Angiotensin II-Induced Cardiac Effects Are Modulated by Endocannabinoid-Mediated CB1 Receptor Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Langendorff Heart Preparation
2.3. Experimental Protocol
2.4. Immunohistochemistry
2.5. Immunofluorescent Visualization of CB1 Receptors in Rat Cardiac Tissue
2.6. Statistical Analysis and Interpretation
3. Results
3.1. CB1 Receptors in Rat Cardiac Tissue
3.2. The Effects of Ang II Infusion on Isolated Rat Hearts
3.3. Effects of Repetitive Ang II Infusion on Coronary Flow and Contractile Function
3.4. Effects of CB Receptor Agonists and CB1R Antagonist O2050 on Coronary Flow and Contractile Function
3.5. The Influence of CB1R Antagonist O2050 and DAGL Inhibitor Orlistat on Ang II Effects
4. Discussion
4.1. Effects of Endocannabinoids on Cardiac Function
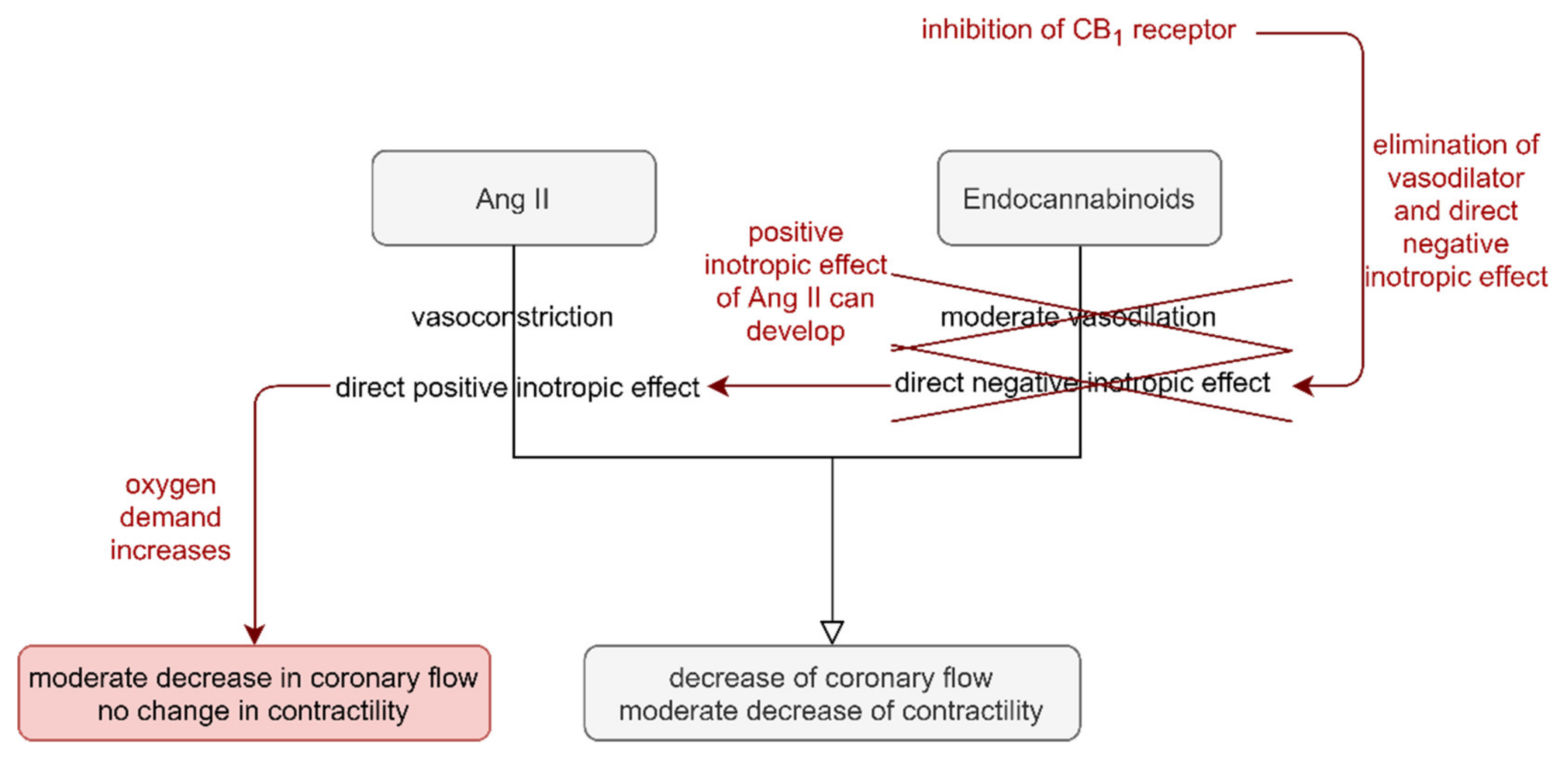
4.2. Role of Angiotensin II-Induced Endocannabinoid Release in Cardiac Function
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lavoie, J.L.; Sigmund, C.D. Minireview: Overview of the Renin-Angiotensin System—An Endocrine and Paracrine System. Endocrinology 2003, 144, 2179–2183. [Google Scholar] [CrossRef]
- Kaschina, E.; Unger, T. Angiotensin AT1/AT2 receptors: Regulation, signalling and function. Blood Press. 2003, 12, 70–88. [Google Scholar] [CrossRef]
- Mehta, P.K.; Griendling, K.K. Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system. Am. J. Physiol. Physiol. 2007, 292, C82–C97. [Google Scholar] [CrossRef]
- Van Esch, J.H.; Gembardt, F.; Sterner-Kock, A.; Heringer-Walther, S.; Le, T.H.; Lassner, D.; Stijnen, T.; Coffman, T.M.; Schultheiss, H.-P.; Danser, A.J.; et al. Cardiac phenotype and angiotensin II levels in AT1a, AT1b, and AT2 receptor single, double, and triple knockouts. Cardiovasc. Res. 2010, 86, 401–409. [Google Scholar] [CrossRef]
- Pörsti, I.; Hecker, M.; Bassenge, E.; Busse, R. Dual action of angiotensin II on coronary resistance in the isolated perfused rabbit heart. Naunyn Schmiedeberg Arch. Pharmacol. 1993, 348, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Booz, G.W.; Baker, K.M. Actions of Angiotensin II on Isolated Cardiac Myocytes. Hear. Fail. Rev. 1998, 3, 125–130. [Google Scholar] [CrossRef]
- Mattiazzi, A. Positive inotropic effect of Angiotensin II. Increases in intracellular Ca2+ or changes in myofilament Ca2+ responsiveness? J. Pharmacol. Toxicol. Methods 1997, 37, 205–214. [Google Scholar] [CrossRef]
- Barry, W.H.; Matsui, H.; Bridge, J.H.B.; Spitzer, K.W. Excitation-Contraction Coupling in Ventricular Myocytes: Effects of Angiotensin II. In Tissue Engineering; Metzler, J.B., Ed.; Springer: Boston, MA, USA, 1995; Volume 382, pp. 31–39. [Google Scholar]
- Lefroy, D.C.; Crake, T.; Del Monte, F.; Vescovo, G.; Libera, L.D.; Harding, S.; Poole-Wilson, P.A. Angiotensin II and contraction of isolated myocytes from human, guinea pig, and infarcted rat hearts. Am. J. Physiol. Content 1996, 270, H2060–H2069. [Google Scholar] [CrossRef]
- Paul, M.; Mehr, A.P.; Kreutz, R. Physiology of Local Renin-Angiotensin Systems. Physiol. Rev. 2006, 86, 747–803. [Google Scholar] [CrossRef] [PubMed]
- Hunyady, L.; Catt, K.J. Pleiotropic AT1 Receptor Signaling Pathways Mediating Physiological and Pathogenic Actions of Angiotensin II. Mol. Endocrinol. 2006, 20, 953–970. [Google Scholar] [CrossRef]
- Turu, G.; Várnai, P.; Gyombolai, P.; Szidonya, L.; Offertaler, L.; Bagdy, G.; Kunos, G.; Hunyady, L. Paracrine Transactivation of the CB1 Cannabinoid Receptor by AT1 Angiotensin and Other Gq/11 Protein-coupled Receptors. J. Biol. Chem. 2009, 284, 16914–16921. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, M.; Nádasy, G.L.; Turu, G.; Soltész-Katona, E.; Tóth, Z.E.; Balla, A.; Catt, K.J.; Hunyady, L. Angiotensin II Induces Vascular Endocannabinoid Release, Which Attenuates Its Vasoconstrictor Effect via CB1 Cannabinoid Receptors. J. Biol. Chem. 2012, 287, 31540–31550. [Google Scholar] [CrossRef] [PubMed]
- Benyó, Z.; Ruisanchez, É.; Leszl-Ishiguro, M.; Sándor, P.; Pacher, P. Endocannabinoids in cerebrovascular regulation. Am. J. Physiol. Circ. Physiol. 2016, 310, H785–H801. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, M.; Nadasy, G.L.; Turu, G.; Soltész-Katona, E.; Benyó, Z.; Offermanns, S.; Ruisanchez, É.; Szabó, E.; Takats, Z.; Batkai, S.; et al. Endocannabinoid-mediated modulation of Gq/11 protein-coupled receptor signaling-induced vasoconstriction and hypertension. Mol. Cell. Endocrinol. 2015, 403, 46–56. [Google Scholar] [CrossRef]
- Cajanus, K.; Holmström, E.J.; Wessman, M.; Anttila, V.; Kaunisto, M.A.; Kalso, E. Effect of endocannabinoid degradation on pain. Pain 2016, 157, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Cedernaes, J.; Fanelli, F.; Fazzini, A.; Pagotto, U.; Broman, J.-E.; Vogel, H.; Dickson, S.L.; Schiöth, H.B.; Benedict, C. Sleep restriction alters plasma endocannabinoids concentrations before but not after exercise in humans. Psychoneuroendocrinology 2016, 74, 258–268. [Google Scholar] [CrossRef]
- Pacher, P.; Bátkai, S.; Kunos, G. The Endocannabinoid System as an Emerging Target of Pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. [Google Scholar] [CrossRef]
- Hillard, C.J. Circulating Endocannabinoids: From Whence Do They Come and Where are They Going? Neuropsychopharmacology 2018, 43, 155–172. [Google Scholar] [CrossRef]
- Freund, T.F.; Katona, I.; Piomelli, D. Role of Endogenous Cannabinoids in Synaptic Signaling. Physiol. Rev. 2003, 83, 1017–1066. [Google Scholar] [CrossRef]
- Wagner, J.A.; Abesser, M.; Harvey-White, J.; Ertl, G. 2-Arachidonylglycerol Acting on CB1 Cannabinoid Receptors Mediates Delayed Cardioprotection Induced by Nitric Oxide in Rat Isolated Hearts. J. Cardiovasc. Pharmacol. 2006, 47, 650–655. [Google Scholar] [CrossRef]
- Wagner, J.A.; Abesser, M.; Karcher, J.; Laser, M.; Kunos, G. Coronary Vasodilator Effects of Endogenous Cannabinoids in Vasopressin-Preconstricted Unpaced Rat Isolated Hearts. J. Cardiovasc. Pharmacol. 2005, 46, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Stanley, C.; O′Sullivan, S.E. Vascular targets for cannabinoids: Animal and human studies. Br. J. Pharmacol. 2014, 171, 1361–1378. [Google Scholar] [CrossRef]
- Iring, A.; Ruisanchez, E.; Leszl-Ishiguro, M.; Horváth, B.; Benko, R.; Lacza, Z.; Járai, Z.; Sandor, P.; Di Marzo, V.; Pacher, P.; et al. Role of Endocannabinoids and Cannabinoid-1 Receptors in Cerebrocortical Blood Flow Regulation. PLoS ONE 2013, 8, e53390. [Google Scholar] [CrossRef]
- Hiley, C.R. Endocannabinoids and the Heart. J. Cardiovasc. Pharmacol. 2009, 53, 267–276. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Chapnick, B.M.; Howlett, A.C. Anandamide-induced vasorelaxation in rabbit aortic rings has two components: G protein dependent and independent. Am. J. Physiol. Circ. Physiol. 2002, 282, H2046–H2054. [Google Scholar] [CrossRef]
- Bondarenko, A.I. Endothelial atypical cannabinoid receptor: Do we have enough evidence? Br. J. Pharmacol. 2014, 171, 5573–5588. [Google Scholar] [CrossRef] [PubMed]
- Lípez-Miranda, V.; Herradón, E.; Martín, M.I. Vasorelaxation caused by cannabinoids: Mechanisms in different vascular beds. Curr. Vasc. Pharmacol 2008, 6, 335–346. [Google Scholar] [CrossRef]
- Turu, G.; Simon, A.; Gyombolai, P.; Szidonya, L.; Bagdy, G.; Lenkei, Z.; Hunyady, L. The Role of Diacylglycerol Lipase in Constitutive and Angiotensin AT1 Receptor-stimulated Cannabinoid CB1 Receptor Activity. J. Biol. Chem. 2007, 282, 7753–7757. [Google Scholar] [CrossRef]
- Dell′Italia, L.J. Translational Success Stories: Angiotensin Receptor 1 Antagonists in Heart Failure. Circ. Res. 2011, 109, 437–452. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Kobayashi, M.; Kurebayashi, N.; Yoshikawa, D.; Suzuki, S.; Ichimiya, S.; Kanashiro, M.; Sone, T.; Tsuboi, H.; Amano, T.; et al. Impact of Angiotensin II Receptor Blocker Therapy (Olmesartan or Valsartan) on Coronary Atherosclerotic Plaque Volume Measured by Intravascular Ultrasound in Patients With Stable Angina Pectoris. Am. J. Cardiol. 2013, 112, 363–368. [Google Scholar] [CrossRef]
- Heeneman, S.; Sluimer, J.C.; Daemen, M.J.A.P. Angiotensin-Converting Enzyme and Vascular Remodeling. Circ. Res. 2007, 101, 441–454. [Google Scholar] [CrossRef]
- Wafa, D.; Koch, N.; Kovács, J.; Kerék, M.; Proia, R.L.; Tigyi, G.J.; Benyó, Z.; Miklós, Z. Opposing Roles of S1P3 Receptors in Myocardial Function. Cells 2020, 9, 1770. [Google Scholar] [CrossRef]
- Kemecsei, P.; Miklós, Z.; Bíró, T.; Marincsák, R.; Tóth, B.I.; Komlodi-Pasztor, E.; Barnucz, E.; Mirk, É.; Van Der Vusse, G.J.; Ligeti, L.; et al. Hearts of surviving MLP-KO mice show transient changes of intracellular calcium handling. Mol. Cell. Biochem. 2010, 342, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Miklós, Z.; Kemecsei, P.; Biro, T.; Marincsak, R.; Toth, B.I.; Buijs, J.; Benis, E.; Drozgyik, A.; Ivanics, T. Early cardiac dysfunction is rescued by upregulation of SERCA2a pump activity in a rat model of metabolic syndrome. Acta Physiol. 2012, 205, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, A.; Zimmer, A.M.; Hohmann, A.G.; Herkenham, M.; Bonner, T.I. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc. Natl. Acad. Sci. USA 1999, 96, 5780–5785. [Google Scholar] [CrossRef] [PubMed]
- Tóth, Z.E.; Mezey, É. Simultaneous Visualization of Multiple Antigens with Tyramide Signal Amplification using Antibodies from the same Species. J. Histochem. Cytochem. 2007, 55, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Krenacs, T.; Meggyeshazi, N.; Forika, G.; Kiss, E.; Hamar, P.; Szekely, T.; Vancsik, T. Modulated Electro-Hyperthermia-Induced Tumor Damage Mechanisms Revealed in Cancer Models. Int. J. Mol. Sci. 2020, 21, 6270. [Google Scholar] [CrossRef] [PubMed]
- Bátkai, S.; Járai, Z.; Wagner, J.A.; Goparaju, S.K.; Varga, K.; Liu, J.; Wang, L.; Mirshahi, F.; Khanolkar, A.D.; Makriyannis, A.; et al. Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nat. Med. 2001, 7, 827–832. [Google Scholar] [CrossRef]
- Bátkai, S.; Pacher, P.; Osei-Hyiaman, D.; Radaeva, S.; Liu, J.; Harvey-White, J.; Offertáler, L.; Mackie, K.; Rudd, M.A.; Bukoski, R.D.; et al. Endocannabinoids Acting at Cannabinoid-1 Receptors Regulate Cardiovascular Function in Hypertension. Circulation 2004, 110, 1996–2002. [Google Scholar] [CrossRef]
- Randall, M.D.; Kendall, D.A.; O′Sullivan, S. The complexities of the cardiovascular actions of cannabinoids. Br. J. Pharmacol. 2004, 142, 20–26. [Google Scholar] [CrossRef]
- Dannert, M.; Alsasua, A.; Herradón, E.; Martin, M.; López-Miranda, V. Vasorelaxant effect of Win 55,212-2 in rat aorta: New mechanisms involved. Vasc. Pharmacol. 2007, 46, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Bonz, A.; Laser, M.; Küllmer, S.; Kniesch, S.; Babin-Ebell, J.; Popp, V.; Ertl, G.; Wagner, J.A. Cannabinoids Acting on CB1 Receptors Decrease Contractile Performance in Human Atrial Muscle. J. Cardiovasc. Pharmacol. 2003, 41, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Bátkai, S.; Rajesh, M.; Czifra, N.; Harvey-White, J.; Haskó, G.; Zsengeller, Z.; Gerard, N.P.; Liaudet, L.; Kunos, G.; et al. Pharmacological Inhibition of CB1Cannabinoid Receptor Protects Against Doxorubicin-Induced Cardiotoxicity. J. Am. Coll. Cardiol. 2007, 50, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Högestätt, E.D.; Zygmunt, P. Cardiovascular pharmacology of anandamide. Prostaglandins Leukot. Essent. Fat. Acids 2002, 66, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Lépicier, P.; Bouchard, J.-F.; Lagneux, C.; Lamontagne, D. Endocannabinoids protect the rat isolated heart against ischaemia. Br. J. Pharmacol. 2003, 139, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, K.M.; Baewer, D.V.; Hittner, S.; Hillard, C.J.; Nithipatikom, K.; Reddy, D.S.; Falck, J.R.; Campbell, W.B. Endothelium-derived 2-arachidonylglycerol: An intermediate in vasodilatory eicosanoid release in bovine coronary arteries. Am. J. Physiol. Circ. Physiol. 2005, 288, H1344–H1351. [Google Scholar] [CrossRef]
- Gorbunov, A.S.; Maslov, L.N.; Tsibulnikov, S.Y.; Khaliulin, I.G.; Tsepokina, A.V.; Khutornaya, M.V.; Kutikhin, A.G. CB-Receptor Agonist HU-210 Mimics the Postconditioning Phenomenon of Isolated Heart. Bull. Exp. Biol. Med. 2016, 162, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Ford, W.R.; A Honan, S.; White, R.; Hiley, C.R. Evidence of a novel site mediating anandamide-induced negative inotropic and coronary vasodilatator responses in rat isolated hearts. Br. J. Pharmacol. 2002, 135, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, M.; Nádasy, G.L.; Soltész-Katona, E.; Hunyady, L. Control of myogenic tone and agonist induced contraction of intramural coronary resistance arterioles by cannabinoid type 1 receptors and endocannabinoids. Prostaglandins Other Lipid Mediat. 2018, 134, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Gyombolai, P.; Pap, D.; Turu, G.; Catt, K.J.; Bagdy, G.; Hunyady, L. Regulation of endocannabinoid release by G proteins: A paracrine mechanism of G protein-coupled receptor action. Mol. Cell. Endocrinol. 2012, 353, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Fride, E.; Ben-Shabat, S.; Meiri, U.; Horowitz, M. Carbachol, an acetylcholine receptor agonist, enhances production in rat aorta of 2-arachidonoyl glycerol, a hypotensive endocannabinoid. Eur. J. Pharmacol. 1998, 362, R1–R3. [Google Scholar] [CrossRef]
- França, L.P.; Pacheco, N.A.; Correa, S.A.; Han, S.W.; Nakaie, C.R.; Paiva, A.C.M.; Shimuta, S.I. Angiotensin II-mediated cellular responses: A role for the 3’-untranslated region of the angiotensin AT1 receptor. Eur. J. Pharmacol. 2003, 476, 25–30. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miklós, Z.; Wafa, D.; Nádasy, G.L.; Tóth, Z.E.; Besztercei, B.; Dörnyei, G.; Laska, Z.; Benyó, Z.; Ivanics, T.; Hunyady, L.; et al. Angiotensin II-Induced Cardiac Effects Are Modulated by Endocannabinoid-Mediated CB1 Receptor Activation. Cells 2021, 10, 724. https://doi.org/10.3390/cells10040724
Miklós Z, Wafa D, Nádasy GL, Tóth ZE, Besztercei B, Dörnyei G, Laska Z, Benyó Z, Ivanics T, Hunyady L, et al. Angiotensin II-Induced Cardiac Effects Are Modulated by Endocannabinoid-Mediated CB1 Receptor Activation. Cells. 2021; 10(4):724. https://doi.org/10.3390/cells10040724
Chicago/Turabian StyleMiklós, Zsuzsanna, Dina Wafa, György L. Nádasy, Zsuzsanna E. Tóth, Balázs Besztercei, Gabriella Dörnyei, Zsófia Laska, Zoltán Benyó, Tamás Ivanics, László Hunyady, and et al. 2021. "Angiotensin II-Induced Cardiac Effects Are Modulated by Endocannabinoid-Mediated CB1 Receptor Activation" Cells 10, no. 4: 724. https://doi.org/10.3390/cells10040724






