Functional Characterization of the Photosynthetic Machinery in Smicronix Galls on the Parasitic Plant Cuscuta campestris by JIP-Test
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chlorophyll a Fluorescence Measurement
2.3. JIP-Test Parameters
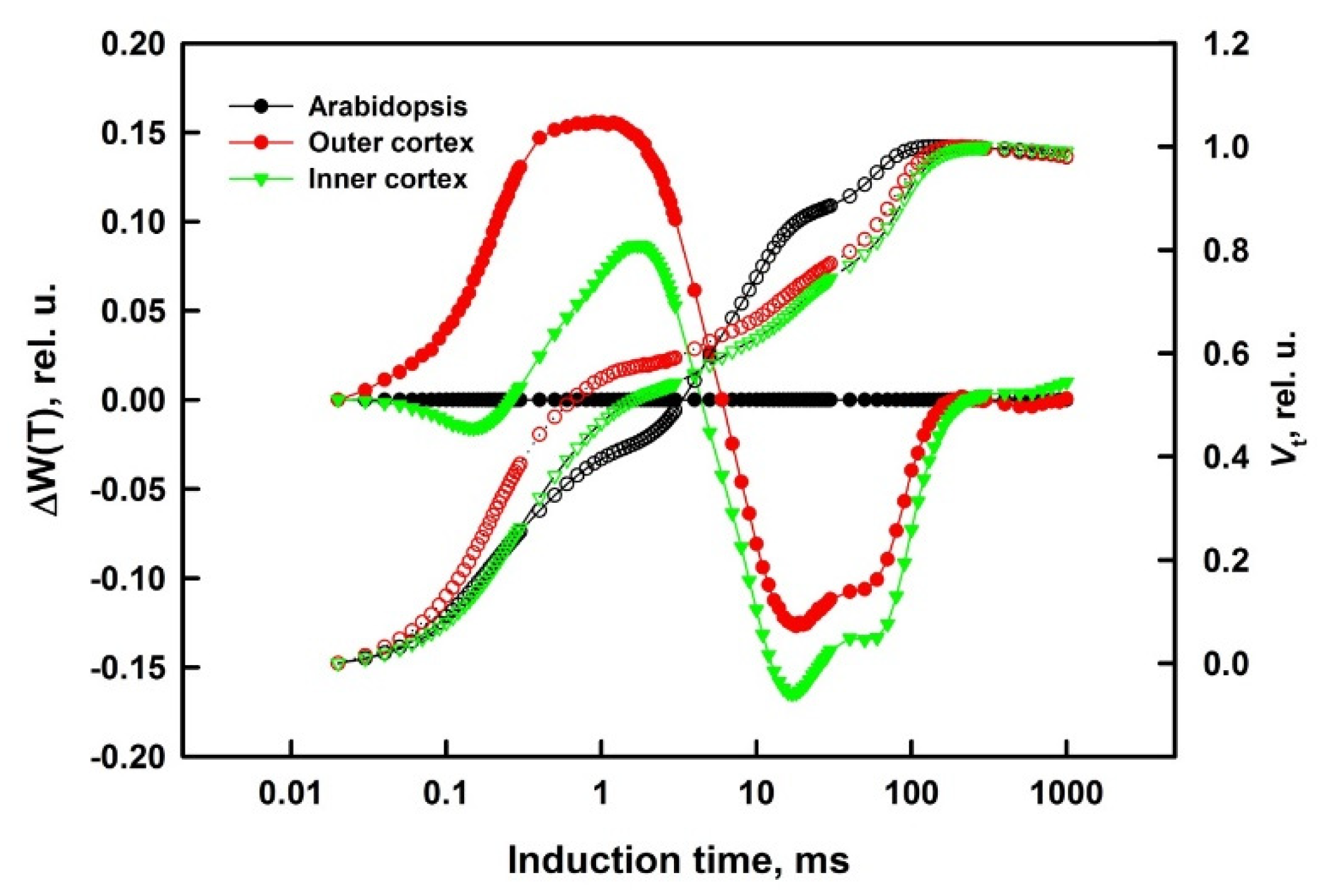
2.4. Difference Curves Calculations
2.5. Statistical Analysis
2.6. Thylakoid Protein Complexes Isolation and Separation
3. Results
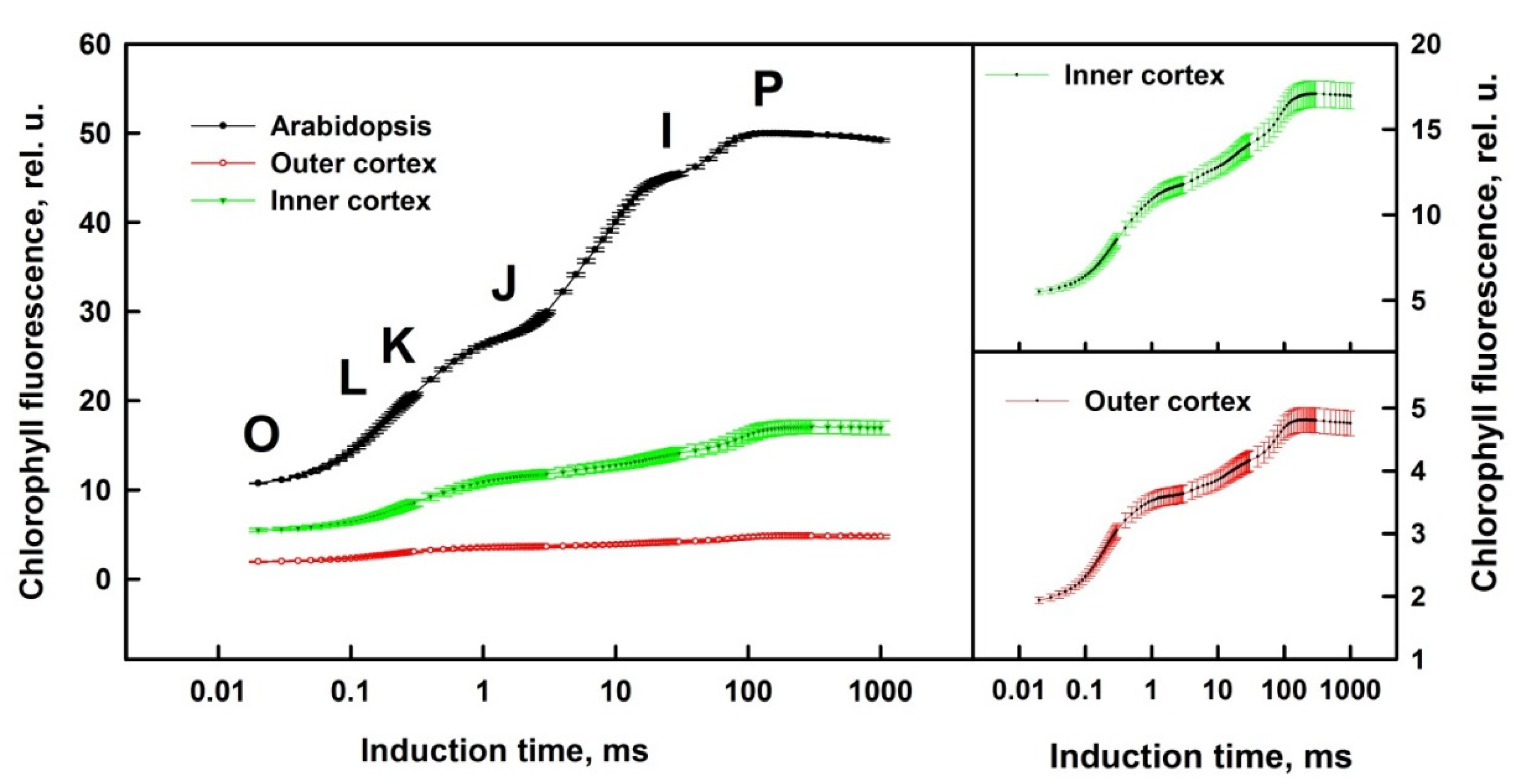
3.1. Prompt Chlorophyll A Fluorescence
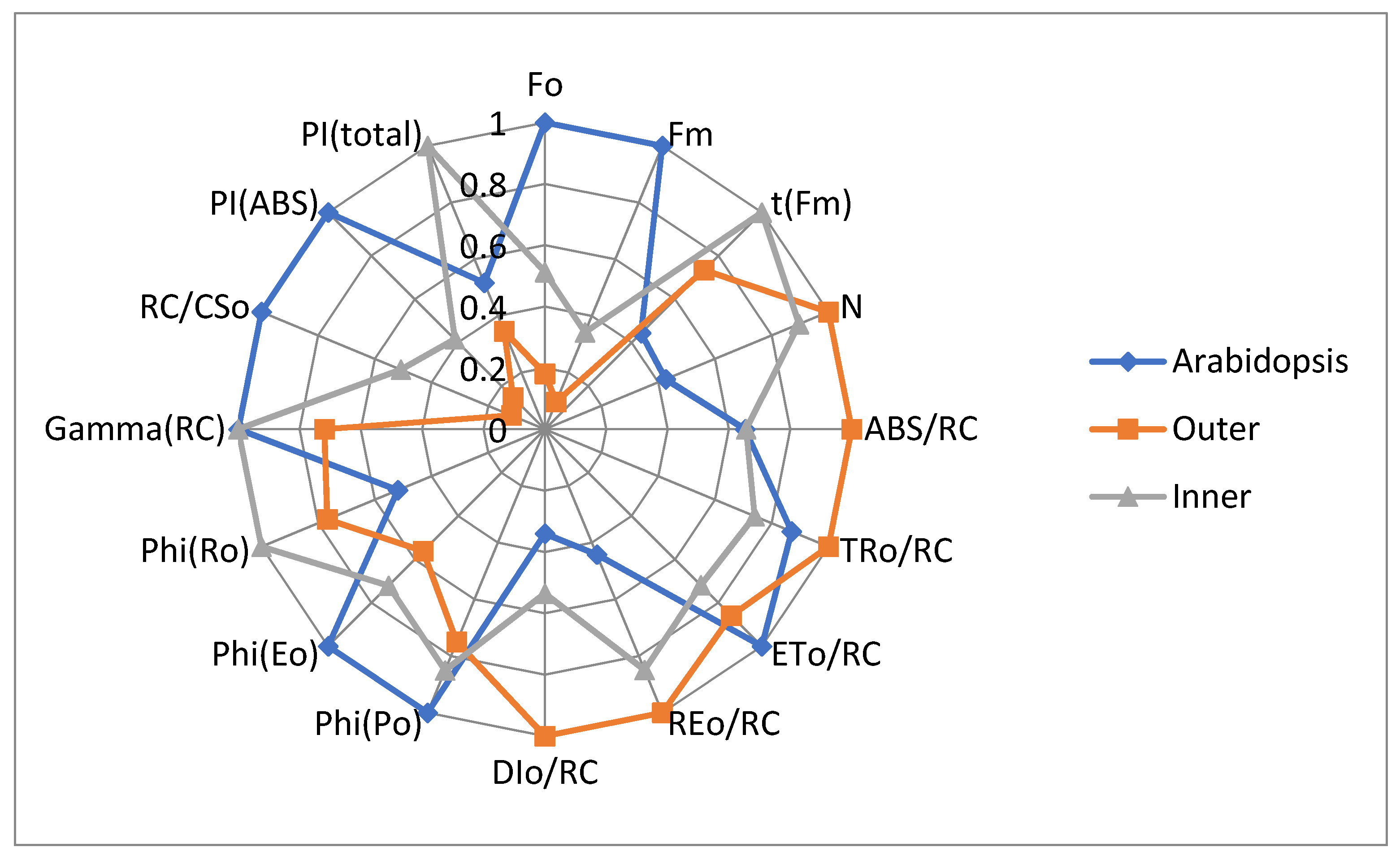
3.2. JIP-Test Parameters
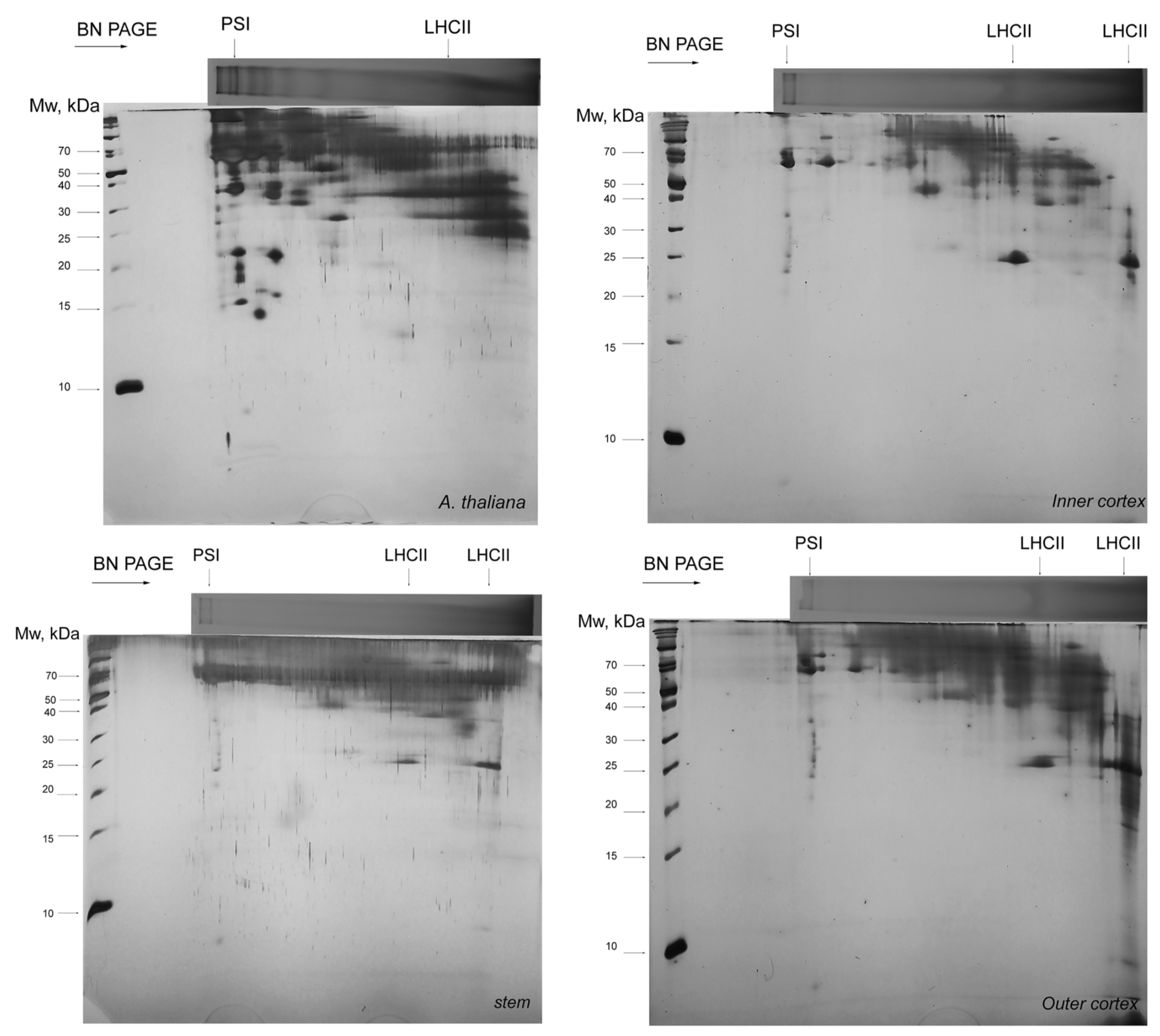
3.3. Thylakoid Complexes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
Appendix A
| Parameter | Definition |
|---|---|
| FO | Minimal fluorescence, when all PSII reaction centers (RCs) are open, fluorescence intensity at 20th µs |
| FM = FP | Maximal fluorescence recorded under saturating illumination at the peak P of OJIP, when all PSII RCs are closed |
| FJ (2 ms) | Fluorescence level at 2nd ms, the J-step of OJIP |
| FI (40 ms) | Fluorescence level at 40th ms, the I-step of OJIP нa гpaφикaтa e 40-тa ms |
| FV | Maximal variable fluorescence, FV = FM − FO |
| Vt | Relative variable fluorescence at time t: Vt = (Ft − FO)/(FM − FO) |
| VL | Relative fluorescence value at L-step [0.15 ms] |
| VK | Relative fluorescence value at K-step [0.3 ms] |
| N | Number indicating how many times QA is reduced until fluorescence reaches its maximal value FM (number of QA redox turnovers until FM is reached) |
| ABS | Absorbed energy flux (exited PSII antennae Chl a molecules) |
| TR0 | Trapped energy, i.e., energy, utilized for reduction of pheophytin and primary quinone acceptor QA |
| ET0 | Electron transport from QA– to the next electron acceptors between the two photosystems. |
| RE0 | Energy/electron flux for reduction of end acceptors in acceptor side of PSI—NADP and ferredoxin. |
| φEo (ET0/ABS) | Efficiency/probability for the electron in PSII to move further than QA–—quantum yield for electron transport. φEo = ET0/ABS = φPo × ψ0 = (1 − F0/FM) × ψ0 |
| ABS/RC | Absorbed energy flux in antenna chlorophylls per PSII reaction center (a measure of PSII apparent antenna size) |
| TR0/RC | Trapped energy flux per RC; TR0/RC = M0 (1/VJ) |
| ET0/RC | Electron transport flux further than QA– per PSII reaction center |
| RE0/RC | Electron flux reducing end electron acceptors at the PSI acceptor side, per PSII reaction center |
| DI0/RC | Heat dissipation of excitation energy by PSII reaction center |
| ET0/RC | Electron transport flux further than QA– per PSII reaction center |
| φPo (TR0/ABS) | Maximum quantum yield of primary photochemical reactions in PSII RC. φPo = TR0/ABS = 1 − FO/FM = FV/FM |
| φRo (RE0/ABS) | Quantum yield of the electron transport from QA– to the end electron acceptors of PSI RE0/ABS = φRo = φPo × ψ0 × δ0 |
| RC/CS0 | Density of active PSII reaction centers (RC) per cross section RC/CS0 = φPo × (VJ/M0) × (ABS/CS0) |
| PIABS | Performance index (potential) for conservation of the energy absorbed by PSII RC until the reduction of intersystem electron acceptors |
| PItotal | Performance index (potential) for conservation of the energy absorbed by PSII until the reduction of PSI end electron acceptors |
References
- Parker, C. Parasitic weeds: A world challenge. Weed Sci. 2012, 60, 269–276. [Google Scholar] [CrossRef]
- Kim, G.; Westwood, J.H. Macromolecule exchange in Cuscuta–host plant interactions. Curr. Opin. Plant Biol. 2015, 26, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Revill, M.J.; Stanley, S.; Hibberd, J.M. Plastid genome structure and loss of photosynthetic ability in the parasitic genus Cuscuta. J. Exp. Bot. 2005, 56, 2477–2486. [Google Scholar] [CrossRef]
- Funk, H.T.; Berg, S.; Krupinska, K.; Maier, U.G.; Krause, K. Complete DNA sequences of the plastid genomes of two parasitic flowering plant species, Cuscuta reflexa and Cuscuta gronovii. BMC Plant Biol. 2007, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- McNeal, J.R.; Kuehl, J.V.; Boore, J.L.; de Pamphilis, C.W. Complete plastid genome sequences suggest strong selection for retention of photosynthetic genes in the parasitic plant genus Cuscuta. BMC Plant Biol. 2007, 7, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Braukmann, T.; Kuzmina, M.; Stefanović, S. Plastid genome evolution across the genus Cuscuta (Convolvulaceae): Two clades within subgenus Grammica exhibit extensive gene loss. J. Exp. Bot. 2013, 64, 977–989. [Google Scholar] [CrossRef] [Green Version]
- Vogel, A.; Schwacke, R.; Denton, A.K.; Usadel, B.; Hollmann, J.; Fischer, K.; Bolger, A.; Schmidt, M.H.-W.; Bolger, M.E.; Gundlach, H. Footprints of parasitism in the genome of the parasitic flowering plant Cuscuta campestris. Nat. Commun. 2018, 9, 2515. [Google Scholar] [CrossRef] [PubMed]
- Van der Kooij, T.; Krause, K.; Dörr, I.; Krupinska, K. Molecular, functional and ultrastructural characterisation of plastids from six species of the parasitic flowering plant genus Cuscuta. Planta 2000, 210, 701–707. [Google Scholar] [CrossRef]
- Aistova, E.; Bezborodov, V. Weevils belonging to the Genus Smicronyx Schönherr, 1843 (Coleoptera, Curculionidae) affecting dodders (Cuscuta Linnaeus, 1753) in the Russian Far East. Russ. J. Biol. Invasions 2017, 8, 184–188. [Google Scholar] [CrossRef]
- De Oliveira, D.C.; Moreira, A.S.F.P.; dos Santos Isaias, R.M. Functional gradients in insect gall tissues: Studies on Neotropical host plants. In Neotropical Insect Galls; Springer: New York, NY, USA, 2014; pp. 35–49. [Google Scholar]
- Anikin, V.; Nikelshparg, M.; Nikelshparg, E.; Konyukhov, I. Photosynthetic activity of the dodder Cuscuta campestris (Convolvulaceae) in case of plant inhabitation by the gallformed weevil Smicronyx smreczynskii (Coleoptera, Curculionidae). Izv. Saratov Univ. New Ser. Chem. Biol. Ecol. 2017, 17, 42–47. [Google Scholar]
- Zagorchev, L.I.; Albanova, I.A.; Tosheva, A.G.; Li, J.; Teofanova, D.R. Metabolic and functional distinction of the Smicronyx sp. galls on Cuscuta campestris. Planta 2018, 248, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Zhekova, E.; Petkova, D.; Ivanova, I. Smicronyx smreczynskii F. Solari, 1952 (Insecta: Curculionidae): Possibilities for biological control of two Cuscuta species (Cuscutaceae) in district of Ruse. Acta Zool. Bulg. 2014, 66, 431–432. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence; Springer: New York, NY, USA, 2004; pp. 321–362. [Google Scholar]
- Stirbet, A.; Govindjee. Chlorophyll a fluorescence induction: A personal perspective of the thermal phase, the J-I-P rise. Photosynth. Res. 2012, 113, 15–61. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, S.; Paunov, M.; Pavlova, B.; Dankov, K.; Kouzmanova, M.; Velikova, V.; Tsonev, T.; Kalaji, H.; Goltsev, V. Photosynthetic efficiency of two Platanus orientalis L. ecotypes exposed to moderately high temperature–JIP-test analysis. Photosynthetica 2020, 58, 657–670. [Google Scholar] [CrossRef] [Green Version]
- Järvi, S.; Suorsa, M.; Paakkarinen, V.; Aro, E.-M. Optimized native gel systems for separation of thylakoid protein complexes: Novel super-and mega-complexes. Biochem. J. 2011, 439, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kügler, M.; Jänsch, L.; Kruft, V.; Schmitz, U.K.; Braun, H.-P. Analysis of the chloroplast protein complexes by blue-native polyacrylamide gel electrophoresis (BN-PAGE). Photosynth. Res. 1997, 53, 35–44. [Google Scholar] [CrossRef]
- Schägger, H.; von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987, 166, 368–379. [Google Scholar] [CrossRef]
- Tsimilli-Michael, M.; Strasser, R.J. The energy flux theory 35 years later: Formulations and applications. Photosynth. Res. 2013, 117, 289–320. [Google Scholar] [CrossRef]
- Stirbet, A. Excitonic connectivity between photosystem II units: What is it, and how to measure it? Photosynth. Res. 2013, 116, 189–214. [Google Scholar] [CrossRef]
- Strasser, R.J.; Stirbet, A.D. Estimation of the energetic connectivity of PS II centres in plants using the fluorescence rise O–J–I–P: Fitting of experimental data to three different PS II models. Math. Comput. Simul. 2001, 56, 451–462. [Google Scholar] [CrossRef]
- Srivastava, A.; Strasser, R.J. Stress and stress management of land plants during a regular day. J. Plant Physiol. 1996, 148, 445–455. [Google Scholar] [CrossRef]
- Srivastava, A.; Guisse, B.; Greppin, H.; Strasser, R.J. Regulation of antenna structure and electron transport in photosystem II of Pisum sativum under elevated temperature probed by the fast polyphasic chlorophyll a fluorescence transient: OKJIP. Biochim. Biophys. Acta (BBA) Bioenerg. 1997, 1320, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Strasser, B.J. Donor side capacity of photosystem II probed by chlorophyll a fluorescence transients. Photosynth. Res. 1997, 52, 147–155. [Google Scholar] [CrossRef]
- Stirbet, A.; Riznichenko, G.Y.; Rubin, A. Modeling chlorophyll a fluorescence transient: Relation to photosynthesis. Biochemistry 2014, 79, 291–323. [Google Scholar] [CrossRef] [PubMed]
- Guisse, B.; Srivastava, A.; Strasser, R. The polyphasic rise of the chlorophyll a fluorescence (OKJIP) in heat-stressed leaves. Arch. Sci. 1995, 48, 147–160. [Google Scholar]
- Ceppi, M.G.; Oukarroum, A.; Çiçek, N.; Strasser, R.J.; Schansker, G. The IP amplitude of the fluorescence rise OJIP is sensitive to changes in the photosystem I content of leaves: A study on plants exposed to magnesium and sulfate deficiencies, drought stress and salt stress. Physiol. Plant. 2012, 144, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Blankenship, R.E. Molecular Mechanisms of Photosynthesis; Wiley-Blackwell Publisher: Chichester, West Sussex, UK, 2014; p. 312. [Google Scholar]
- Ke, B. Photosynthesis: Photobiochemistry and Photobiophysics; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Dinelli, G.; Bonetti, A.; Tibiletti, E. Photosynthetic and accessory pigments in Cuscuta campestris Yuncker and some host species. Weed Res. 1993, 33, 253–260. [Google Scholar] [CrossRef]
- Sherman, T.D.; Pettigrew, W.T.; Vaughn, K.C. Structural and immunological characterization of the Cuscuta pentagona L. chloroplast. Plant Cell Physiol. 1999, 40, 592–603. [Google Scholar] [CrossRef]
- Kobayashi, K.; Baba, S.; Obayashi, T.; Sato, M.; Toyooka, K.; Keranen, M.; Aro, E.-M.; Fukaki, H.; Ohta, H.; Sugimoto, K.; et al. Regulation of root greening by light and auxin/cytokinin signaling in Arabidopsis. Plant Cell 2012, 24, 1081–1095. [Google Scholar] [CrossRef] [Green Version]
- Machado, M.; Zetsche, K. A structural, functional and molecular analysis of plastids of the holoparasites Cuscuta reflexa and Cuscuta europaea. Planta 1990, 181, 91–96. [Google Scholar] [CrossRef]
- McNeal, J.R.; Arumugunathan, K.; Kuehl, J.V.; Boore, J.L.; Claude, W.D. Systematics and plastid genome evolution of the cryptically photosynthetic parasitic plant genus Cuscuta (Convolvulaceae). BMC Biol. 2007, 5, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Jadhav, S.; Toro, S. Vestigial root structure of Cuscuta reflexa and C. chinensis. BIOINFOLET-A Q. J. Life Sci. 2020, 17, 53–55. [Google Scholar]
- Snyder, A.M.; Clark, B.M.; Bungard, R.A. Light-dependent conversion of carotenoids in the parasitic angiosperm Cuscuta reflexa L. Plant Cell Environ. 2005, 28, 1326–1333. [Google Scholar] [CrossRef]
- Snyder, A.M.; Clark, B.M.; Robert, B.; Ruban, A.V.; Bungard, R.A. Carotenoid specificity of light-harvesting complex II binding sites: Occurrence of 9-cis-violaxanthin in the neoxanthin-binding site in the parasitic angiosperm Cuscuta reflexa. J. Biol. Chem. 2004, 279, 5162–5168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toth, P.; Tothova, M.; Cagáň, L. Potential biological control agents of field bindweed, common teasel and field dodder from Slovakia. In Proceedings of the 12th International Symposium on Biological Control of Weeds, La Grande Motte, France, 22–27 April 2008; pp. 216–220. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagorchev, L.; Atanasova, A.; Albanova, I.; Traianova, A.; Mladenov, P.; Kouzmanova, M.; Goltsev, V.; Kalaji, H.M.; Teofanova, D. Functional Characterization of the Photosynthetic Machinery in Smicronix Galls on the Parasitic Plant Cuscuta campestris by JIP-Test. Cells 2021, 10, 1399. https://doi.org/10.3390/cells10061399
Zagorchev L, Atanasova A, Albanova I, Traianova A, Mladenov P, Kouzmanova M, Goltsev V, Kalaji HM, Teofanova D. Functional Characterization of the Photosynthetic Machinery in Smicronix Galls on the Parasitic Plant Cuscuta campestris by JIP-Test. Cells. 2021; 10(6):1399. https://doi.org/10.3390/cells10061399
Chicago/Turabian StyleZagorchev, Lyuben, Alexandra Atanasova, Ivanela Albanova, Anelia Traianova, Petko Mladenov, Margarita Kouzmanova, Vasilij Goltsev, Hazem M. Kalaji, and Denitsa Teofanova. 2021. "Functional Characterization of the Photosynthetic Machinery in Smicronix Galls on the Parasitic Plant Cuscuta campestris by JIP-Test" Cells 10, no. 6: 1399. https://doi.org/10.3390/cells10061399
APA StyleZagorchev, L., Atanasova, A., Albanova, I., Traianova, A., Mladenov, P., Kouzmanova, M., Goltsev, V., Kalaji, H. M., & Teofanova, D. (2021). Functional Characterization of the Photosynthetic Machinery in Smicronix Galls on the Parasitic Plant Cuscuta campestris by JIP-Test. Cells, 10(6), 1399. https://doi.org/10.3390/cells10061399







