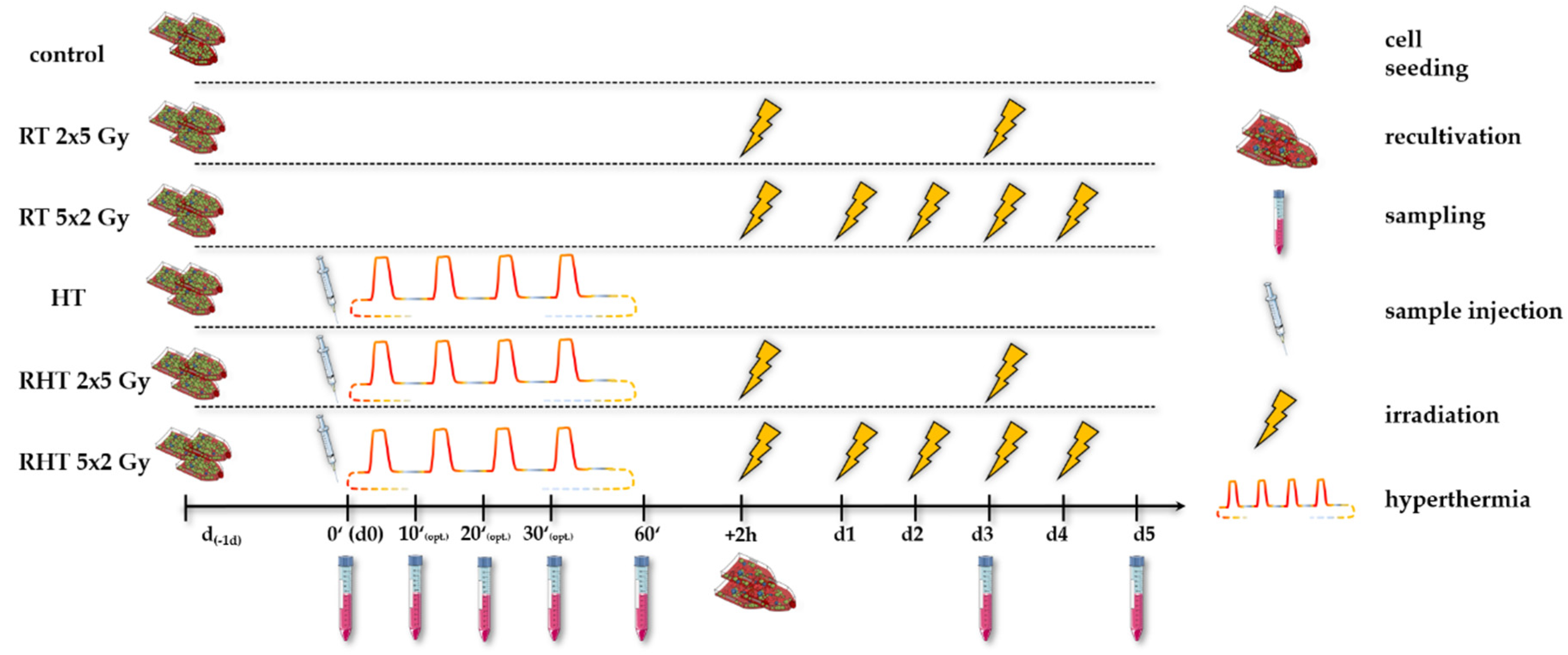
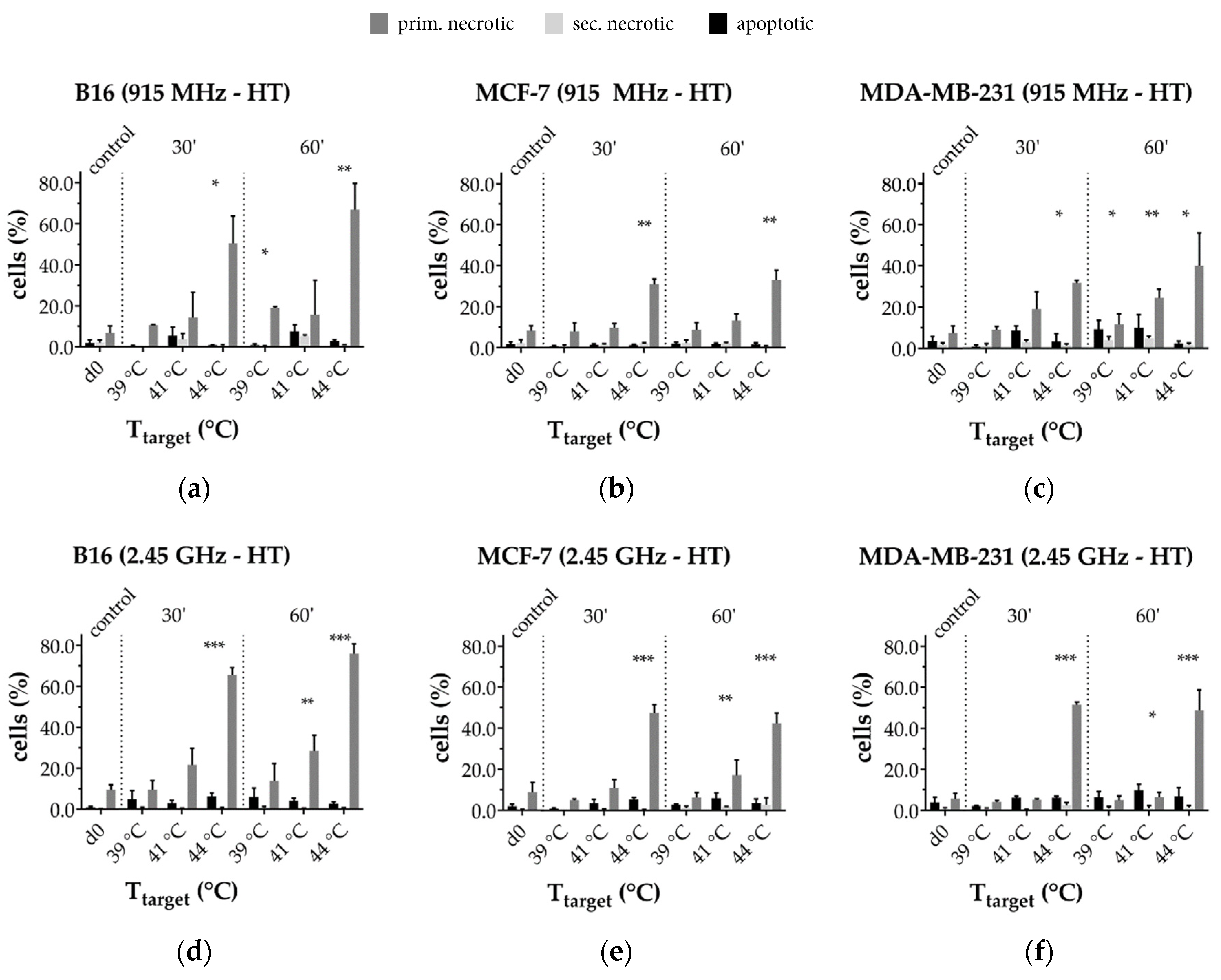
3.3.1. Radiative-Based Hyperthermia at Both 915 MHz and 2.45 GHz Significantly Inactivates Tumor Cells at 44 °C
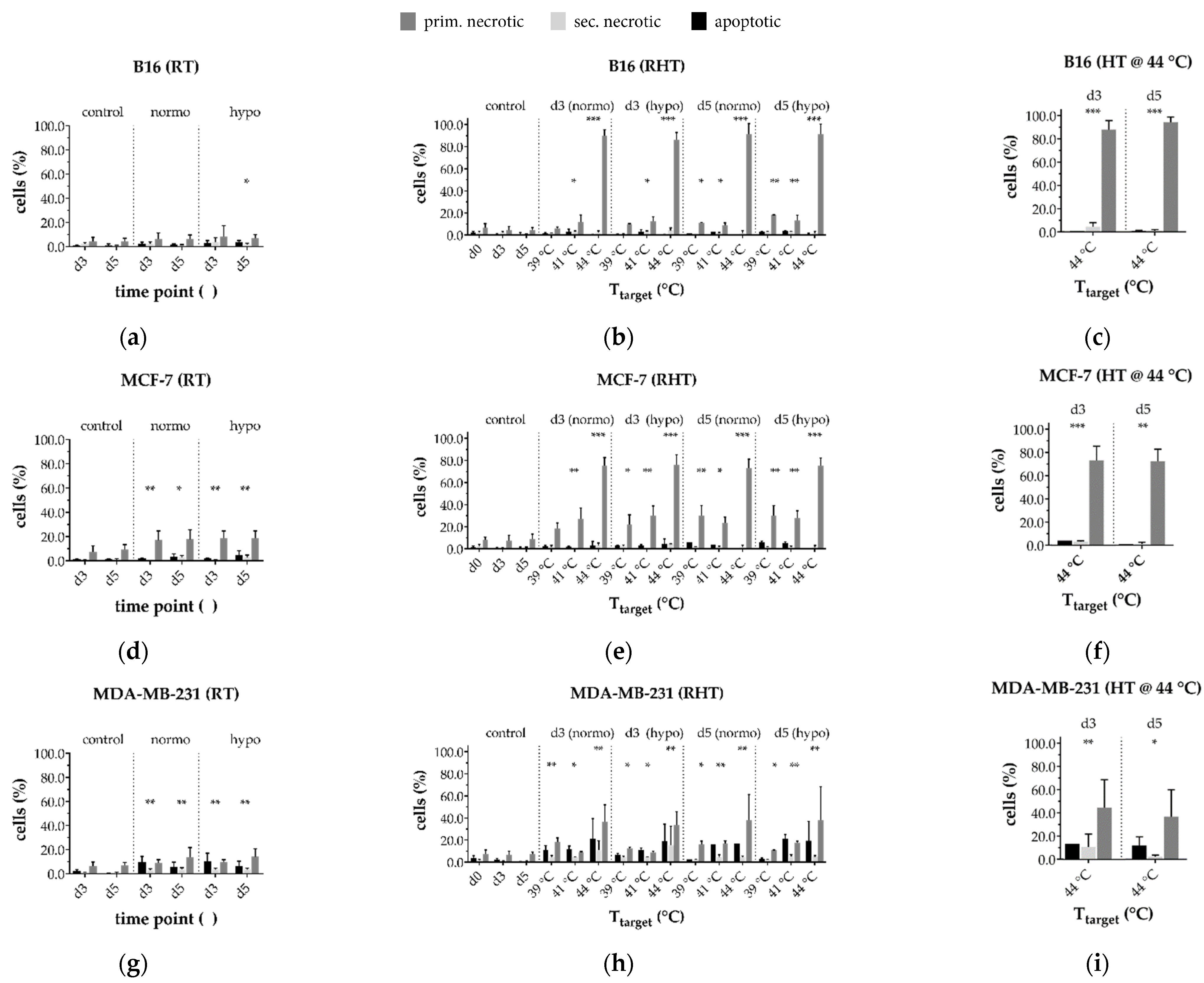
3.3.2. In a Long-Term Follow-Up, at Temperatures of 39 °C and 41 °C Induced by 915 MHz Hyperthermia, Radiotherapy Is the Main Cell Death Inducer, While at 44 °C, It Is the Heat Application
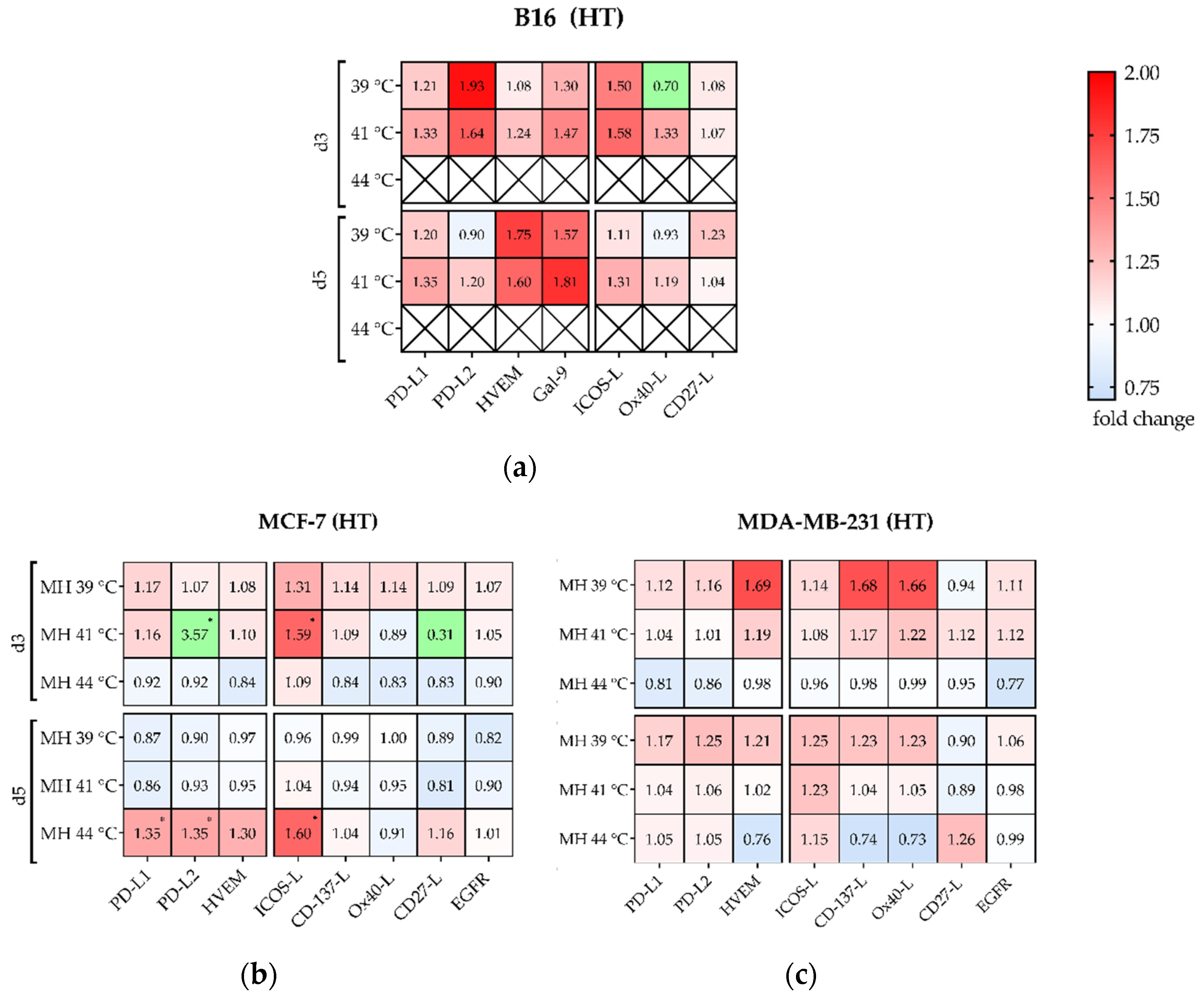
3.4. Hyperthermia with 915 MHz Barely Impacts on the Immune Checkpoint Molecule Expression
Significant Increase of Several Immune Checkpoint Molecules by Multimodal Treatment Using 915 MHz Hyperthermia in Combination with Radiotherapy
3.5. Local Absorption in Cell Membrane Components Show Frequency Dependent Correlations
4. Discussion
4.1. In Silico Simulations on Cells and Biomacromolecules Are Helpful to Describe Effects of Non-Ionizing Electromagnetic Radiation
4.2. A Mixed Temperature Profile during Hyperthermia in Combination with Radiotherapy Could Foster Anti-Tumor Immunreactions and Effectiveness of Immune Checkpoint Molecule Drugs
4.3. Preclinical Experiments Open New Therapeutic Fields and Show a Highly Dynamic Expression of Immune Checkpoint Molecules
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stewart, B.W. (Ed.) World Cancer Report 2014; IARC Press: Lyon, France, 2014; ISBN 9789283204299. [Google Scholar]
- OECD. Health at a Glance 2019. OECD Indicators, 2019th ed.; OECD Publishing: Paris, France, 2019; ISBN 9789264807662. [Google Scholar]
- Datta, N.R.; Ordóñez, S.G.; Gaipl, U.S.; Paulides, M.M.; Crezee, H.; Gellermann, J.; Marder, D.; Puric, E.; Bodis, S. Local hyperthermia combined with radiotherapy and-/or chemotherapy: Recent advances and promises for the future. Cancer Treat. Rev. 2015, 41, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Hader, M.; Frey, B.; Fietkau, R.; Hecht, M.; Gaipl, U.S. Immune biological rationales for the design of combined radio- and immunotherapies. Cancer Immunol. Immunother. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Issels, R.D.; Lindner, L.H.; Verweij, J.; Wessalowski, R.; Reichardt, P.; Wust, P.; Ghadjar, P.; Hohenberger, P.; Angele, M.; Salat, C.; et al. Effect of Neoadjuvant Chemotherapy Plus Regional Hyperthermia on Long-term Outcomes Among Patients with Localized High-Risk Soft Tissue Sarcoma: The EORTC 62961-ESHO 95 Randomized Clinical Trial. JAMA Oncol. 2018, 4, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Mulder, H.T.; Curto, S.; Paulides, M.M.; Franckena, M.; van Rhoon, G.C. Systematic quality assurance of the BSD2000-3D MR-compatible hyperthermia applicator performance using MR temperature imaging. Int. J. Hyperth. 2018, 35, 305–313. [Google Scholar] [CrossRef] [Green Version]
- Maluta, S.; Kolff, M.W. Role of Hyperthermia in Breast Cancer Locoregional Recurrence: A Review. Breast Care 2015, 10, 408–412. [Google Scholar] [CrossRef] [Green Version]
- Nardecchia, S.; Sánchez-Moreno, P.; de Vicente, J.; Marchal, J.A.; Boulaiz, H. Clinical Trials of Thermosensitive Nanomaterials: An Overview. Nanomaterials 2019, 9, 191. [Google Scholar] [CrossRef] [Green Version]
- Trefná, H.D.; Crezee, H.; Schmidt, M.; Marder, D.; Lamprecht, U.; Ehmann, M.; Hartmann, J.; Nadobny, J.; Gellermann, J.; van Holthe, N.; et al. Quality assurance guidelines for superficial hyperthermia clinical trials: I. Clinical requirements. Int. J. Hyperth. 2017, 33, 471–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulides, M.M.; Dobsicek Trefna, H.; Curto, S.; Rodrigues, D.B. Recent technological advancements in radiofrequency- andmicrowave-mediated hyperthermia for enhancing drug delivery. Adv. Drug Deliv. Rev. 2020. [Google Scholar] [CrossRef]
- Kok, H.P.; Kotte, A.N.T.J.; Crezee, J. Planning, optimisation and evaluation of hyperthermia treatments. Int. J. Hyperth. 2017, 33, 593–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavazzi, S.; van Lier, A.L.H.M.W.; Zachiu, C.; Jansen, E.; Lagendijk, J.J.W.; Stalpers, L.J.A.; Crezee, H.; Kok, H.P. Advanced patient-specific hyperthermia treatment planning. Int. J. Hyperth. 2020, 37, 992–1007. [Google Scholar] [CrossRef]
- Rosin, A.; Hader, M.; Drescher, C.; Suntinger, M.; Gerdes, T.; Willert-Porada, M.; Gaipl, U.S.; Frey, B. Comparative study and simulation of tumor cell inactivation by microwave and conventional heating. Compel 2018, 37, 1893–1904. [Google Scholar] [CrossRef]
- Hader, M.; Savcigil, D.P.; Rosin, A.; Ponfick, P.; Gekle, S.; Wadepohl, M.; Bekeschus, S.; Fietkau, R.; Frey, B.; Schlücker, E.; et al. Differences of the Immune Phenotype of Breast Cancer Cells after Ex Vivo Hyperthermia by Warm-Water or Microwave Radiation in a Closed-Loop System Alone or in Combination with Radiotherapy. Cancers 2020, 12, 1082. [Google Scholar] [CrossRef] [PubMed]
- Komarov, V.V.; Tang, J. Dielectric permittivity and loss factor of tap water at 915 MHz. Microw. Opt. Technol. Lett. 2004, 42, 419–420. [Google Scholar] [CrossRef]
- Von Hippel, A.R. Dielectric Materials and Applications, 2nd ed.; Artech House: Boston, MA, USA, 1995; ISBN 1580531237. [Google Scholar]
- Jänicke, R.U.; Ng, P.; Sprengart, M.L.; Porter, A.G. Caspase-3 is required for alpha-fodrin cleavage but dispensable for cleavage of other death substrates in apoptosis. J. Biol. Chem. 1998, 273, 15540–15545. [Google Scholar] [CrossRef] [Green Version]
- Brooks, S.C.; Locke, E.R.; Soule, H.D. Estrogen receptor in a human cell line (MCF-7) from breast carcinoma. J. Biol. Chem. 1973, 248, 6251–6253. [Google Scholar] [CrossRef]
- Lacroix, M.; Leclercq, G. Relevance of breast cancer cell lines as models for breast tumours: An update. Breast Cancer Res. Treat. 2004, 83, 249–289. [Google Scholar] [CrossRef]
- Young, R.K.; Cailleau, R.M.; Mackay, B.; Reeves, W.J. Establishment of epithelial cell line MDA-MB-157 from metastatic pleural effusion of human breast carcinoma. In Vitro 1974, 9, 239–245. [Google Scholar] [CrossRef]
- Van Leeuwen, C.M.; Oei, A.L.; Chin, K.W.; Crezee, J.; Bel, A.; Westermann, A.M.; Buist, M.R.; Franken, N.A.P.; Stalpers, L.J.A.; Kok, H.P. A short time interval between radiotherapy and hyperthermia reduces in-field recurrence and mortality in women with advanced cervical cancer. Radiat. Oncol. 2017, 12, 75. [Google Scholar] [CrossRef] [PubMed]
- Gaipl, U.S.; Kuenkele, S.; Voll, R.E.; Beyer, T.D.; Kolowos, W.; Heyder, P.; Kalden, J.R.; Herrmann, M. Complement binding is an early feature of necrotic and a rather late event during apoptotic cell death. Cell Death Differ. 2001, 8, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.M.; Hollville, E.; Martin, S.J. Measuring apoptosis by microscopy and flow cytometry. Methods 2013, 61, 90–97. [Google Scholar] [CrossRef]
- Sukhorukov, V.L.; Meedt, G.; Kürschner, M.; Zimmermann, U. A single-shell model for biological cells extended to account for the dielectric anisotropy of the plasma membrane. J. Electrost. 2001, 50, 191–204. [Google Scholar] [CrossRef]
- Gekle, S.; Netz, R.R. Nanometer-resolved radio-frequency absorption and heating in biomembrane hydration layers. J. Phys. Chem. B 2014, 118, 4963–4969. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Kok, H.P.; Crezee, H.; Gaipl, U.S.; Bodis, S. Integrating Loco-Regional Hyperthermia Into the Current Oncology Practice: SWOT and TOWS Analyses. Front. Oncol. 2020, 10, 819. [Google Scholar] [CrossRef]
- Schildkopf, P.; Frey, B.; Ott, O.J.; Rubner, Y.; Multhoff, G.; Sauer, R.; Fietkau, R.; Gaipl, U.S. Radiation combined with hyperthermia induces HSP70-dependent maturation of dendritic cells and release of pro-inflammatory cytokines by dendritic cells and macrophages. Radiother. Oncol. 2011, 101, 109–115. [Google Scholar] [CrossRef]
- Schildkopf, P.; Holmer, R.; Sieber, R.; Ott, O.J.; Janko, C.; Mantel, F.; Frey, B.; Fietkau, R.; Gaipl, U.S. Hyperthermia in combination with X-irradiation induces inflammatory forms of cell death. Autoimmunity 2009, 42, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef] [PubMed]
- Marjanović, A.M.; Pavičić, I.; Trošić, I. Biological indicators in response to radiofrequency/microwave exposure. Arh. Hig. Rada Toksikol. 2012, 63, 407–416. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.-T.; Chang, W.H.-S.; Chang, K.; Hou, R.-J.; Wu, T.-W. Pulsed electromagnetic fields affect osteoblast proliferation and differentiation in bone tissue engineering. Bioelectromagnetics 2007, 28, 519–528. [Google Scholar] [CrossRef]
- Tanim, M.M.Z. Electromagnetic Radiation and Human Health—Technical Report; Tampere University of Applied Science: Tampere, Finland, 2015. [Google Scholar] [CrossRef]
- Cifra, M.; Fields, J.Z.; Farhadi, A. Electromagnetic cellular interactions. Prog. Biophys. Mol. Biol. 2011, 105, 223–246. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, K.P.; Keller, B.M.; Syed, K.M.; Lepock, J.R. Hyperthermia (heat shock)-induced protein denaturation in liver, muscle and lens tissue as determined by differential scanning calorimetry. Int. J. Hyperth. 1994, 10, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Adang, D.; Remacle, C.; Vorst, A.V. Results of a Long-Term Low-Level Microwave Exposure of Rats. IEEE Trans. Microw. Theory Techn. 2009, 57, 2488–2497. [Google Scholar] [CrossRef]
- De Pomerai, D.I.; Smith, B.; Dawe, A.; North, K.; Smith, T.; Archer, D.B.; Duce, I.R.; Jones, D.; Candido, E.M. Microwave radiation can alter protein conformation without bulk heating. FEBS Lett. 2003, 543, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Agnihotri, M.V.; Singer, S.J. Dielectric spectrum of a DNA oligomer. Phys. Rev. E 2018, 98. [Google Scholar] [CrossRef]
- McCarthy, E.F. The Toxins of William, B. Coley and the Treatment of Bone and Soft-Tissue Sarcomas. Iowa Orthop. J. 2006, 26, 154–158. [Google Scholar] [PubMed]
- Barnes, J.A.; Dix, D.J.; Collins, B.W.; Luft, C.; Allen, J.W. Expression of inducible Hsp70 enhances the proliferation of MCF-7 breast cancer cells and protects against the cytotoxic effects of hyperthermia. Cell Stress Chaperones 2001, 6, 316. [Google Scholar] [CrossRef] [Green Version]
- Schildkopf, P.; Ott, O.J.; Frey, B.; Wadepohl, M.; Sauer, R.; Fietkau, R.; Gaipl, U.S. Biological Rationales and Clinical Applications of Temperature Controlled Hyperthermia—Implications for Multimodal Cancer Treatments. CMC 2010, 17, 3045–3057. [Google Scholar] [CrossRef] [PubMed]
- Combs, S.E. Hyperthermie. In Strahlentherapie; Wannenmacher, M., Wenz, F., Debus, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 225–248. ISBN 9783540883043. [Google Scholar]
- Knippertz, I.; Stein, M.F.; Dörrie, J.; Schaft, N.; Müller, I.; Deinzer, A.; Steinkasserer, A.; Nettelbeck, D.M. Mild hyperthermia enhances human monocyte-derived dendritic cell functions and offers potential for applications in vaccination strategies. Int. J. Hyperth. 2011, 27, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Issels, R.D.; Lindner, L.H.; Verweij, J.; Wust, P.; Reichardt, P.; Schem, B.-C.; Abdel-Rahman, S.; Daugaard, S.; Salat, C.; Wendtner, C.-M.; et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: A randomised phase 3 multicentre study. Lancet Oncol. 2010, 11, 561–570. [Google Scholar] [CrossRef] [Green Version]
- Lagendijk, J.J.; van Rhoon, G.C.; Hornsleth, S.N.; Wust, P.; de Leeuw, A.C.; Schneider, C.J.; van Dijk, J.D.; van der Zee, J.; van Heek-Romanowski, R.; Rahman, S.A.; et al. ESHO quality assurance guidelines for regional hyperthermia. Int. J. Hyperth. 1998, 14, 125–133. [Google Scholar] [CrossRef]
- Donaubauer, A.-J.; Becker, I.; Rühle, P.F.; Fietkau, R.; Gaipl, U.S.; Frey, B. Analysis of the immune status from peripheral whole blood with a single-tube multicolor flow cytometry assay. Meth. Enzymol. 2020, 632, 389–415. [Google Scholar] [CrossRef]
- Frey, B.; Rückert, M.; Deloch, L.; Rühle, P.F.; Derer, A.; Fietkau, R.; Gaipl, U.S. Immunomodulation by ionizing radiation-impact for design of radio-immunotherapies and for treatment of inflammatory diseases. Immunol. Rev. 2017, 280, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Deloch, L.; Derer, A.; Hartmann, J.; Frey, B.; Fietkau, R.; Gaipl, U.S. Modern Radiotherapy Concepts and the Impact of Radiation on Immune Activation. Front. Oncol. 2016, 6, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Lim, Y.J.; Koh, J.; Kim, S.; Jeon, S.-R.; Chie, E.K.; Kim, K.; Kang, G.H.; Han, S.-W.; Kim, T.-Y.; Jeong, S.-Y.; et al. Chemoradiation-Induced Alteration of Programmed Death-Ligand 1 and CD8+ Tumor-Infiltrating Lymphocytes Identified Patients With Poor Prognosis in Rectal Cancer: A Matched Comparison Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 1216–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondini, M.; Levy, A.; Meziani, L.; Milliat, F.; Deutsch, E. Radiotherapy-immunotherapy combinations: Perspectives and challenges. Mol. Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Szlasa, W.; Zendran, I.; Zalesińska, A.; Tarek, M.; Kulbacka, J. Lipid composition of the cancer cell membrane. J. Bioenerg. Biomembr. 2020, 52, 321–342. [Google Scholar] [CrossRef]
- Dewey, W.C.; Hopwood, L.E.; Sapareto, S.A.; Gerweck, L.E. Cellular responses to combinations of hyperthermia and radiation. Radiology 1977, 123, 463–474. [Google Scholar] [CrossRef]
- Dobšíček Trefná, H.; Schmidt, M.; van Rhoon, G.C.; Kok, H.P.; Gordeyev, S.S.; Lamprecht, U.; Marder, D.; Nadobny, J.; Ghadjar, P.; Abdel-Rahman, S.; et al. Quality assurance guidelines for interstitial hyperthermia. Int. J. Hyperth. 2019, 36, 277–294. [Google Scholar] [CrossRef] [Green Version]
- Deckers, R.; Debeissat, C.; Fortin, P.-Y.; Moonen, C.T.W.; Couillaud, F. Arrhenius analysis of the relationship between hyperthermia and Hsp70 promoter activation: A comparison between ex vivo and in vivo data. Int. J. Hyperth. 2012, 28, 441–450. [Google Scholar] [CrossRef]
- Van Rhoon, G.C. Is CEM43 still a relevant thermal dose parameter for hyperthermia treatment monitoring? Int. J. Hyperth. 2016, 32, 50–62. [Google Scholar] [CrossRef]
- Gill, J.; Prasad, V. A reality check of the accelerated approval of immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2019, 16, 656–658. [Google Scholar] [CrossRef]
- Erber, R.; Hartmann, A. Understanding PD-L1 Testing in Breast Cancer: A Practical Approach. Breast Care 2020, 15, 481–490. [Google Scholar] [CrossRef]
- Yuan, C.; Liu, Z.; Yu, Q.; Wang, X.; Bian, M.; Yu, Z.; Yu, J. Expression of PD-1/PD-L1 in primary breast tumours and metastatic axillary lymph nodes and its correlation with clinicopathological parameters. Sci. Rep. 2019, 9, 14356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derer, A.; Spiljar, M.; Bäumler, M.; Hecht, M.; Fietkau, R.; Frey, B.; Gaipl, U.S. Chemoradiation Increases PD-L1 Expression in Certain Melanoma and Glioblastoma Cells. Front. Immunol. 2016, 7, 610. [Google Scholar] [CrossRef] [Green Version]
- Buchwald, Z.S.; Wynne, J.; Nasti, T.H.; Zhu, S.; Mourad, W.F.; Yan, W.; Gupta, S.; Khleif, S.N.; Khan, M.K. Radiation, Immune Checkpoint Blockade and the Abscopal Effect: A Critical Review on Timing, Dose and Fractionation. Front. Oncol. 2018, 8, 612. [Google Scholar] [CrossRef] [PubMed]
- Malissen, N.; Macagno, N.; Granjeaud, S.; Granier, C.; Moutardier, V.; Gaudy-Marqueste, C.; Habel, N.; Mandavit, M.; Guillot, B.; Pasero, C.; et al. HVEM has a broader expression than PD-L1 and constitutes a negative prognostic marker and potential treatment target for melanoma. Oncoimmunology 2019, 8, e1665976. [Google Scholar] [CrossRef]
- D’Incecco, A.; Andreozzi, M.; Ludovini, V.; Rossi, E.; Capodanno, A.; Landi, L.; Tibaldi, C.; Minuti, G.; Salvini, J.; Coppi, E.; et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br. J. Cancer 2015, 112, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Obeid, J.M.; Erdag, G.; Smolkin, M.E.; Deacon, D.H.; Patterson, J.W.; Chen, L.; Bullock, T.N.; Slingluff, C.L. PD-L1, PD-L2 and PD-1 expression in metastatic melanoma: Correlation with tumor-infiltrating immune cells and clinical outcome. Oncoimmunology 2016, 5, e1235107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbone, D.P.; Reck, M.; Paz-Ares, L.; Creelan, B.; Horn, L.; Steins, M.; Felip, E.; van den Heuvel, M.M.; Ciuleanu, T.-E.; Badin, F.; et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 376, 2415–2426. [Google Scholar] [CrossRef]
- Barnes, M.; Bai, I.; Nguyen, K.; Bredno, J.; Vennapusa, B.; Fonstad, R.; Agarwal, S.; Patil, S.; Little, E.; Koeppen, H.; et al. P2.01-043 Pathologist Agreement Rates of PD-L1 Tumor and Immune Cell Quantitation Using Digital Read, Field-Of-View, and Whole Tumor Image Analysis. J. Thorac. Oncol. 2017, 12, S811–S812. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Ren, Y.; Wang, Z. Programmed death 1 Ligand 1 expression in breast cancer and its association with patients’ clinical parameters. J. Cancer Res. Ther. 2018, 14, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Kulzer, L.; Rubner, Y.; Deloch, L.; Allgäuer, A.; Frey, B.; Fietkau, R.; Dörrie, J.; Schaft, N.; Gaipl, U.S. Norm- and hypo-fractionated radiotherapy is capable of activating human dendritic cells. J. Immunotoxicol. 2014, 11, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Finkel, P.; Frey, B.; Mayer, F.; Bösl, K.; Werthmöller, N.; Mackensen, A.; Gaipl, U.S.; Ullrich, E. The dual role of NK cells in antitumor reactions triggered by ionizing radiation in combination with hyperthermia. Oncoimmunology 2016, 5, e1101206. [Google Scholar] [CrossRef] [PubMed]




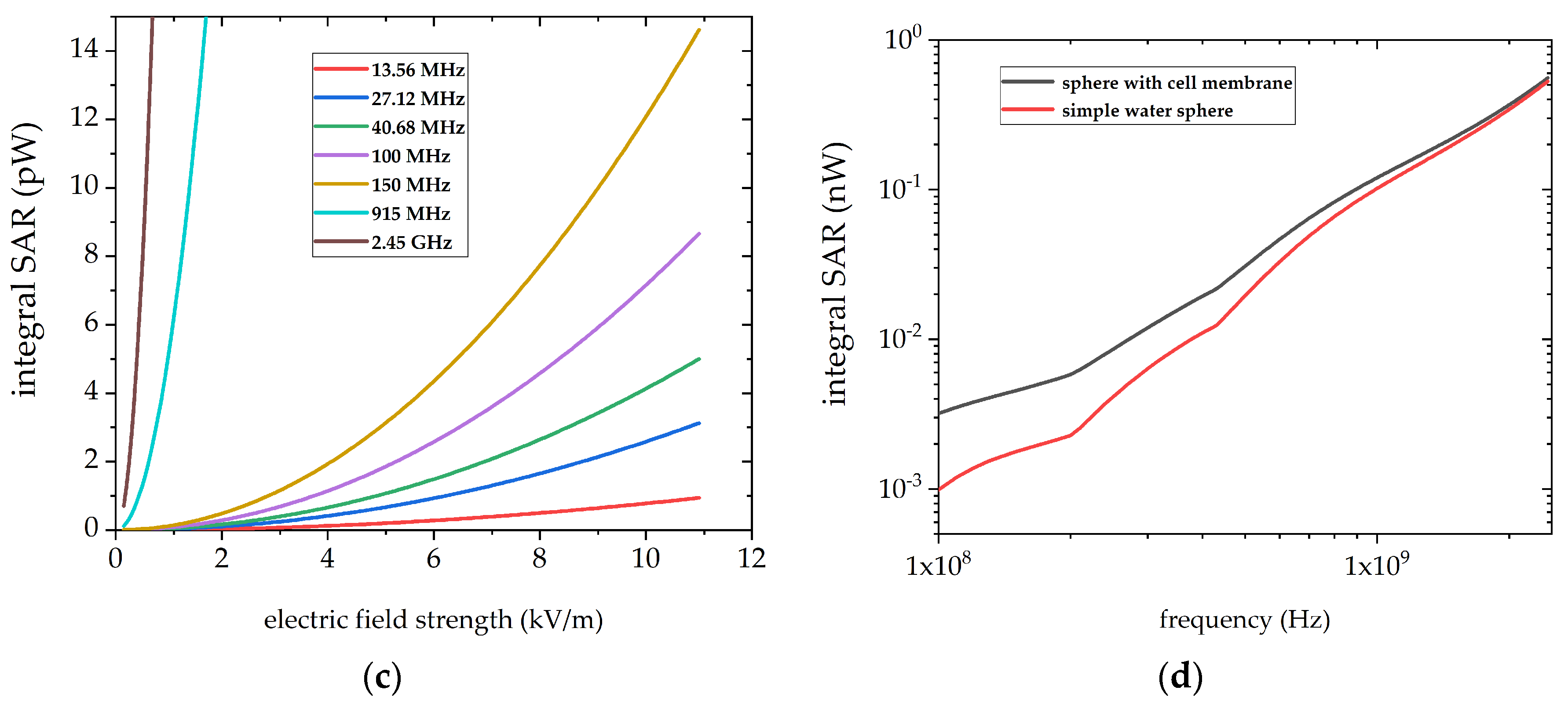
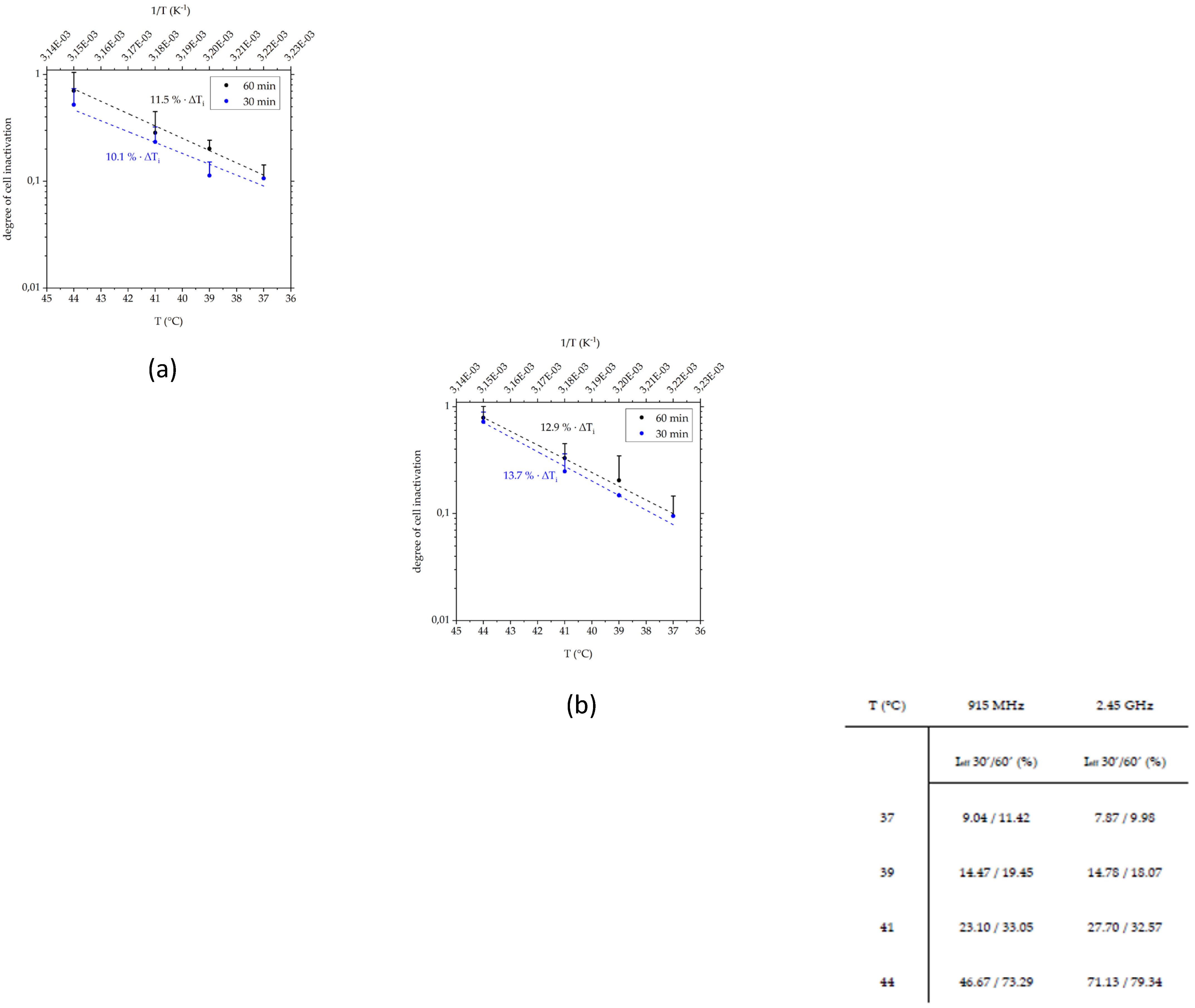
| Parameter | 25 °C | 35 °C | 45 °C | 55 °C |
|---|---|---|---|---|
| 81.26 1/76.70 2 | 75.77 1/74.00 2 | 71.67 1/70.70 2 | 68.40 1/67.50 2 | |
| 3.82 1/12.04 2 | 3.39 1/9.40 2 | 3.09 1/7.50 2 | 2.89 1/6.01 2 |
| Marker | Mastermix #1, Human (µL/Well) | Mastermix #2, Human (µL/Well) | Mastermix #1, Murine (µL/Well) | Mastermix #2, Murine (µL/Well) |
|---|---|---|---|---|
| PD-L1 (CD274) | 0.5 | 0.05 | ||
| PD-L2 (CD273) | 0.5 | 0.1 | ||
| ICOS-L (CD275) | 0.5 | 0.2 | ||
| EGF-Receptor | 0.5 | not detectable | ||
| HVEM (CD270) | 0.5 | 0.05 | ||
| Ox40-L (CD134) | 0.5 | 0.05 | ||
| TNFRSF9 (CD137-L) | 0.5 | not detectable | ||
| CD70 (CD27-L) | 0.5 | 0.2 | ||
| Galectin 9 | 0.05 | |||
| Zombie NIR | 0.1 | 0.1 | 0.1 | |
| Zombie Yellow | 0.1 | |||
| FACS buffer (2% FCS in DPBS) | 97.9 | 97.9 | 99.5 | 99.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hader, M.; Streit, S.; Rosin, A.; Gerdes, T.; Wadepohl, M.; Bekeschus, S.; Fietkau, R.; Frey, B.; Schlücker, E.; Gekle, S.; et al. In Vitro Examinations of Cell Death Induction and the Immune Phenotype of Cancer Cells Following Radiative-Based Hyperthermia with 915 MHz in Combination with Radiotherapy. Cells 2021, 10, 1436. https://doi.org/10.3390/cells10061436
Hader M, Streit S, Rosin A, Gerdes T, Wadepohl M, Bekeschus S, Fietkau R, Frey B, Schlücker E, Gekle S, et al. In Vitro Examinations of Cell Death Induction and the Immune Phenotype of Cancer Cells Following Radiative-Based Hyperthermia with 915 MHz in Combination with Radiotherapy. Cells. 2021; 10(6):1436. https://doi.org/10.3390/cells10061436
Chicago/Turabian StyleHader, Michael, Simon Streit, Andreas Rosin, Thorsten Gerdes, Martin Wadepohl, Sander Bekeschus, Rainer Fietkau, Benjamin Frey, Eberhard Schlücker, Stephan Gekle, and et al. 2021. "In Vitro Examinations of Cell Death Induction and the Immune Phenotype of Cancer Cells Following Radiative-Based Hyperthermia with 915 MHz in Combination with Radiotherapy" Cells 10, no. 6: 1436. https://doi.org/10.3390/cells10061436







