Poly(phenylene ether) Based Amphiphilic Block Copolymers
Abstract
:1. Introduction
2. Materials and Methods
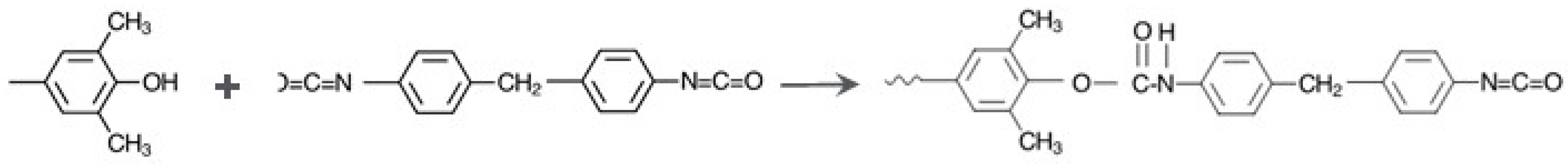
2.1. Preparation of Polyhydroxy Ethers
2.2. Blends and Composites
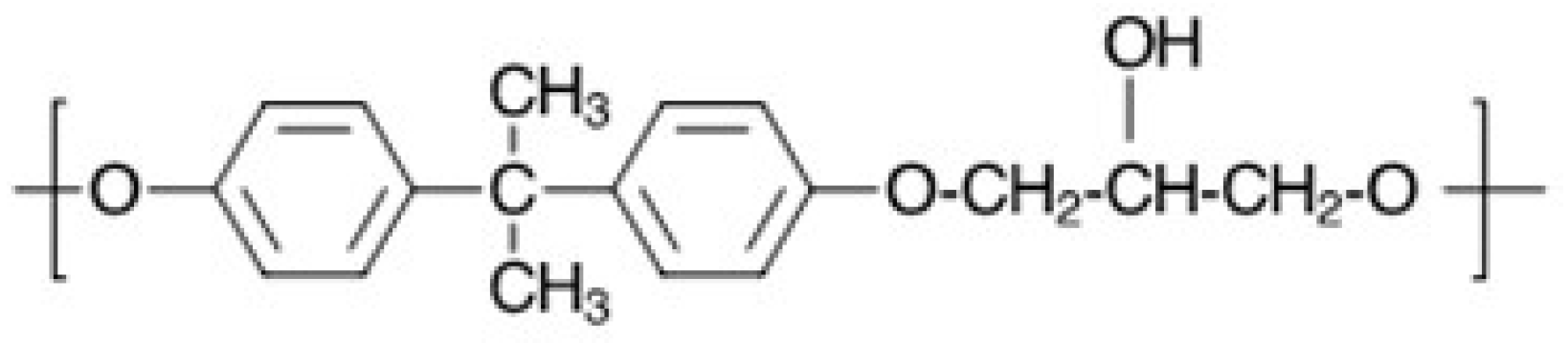
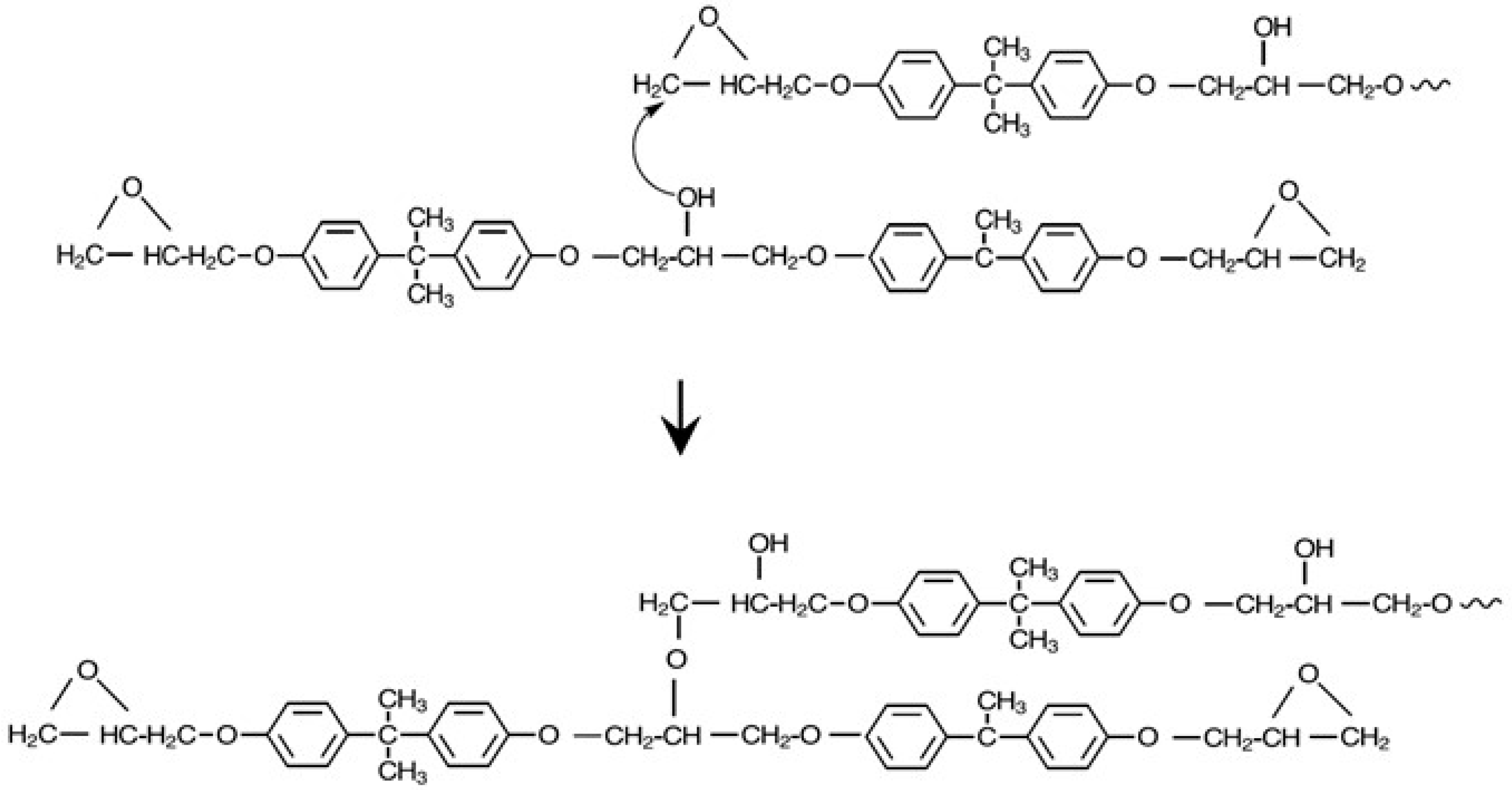
2.3. Preparation of Thermoplastic Polyurethanes
3. Results
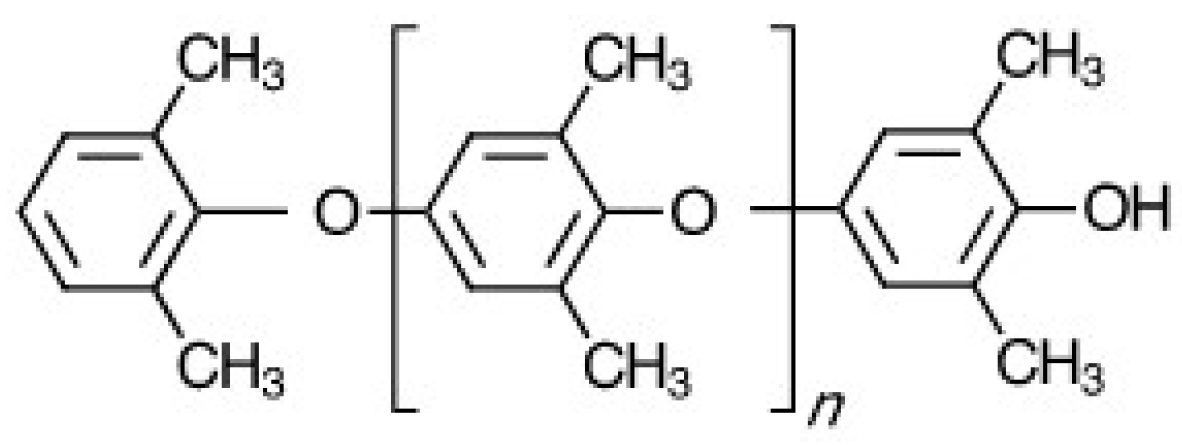
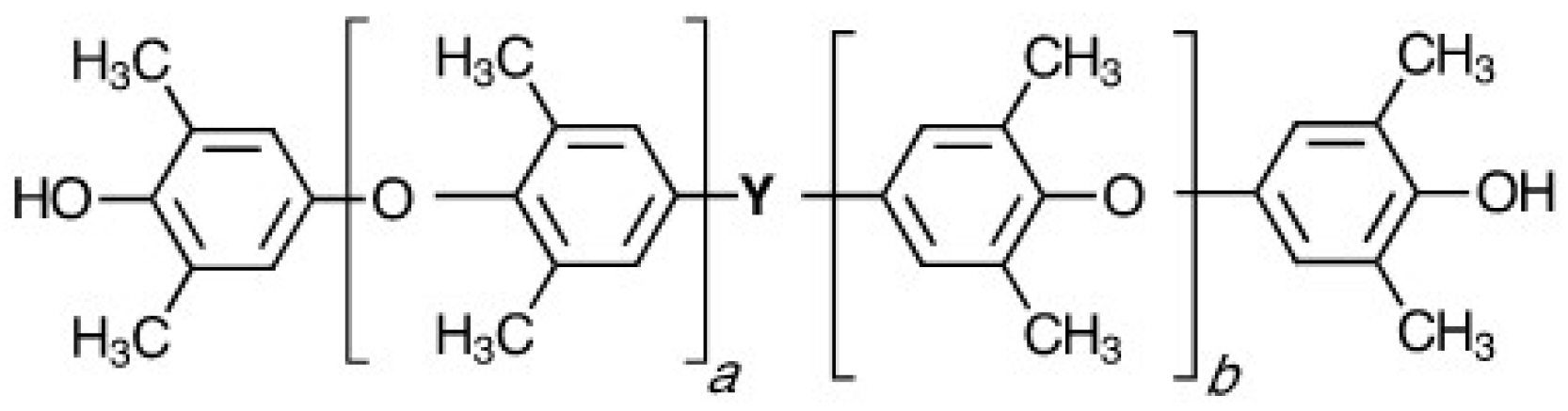
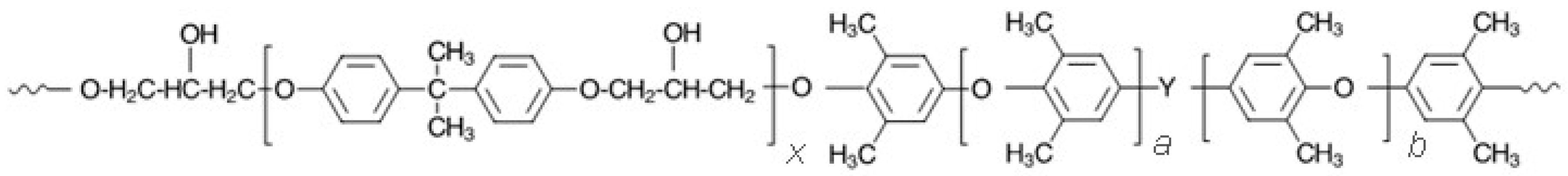
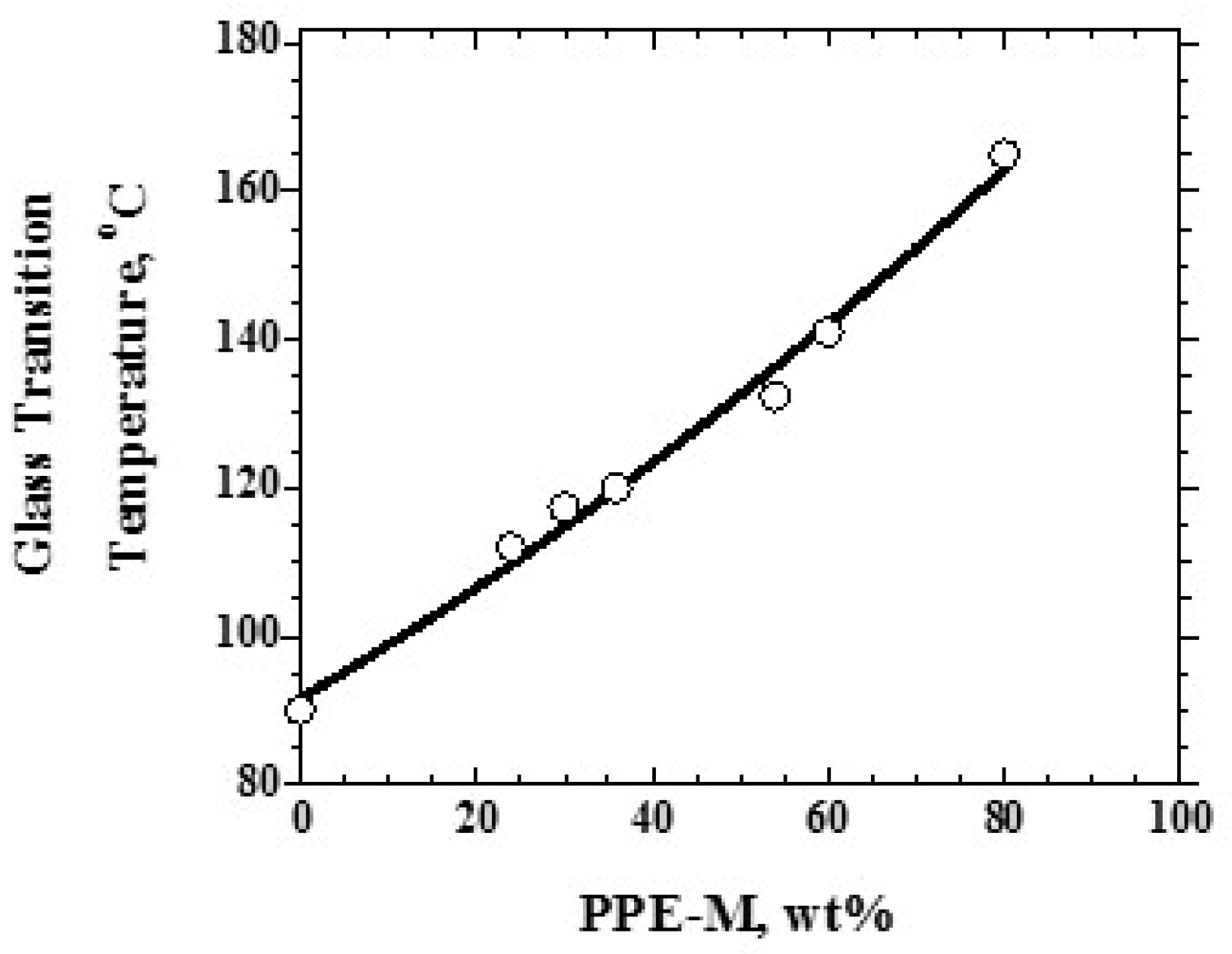
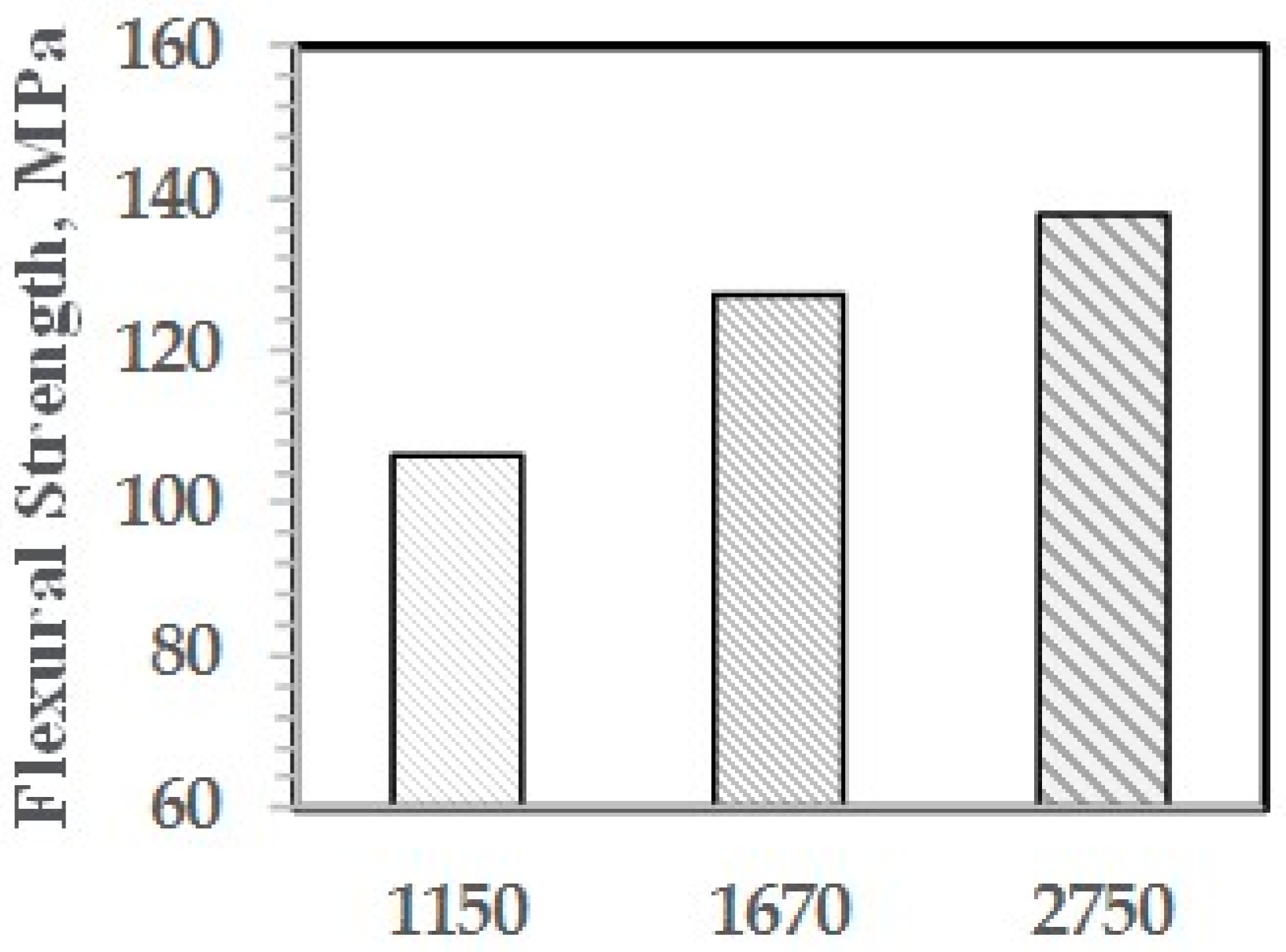
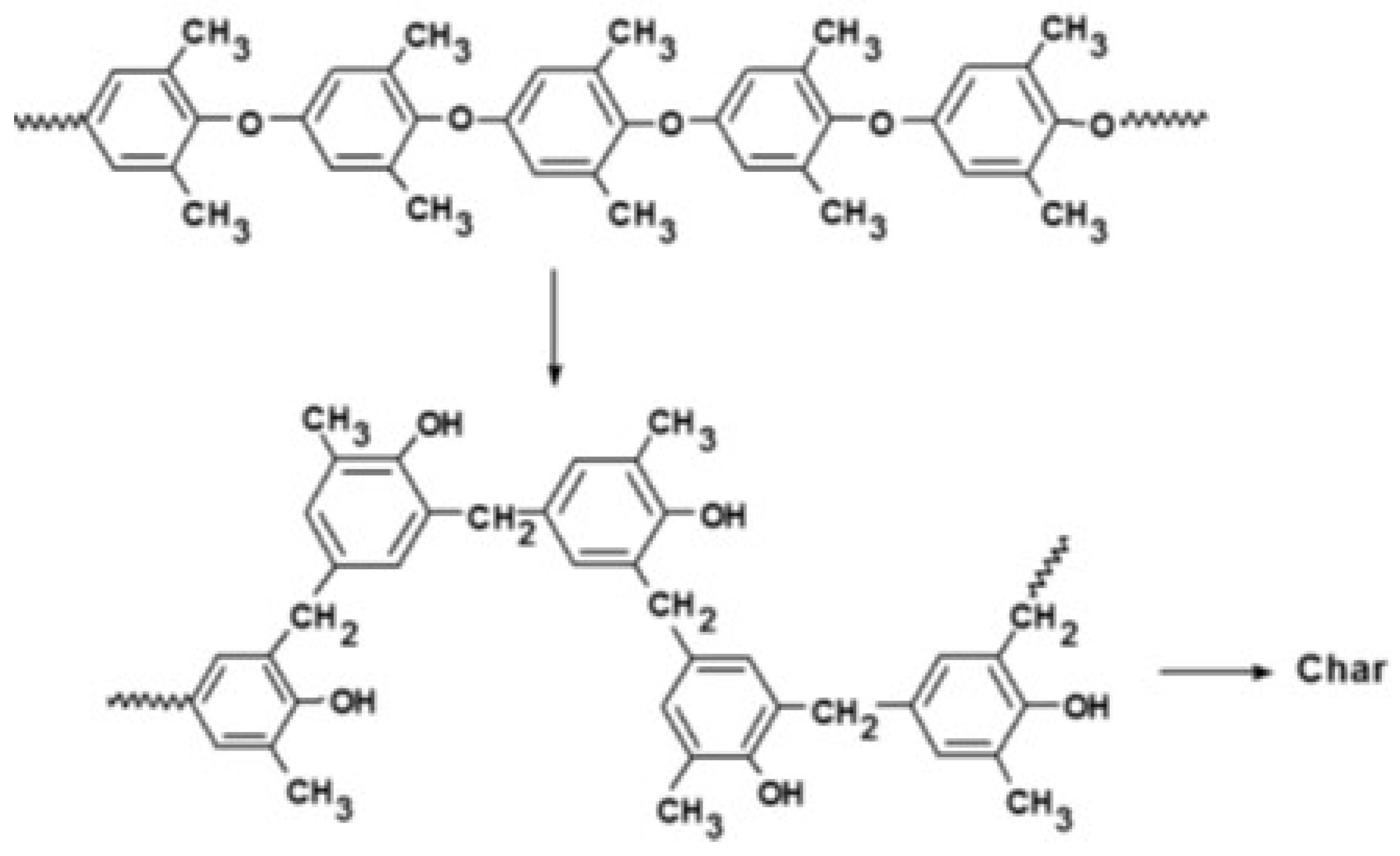
3.1. Properties of PPE-PHE Block Copolymers
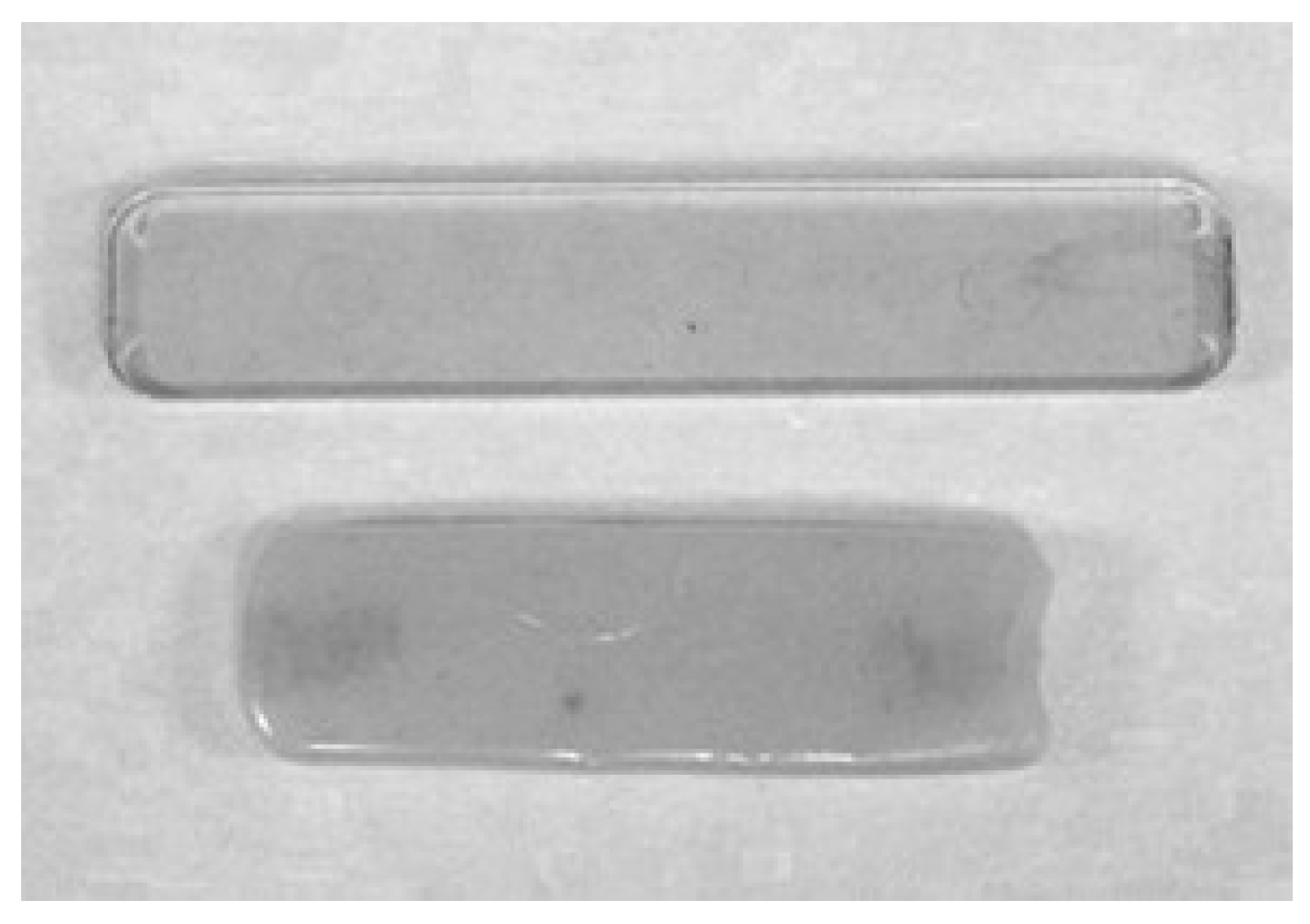
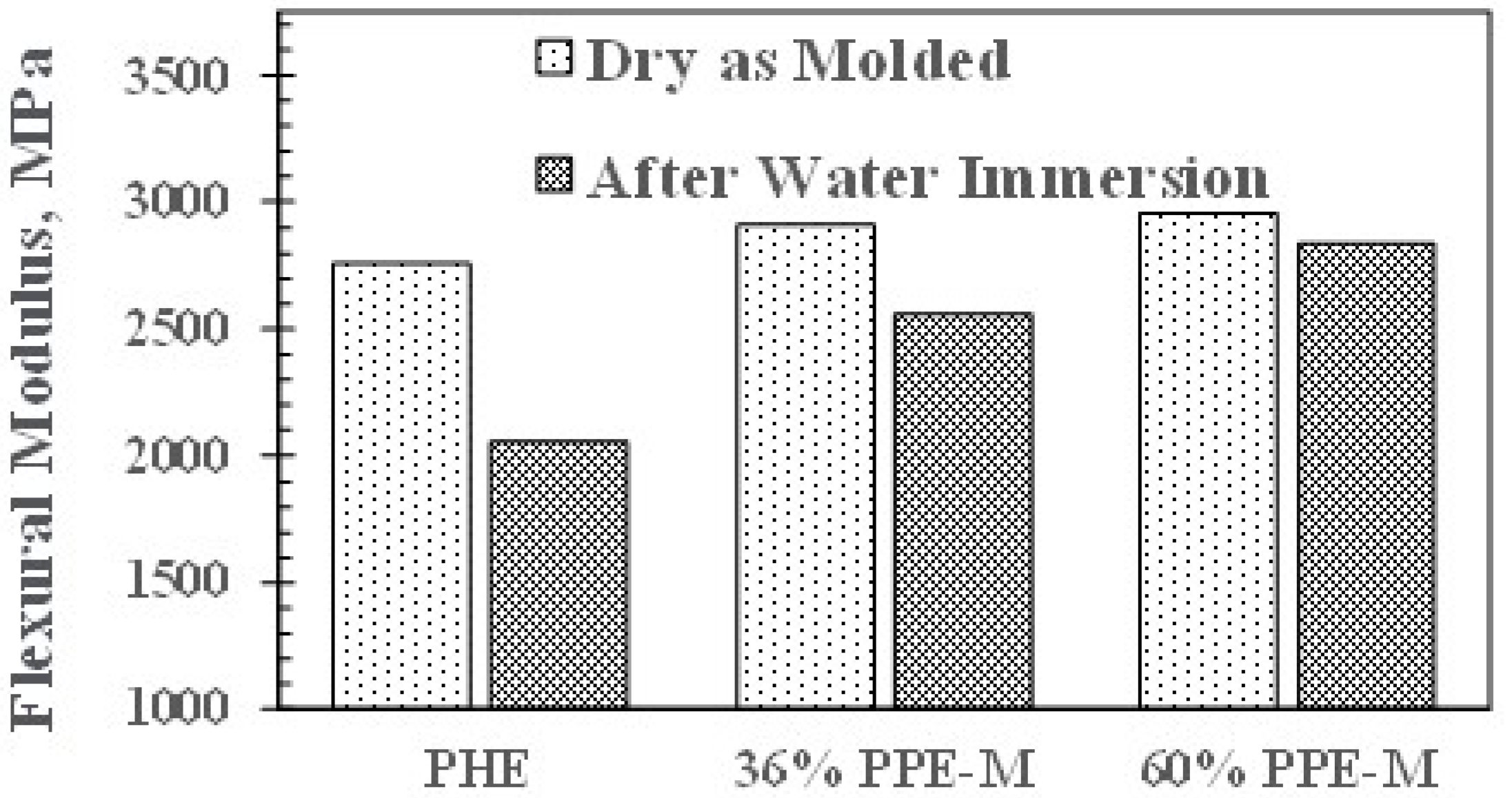
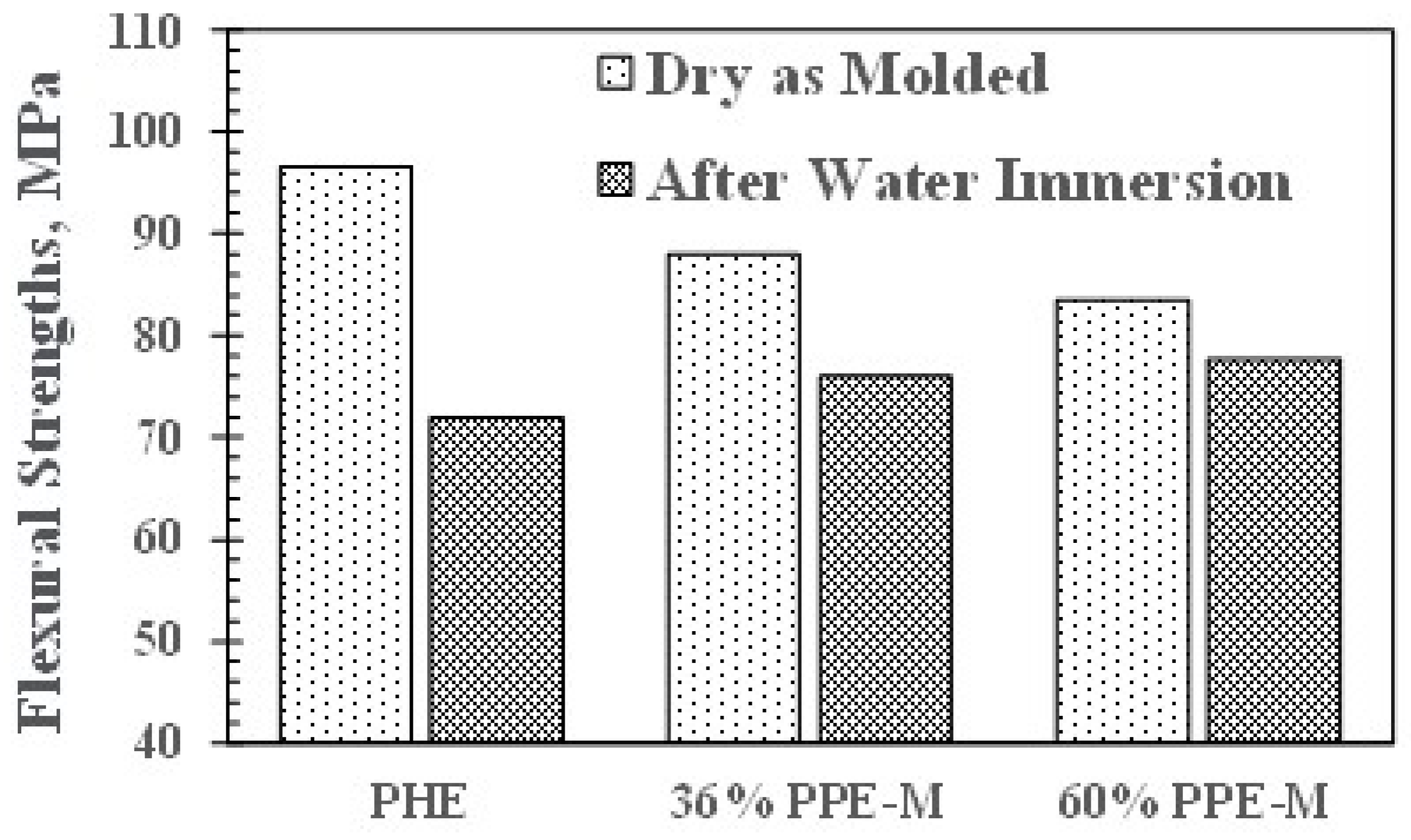
3.2. Blends, Alloys, and Composites of PPE-PHE Block Copolymers
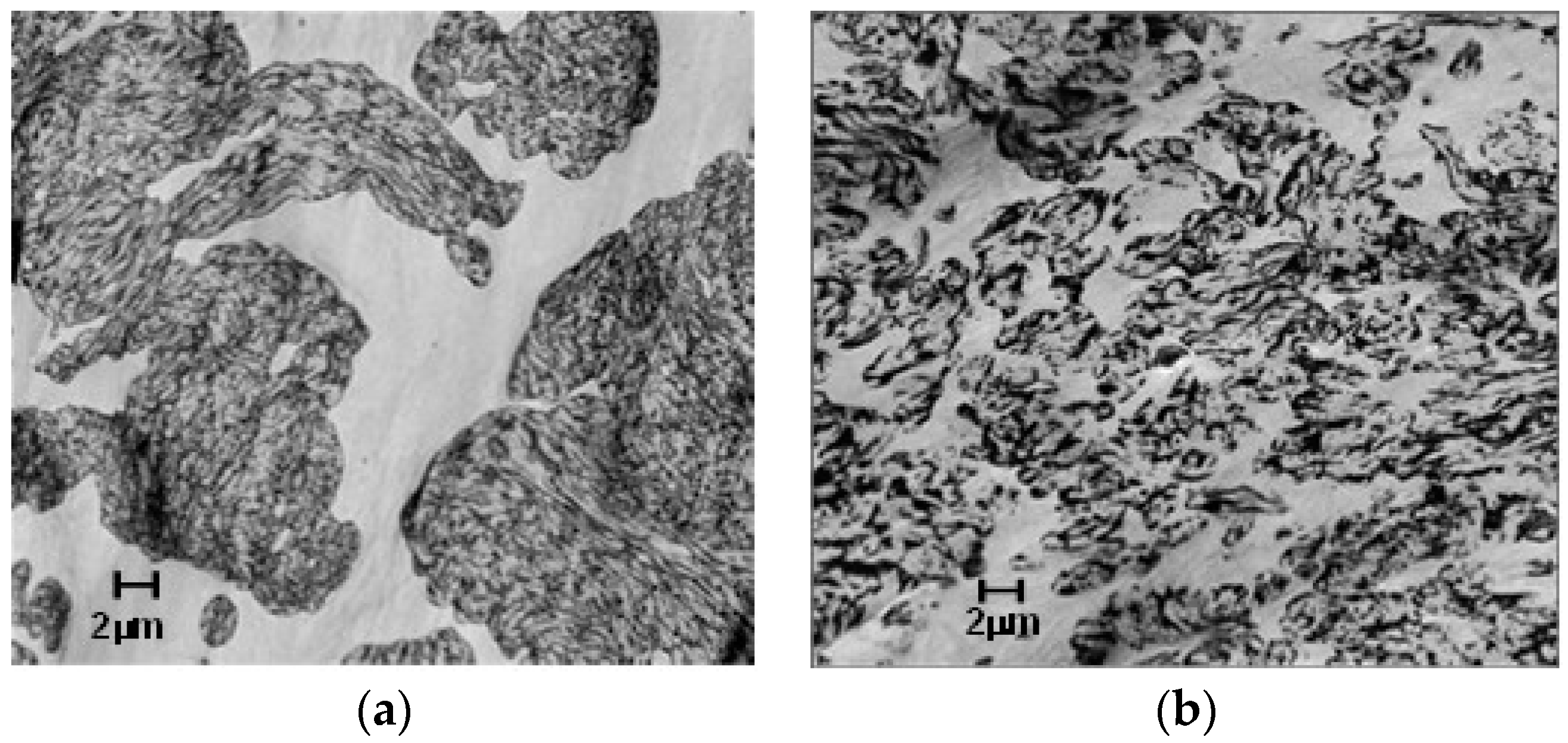
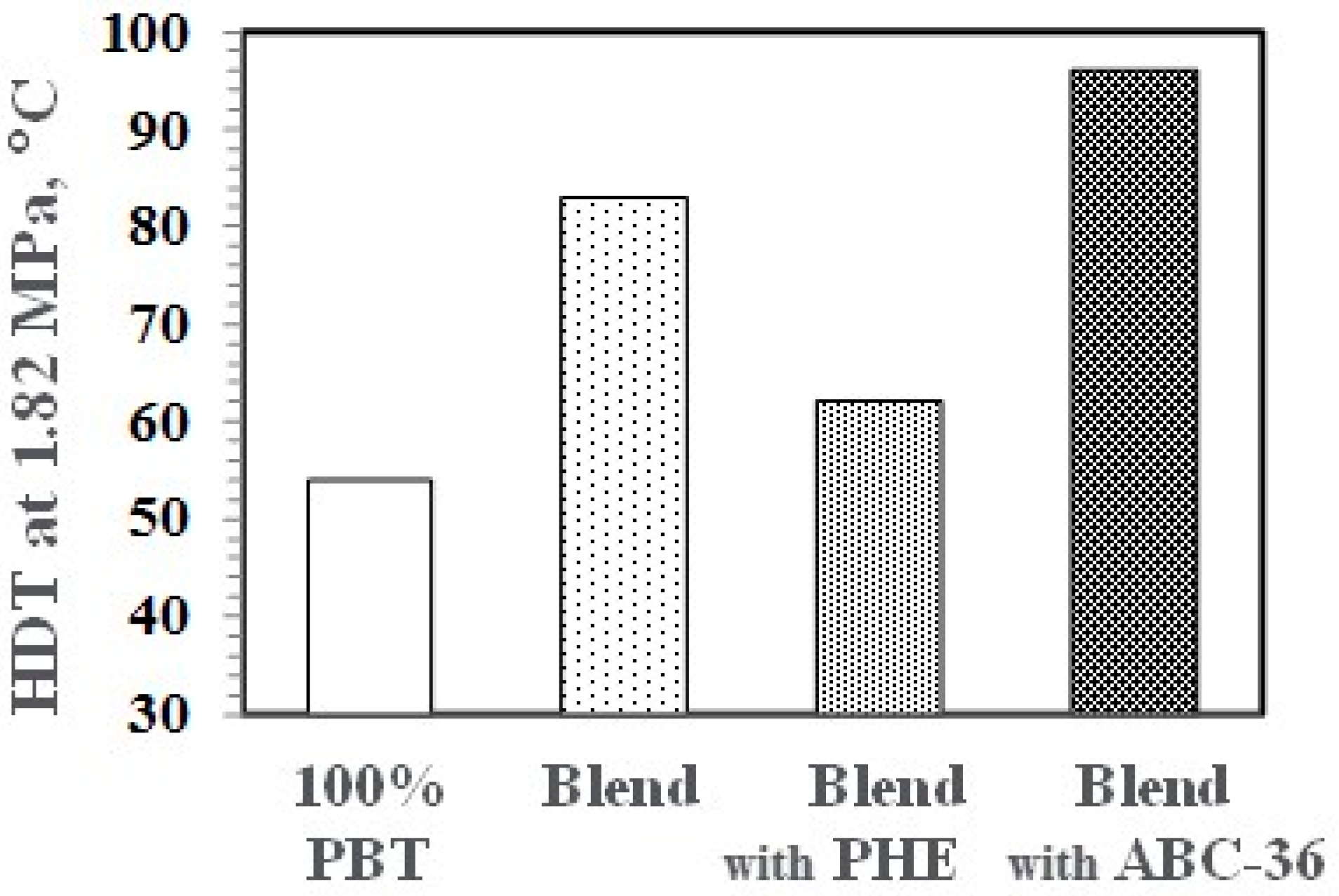
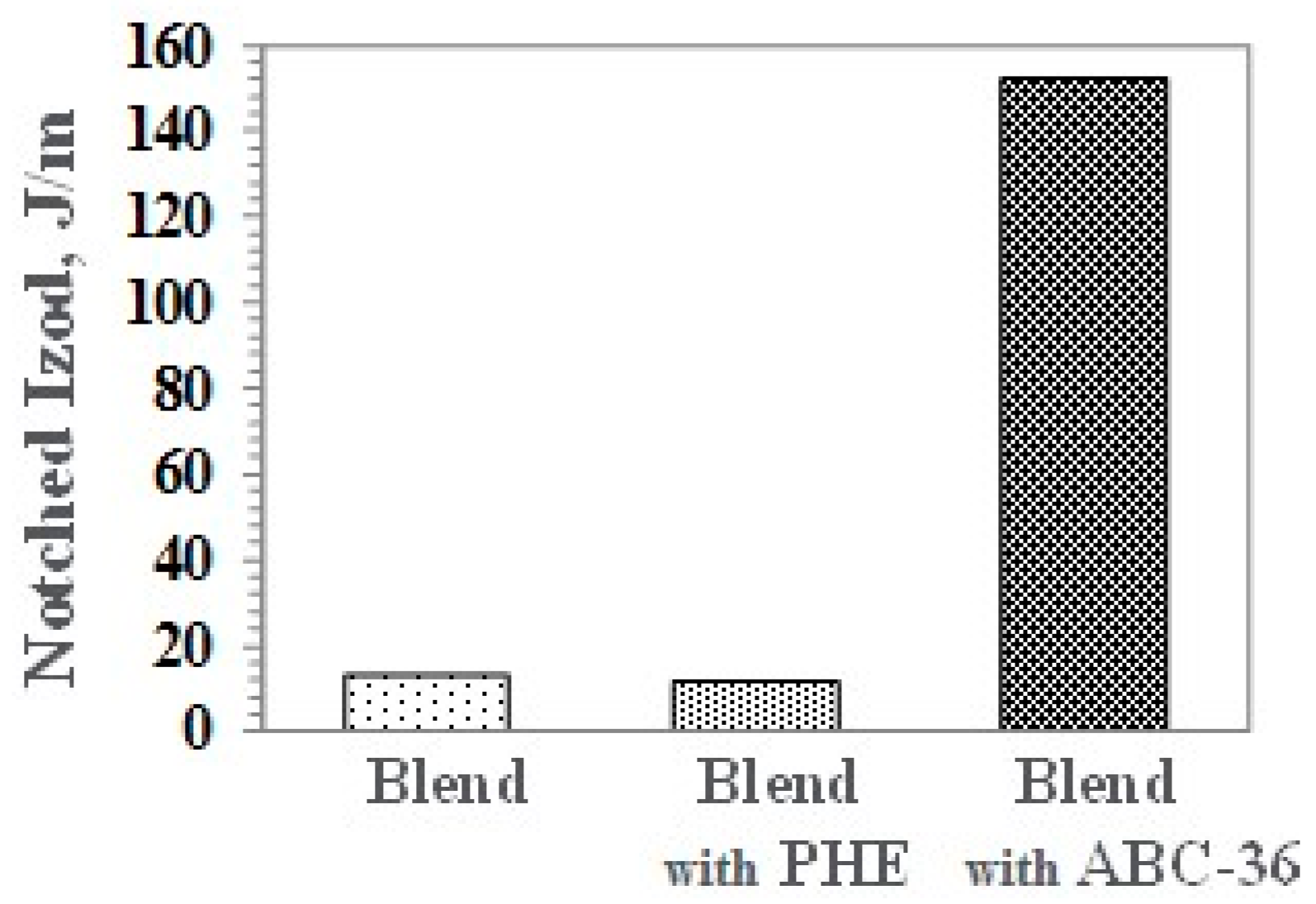
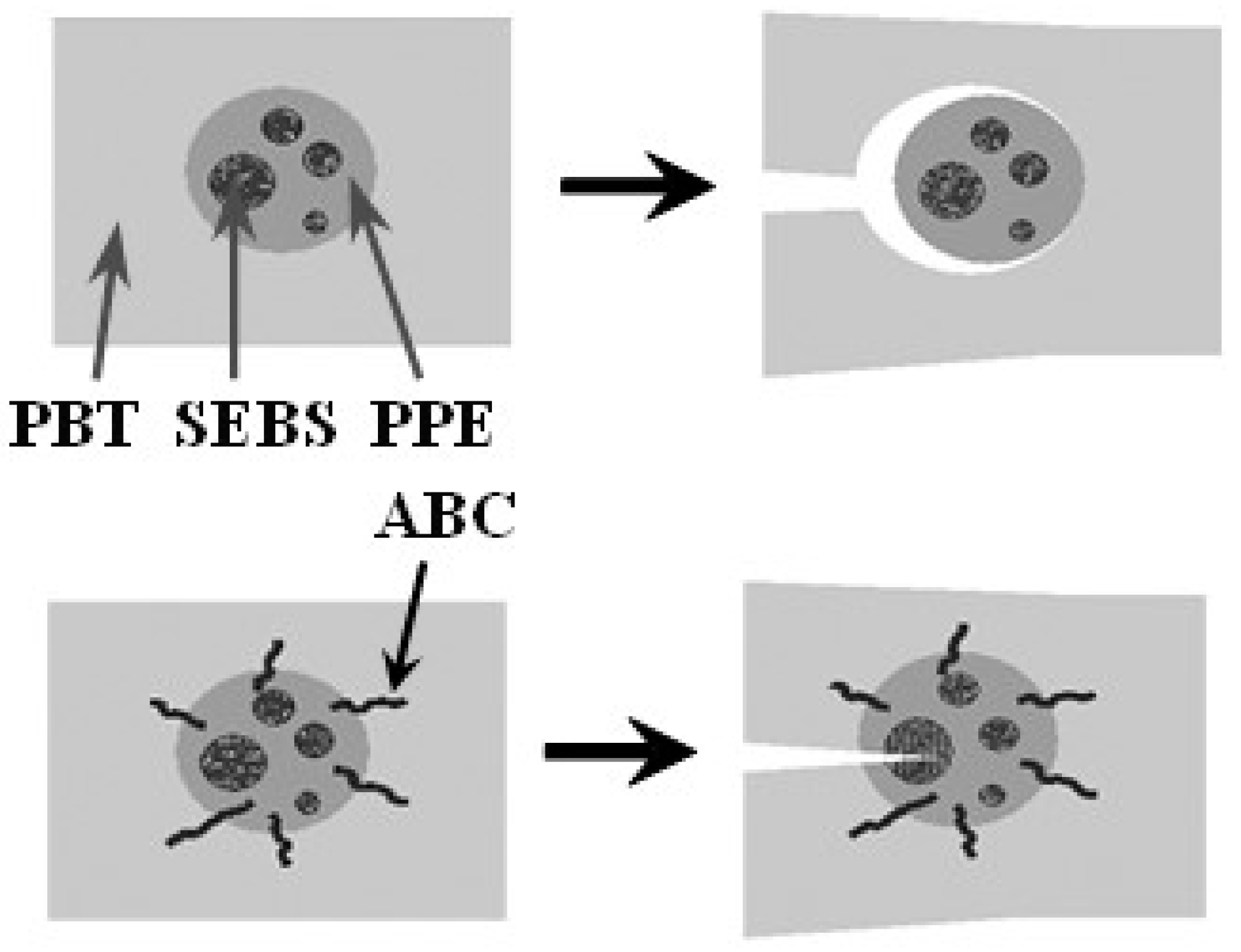
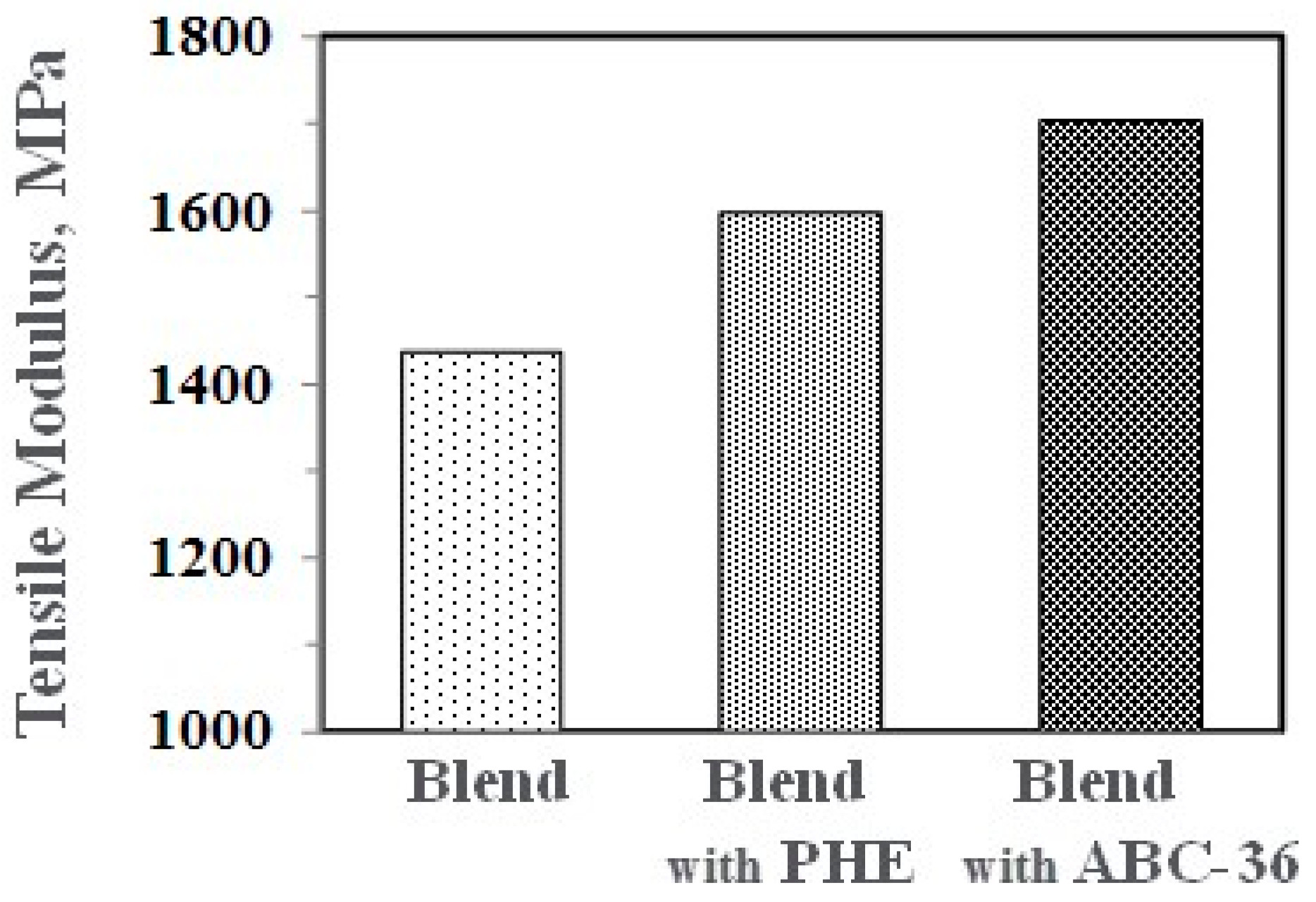
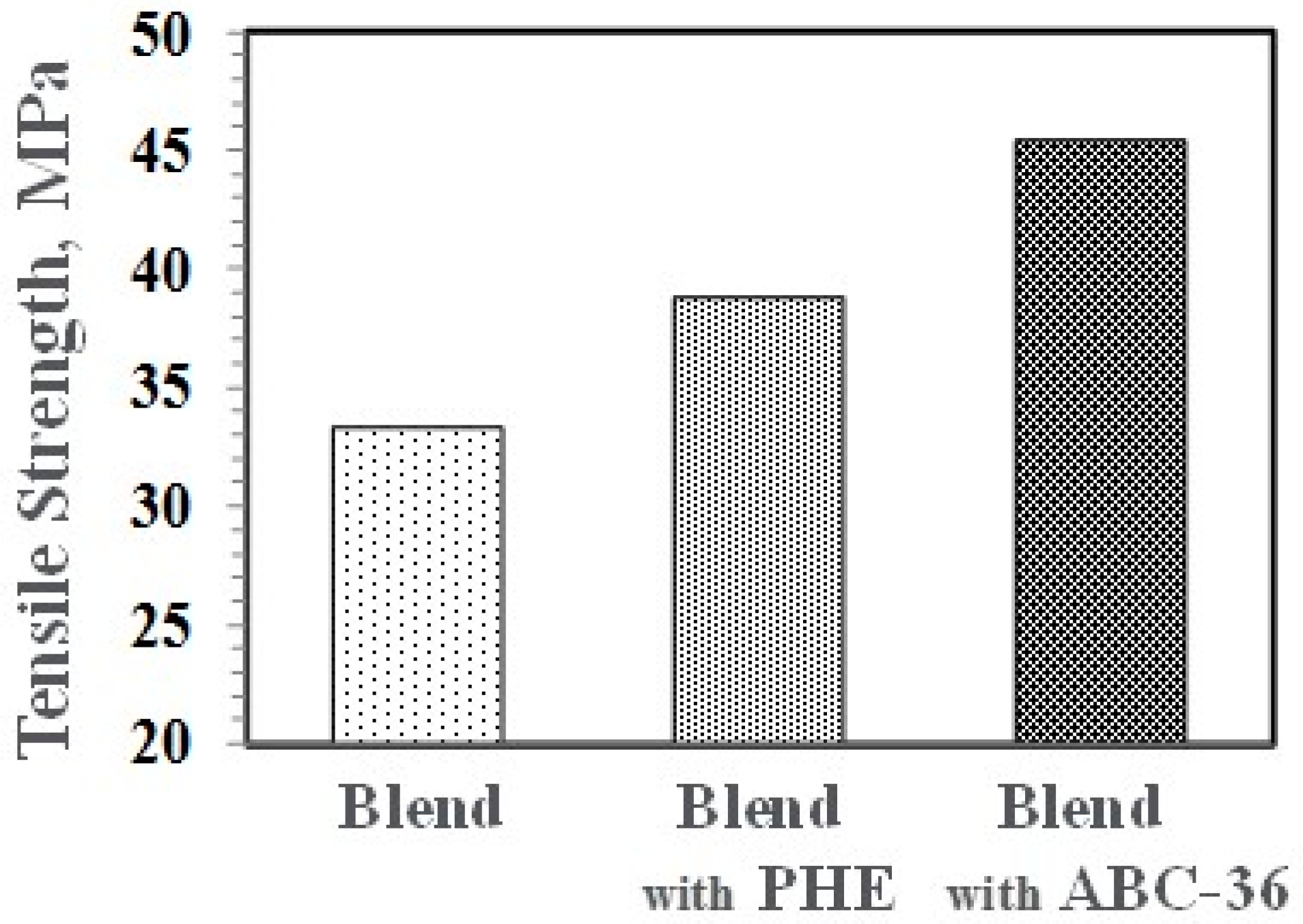
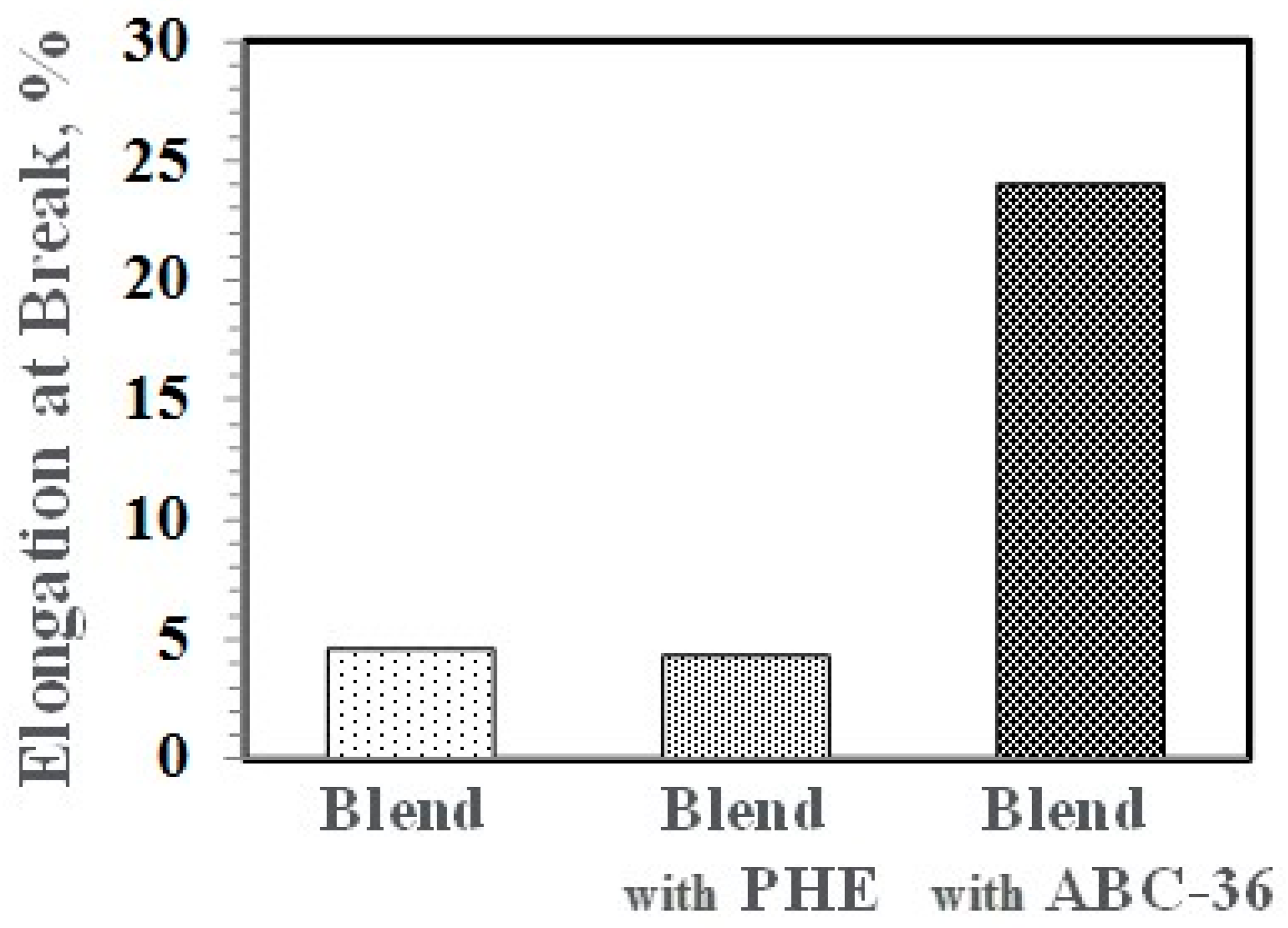
3.2.1. Alloys with Poly(butylene terephthalate)
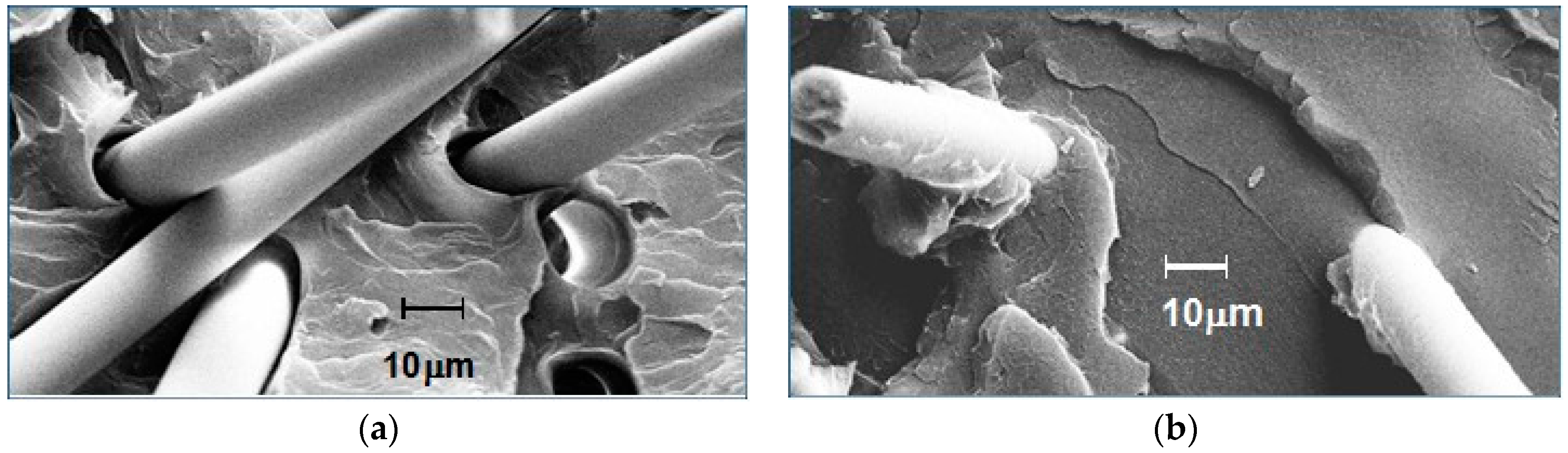
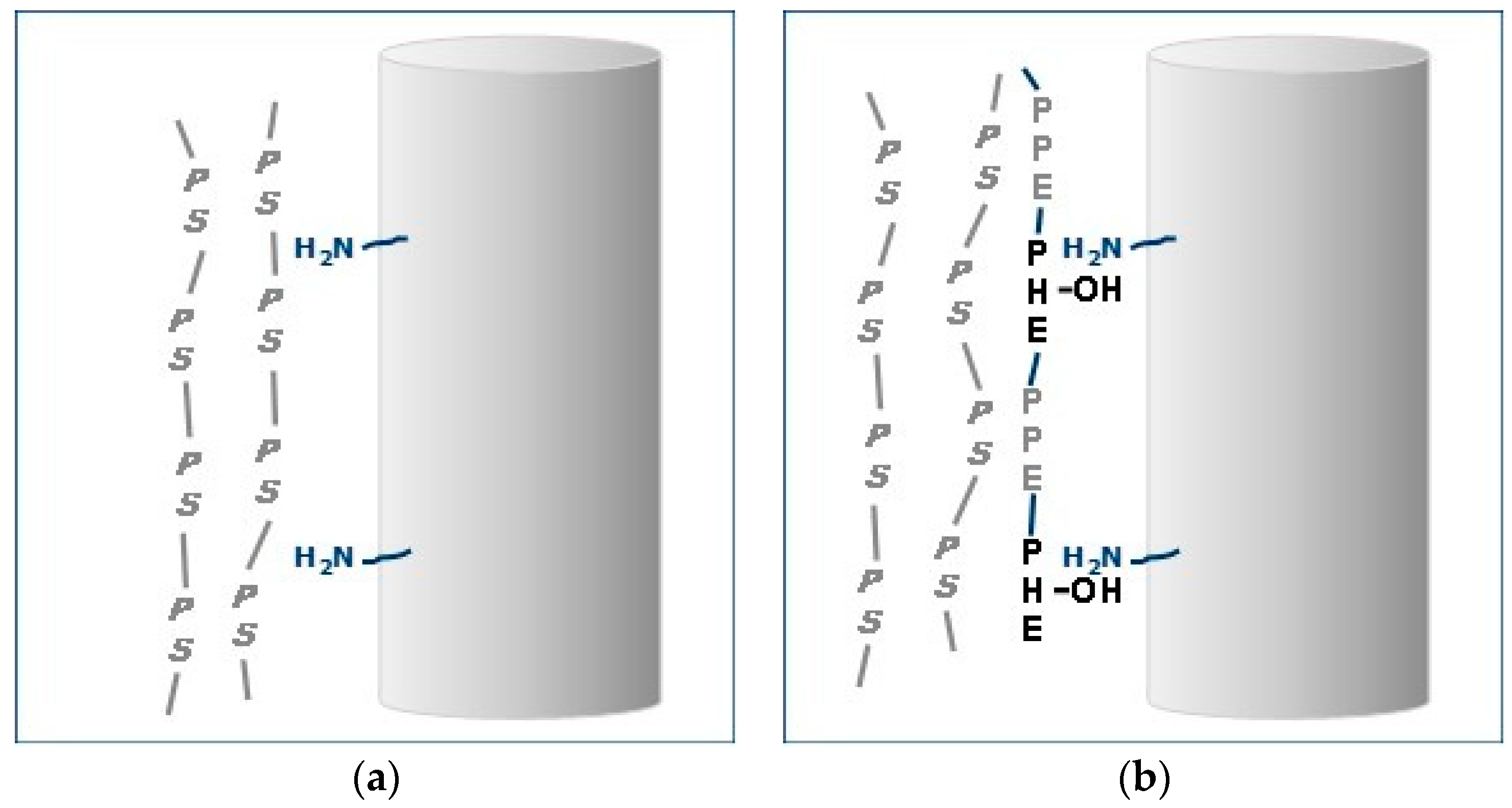
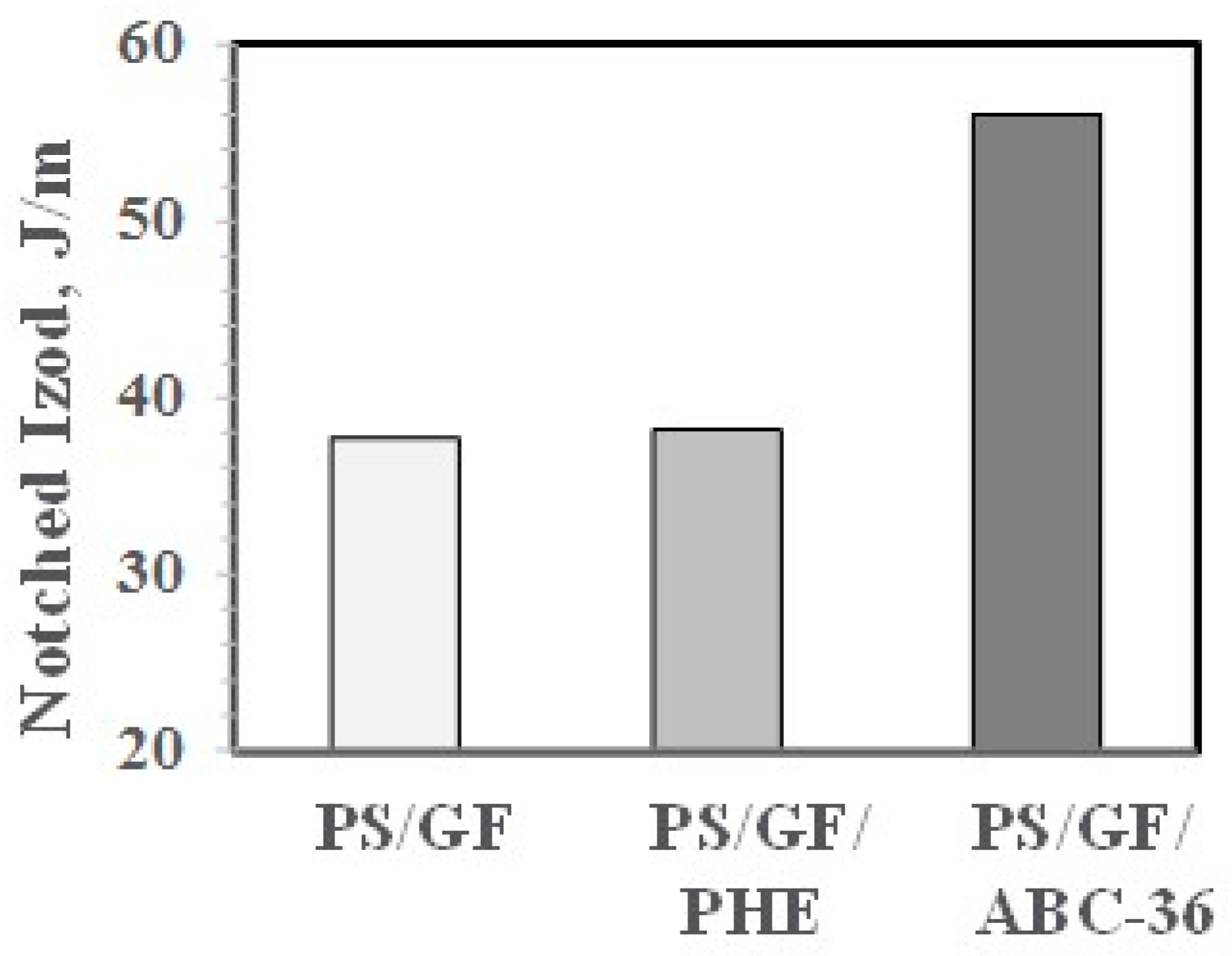
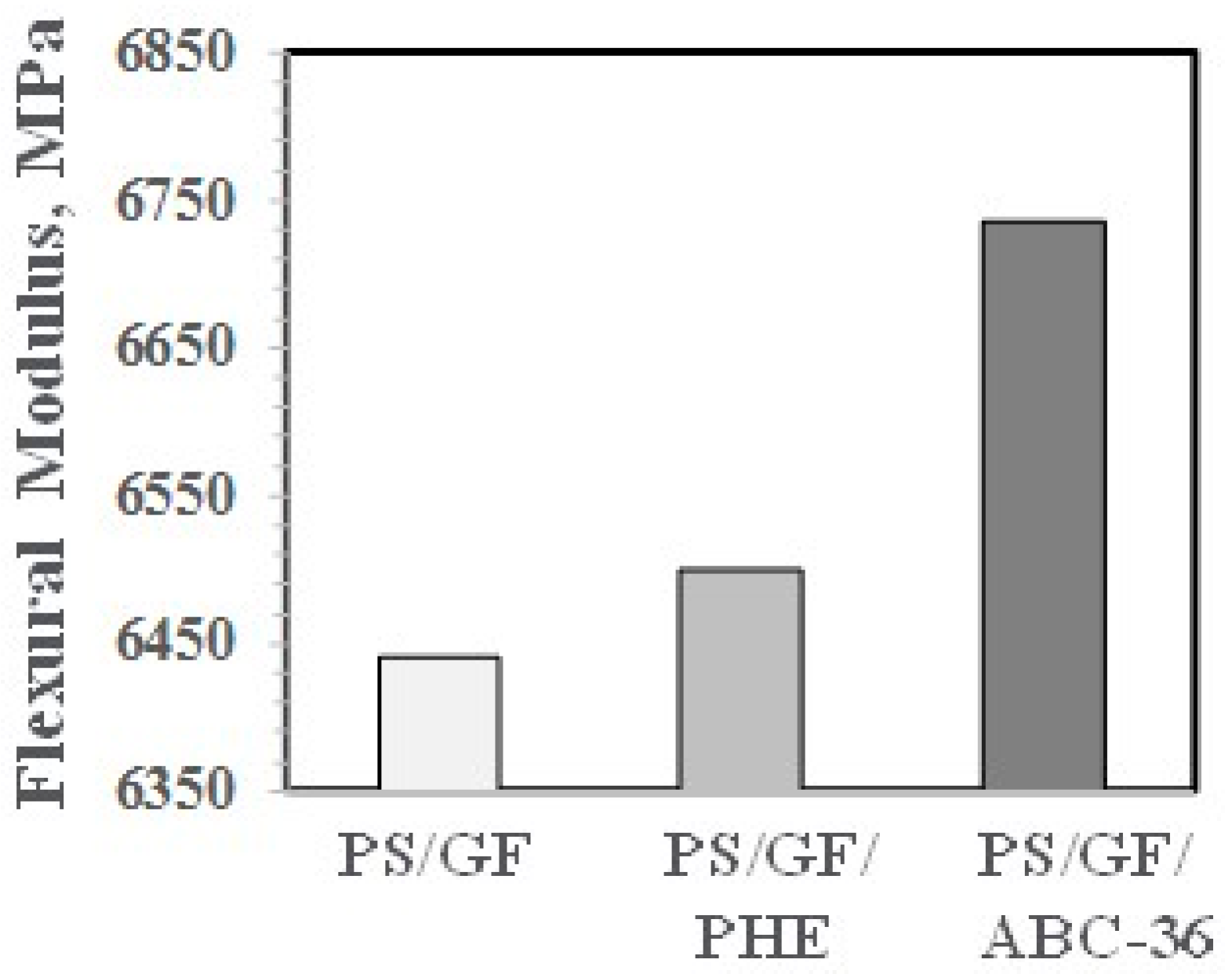
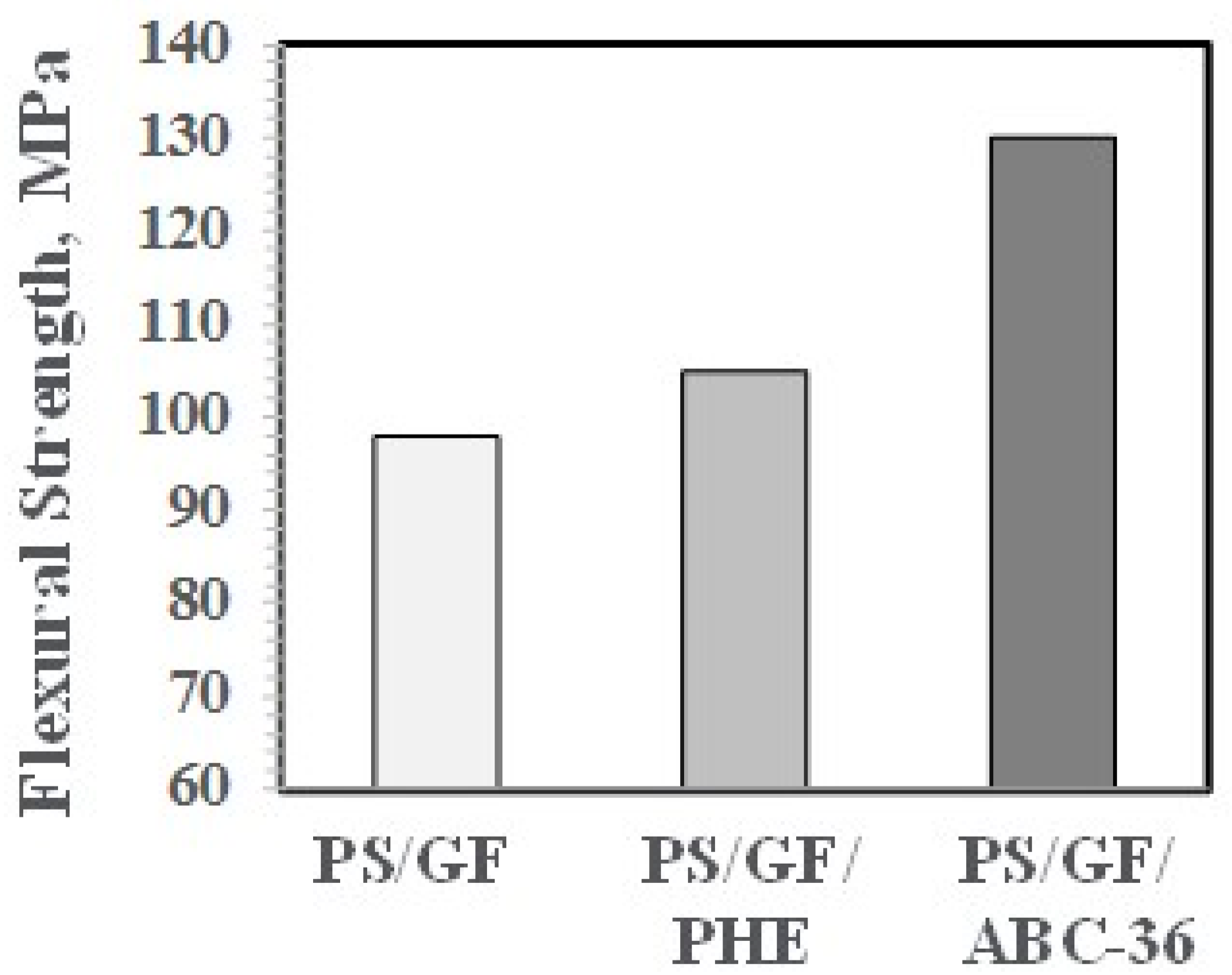
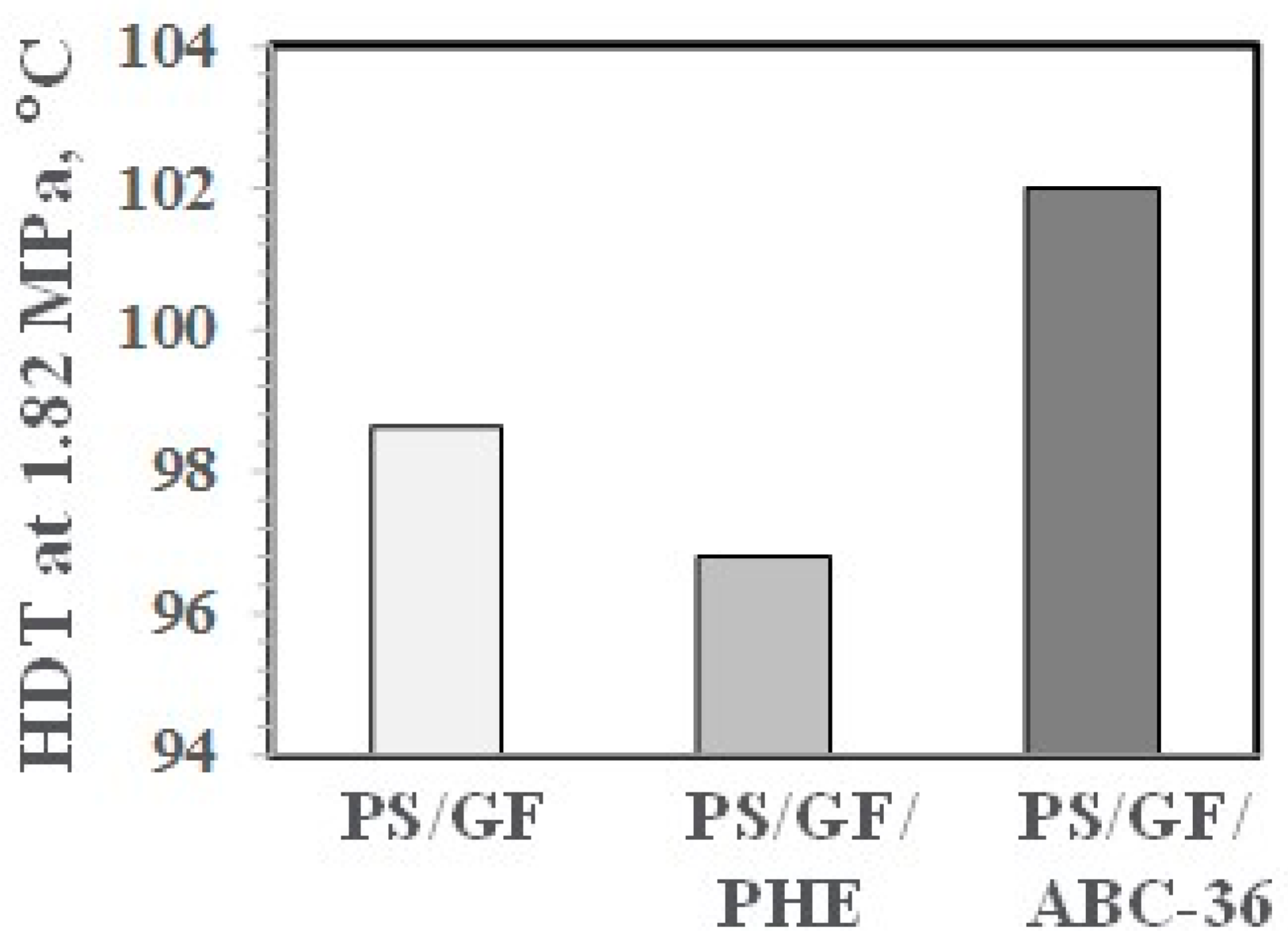
3.2.2. Interface in Composites—Glass Reinforced Polystyrene
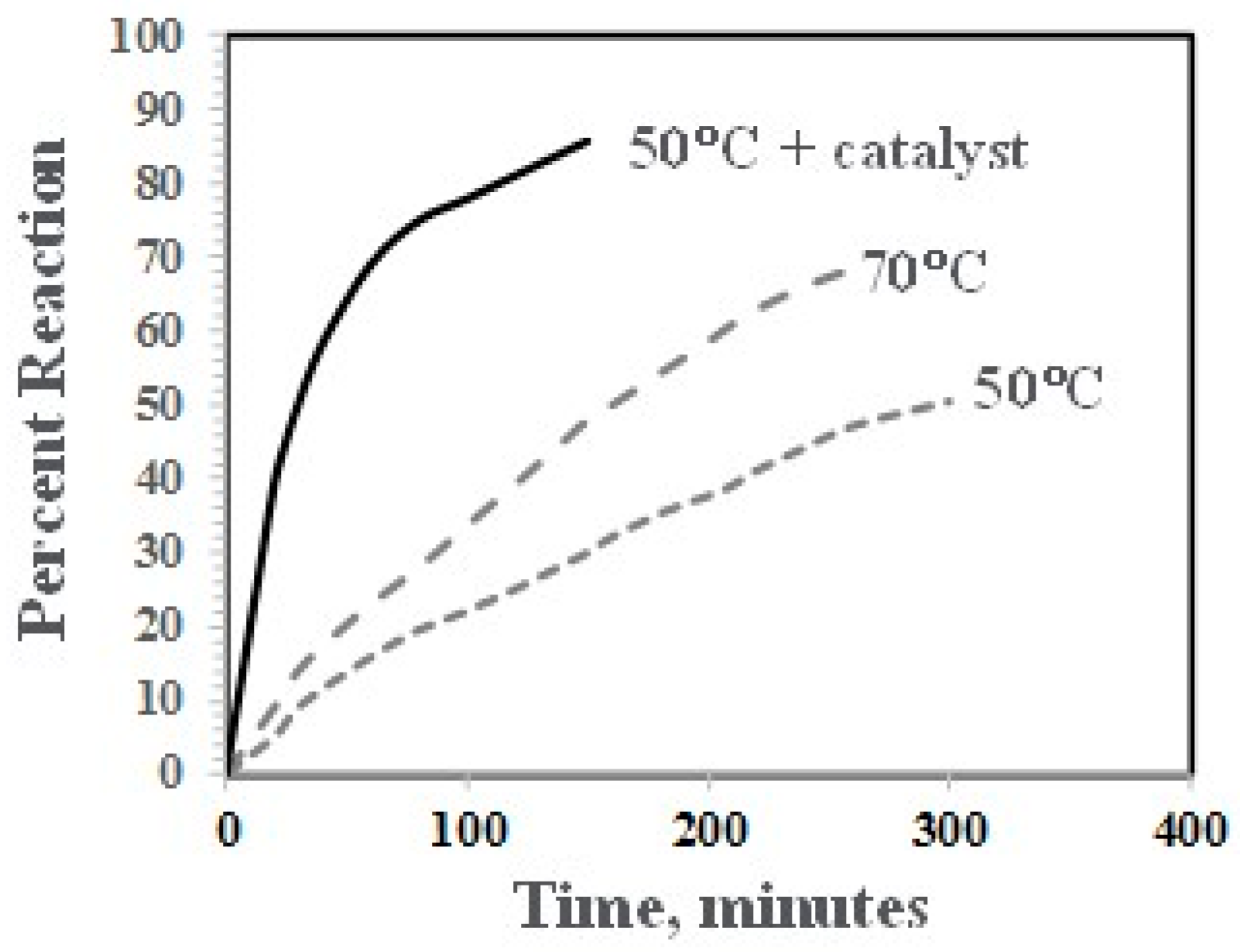
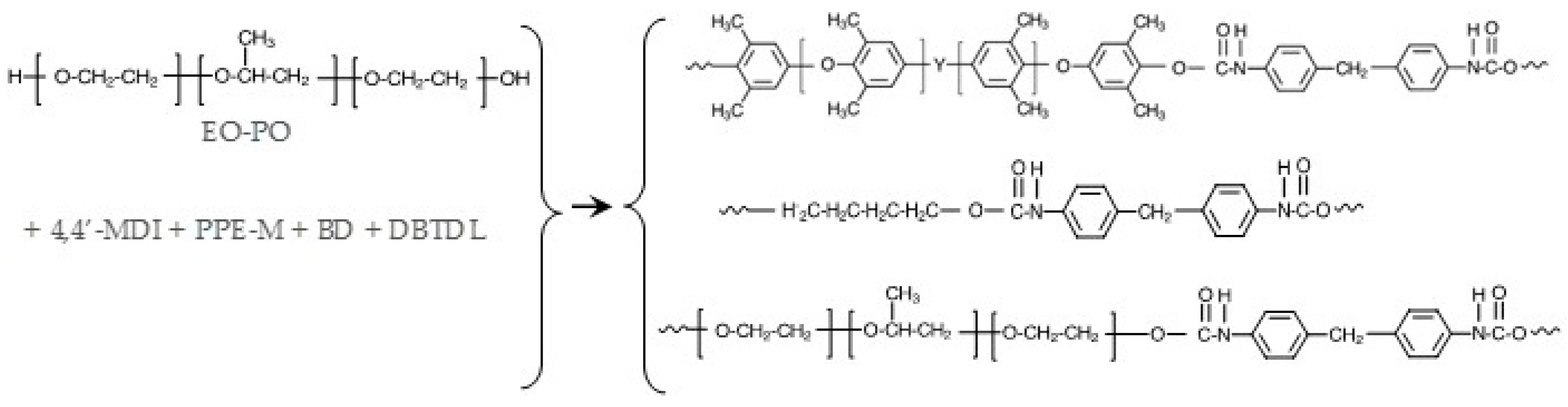
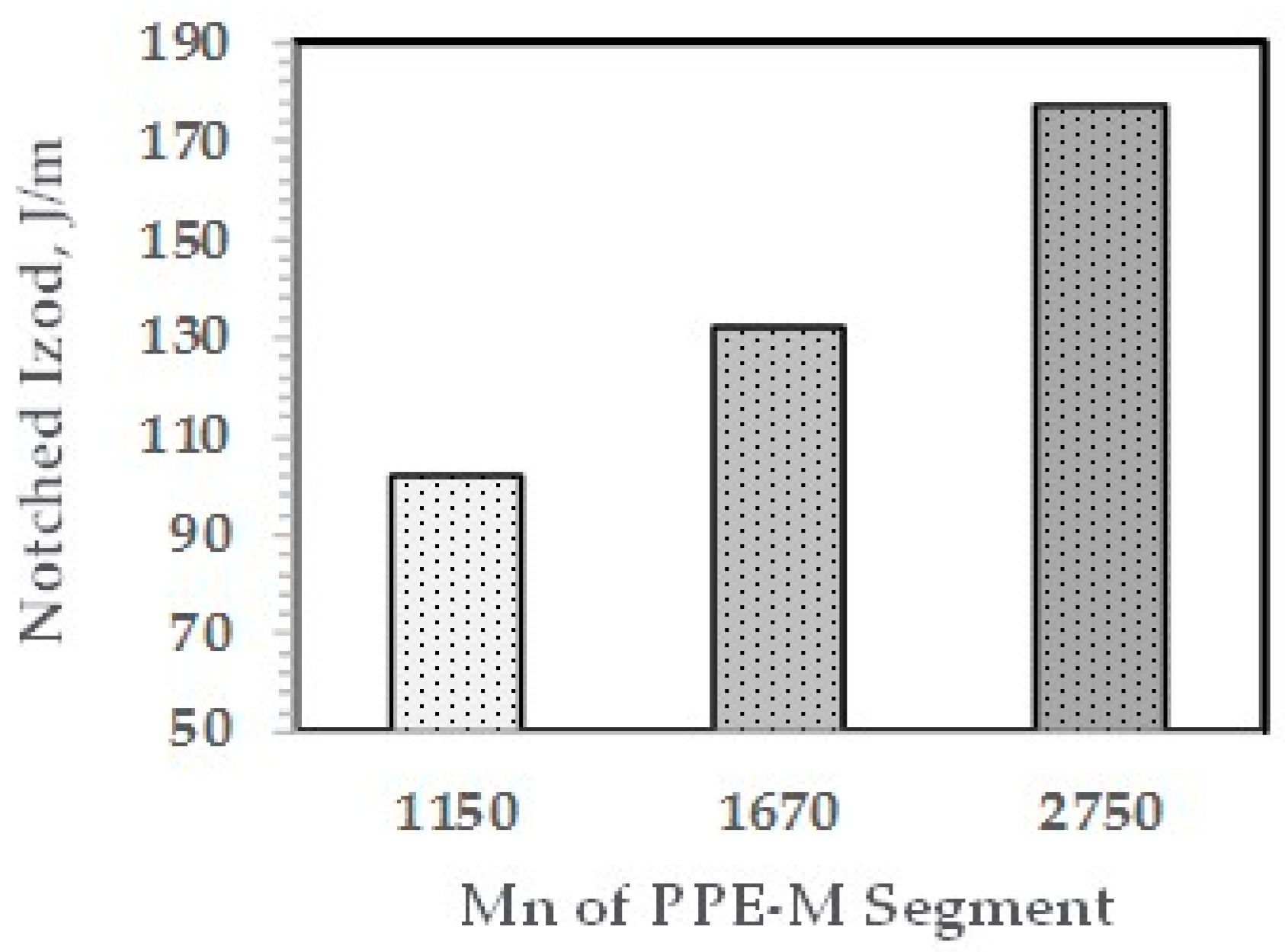
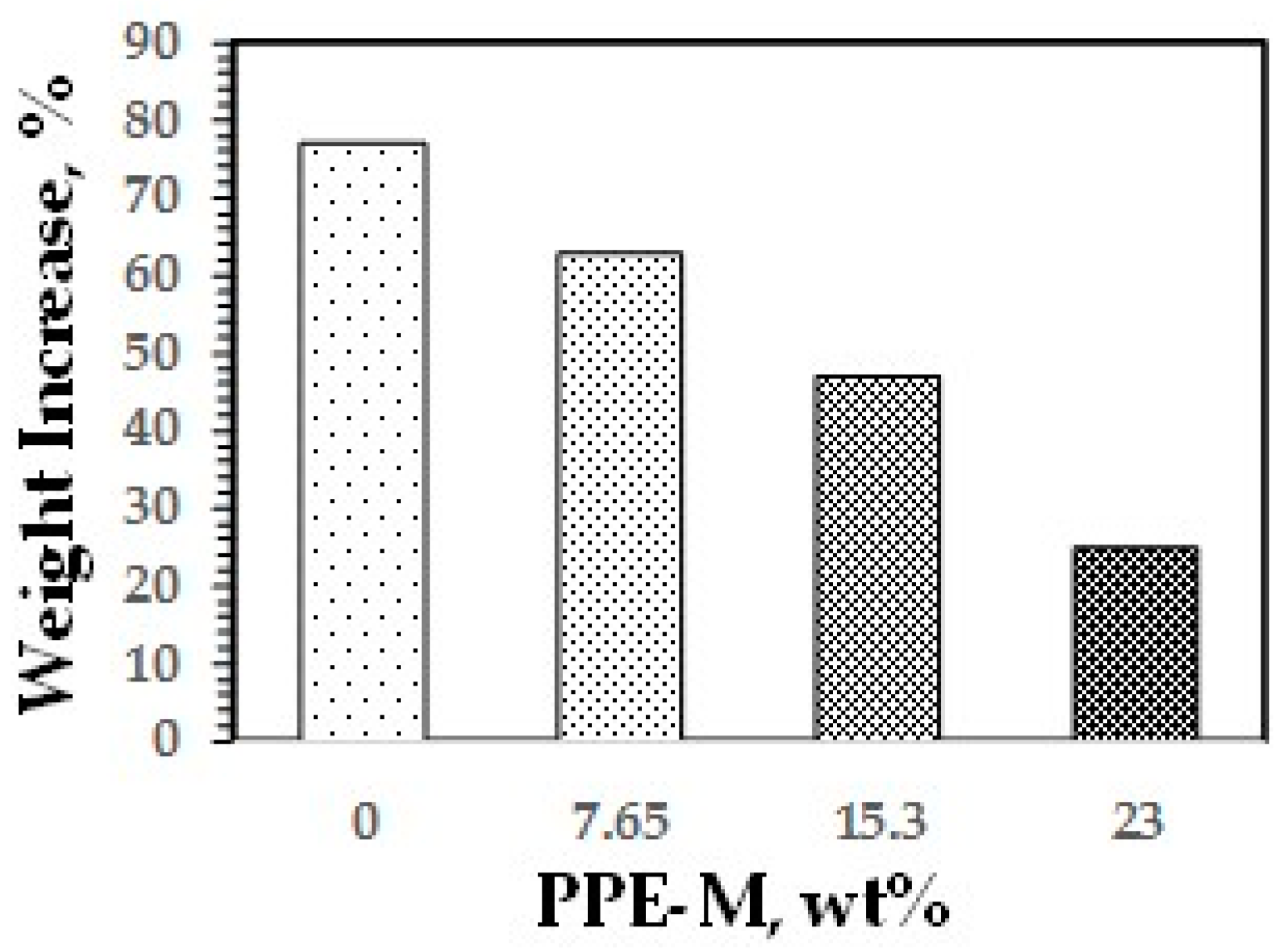
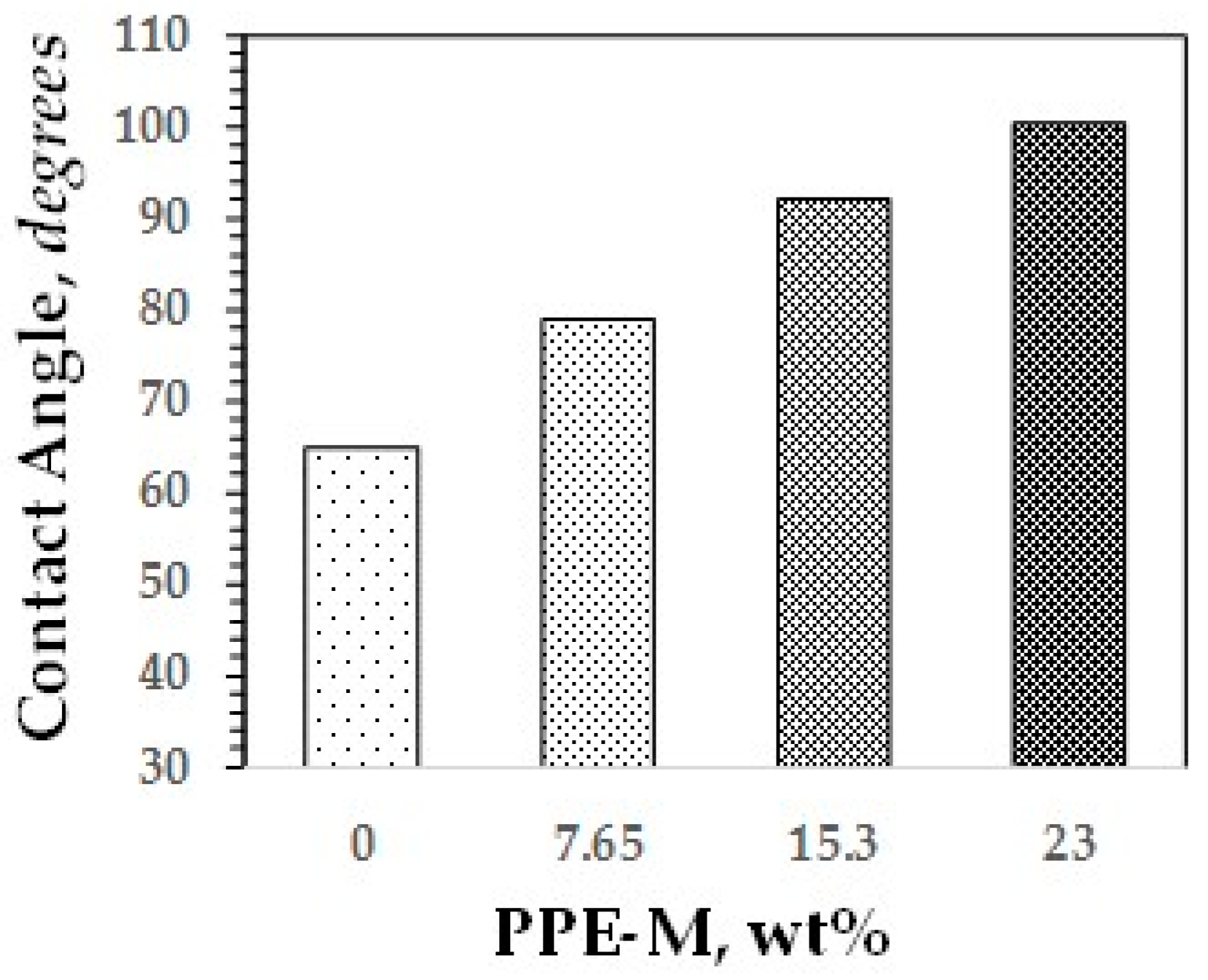
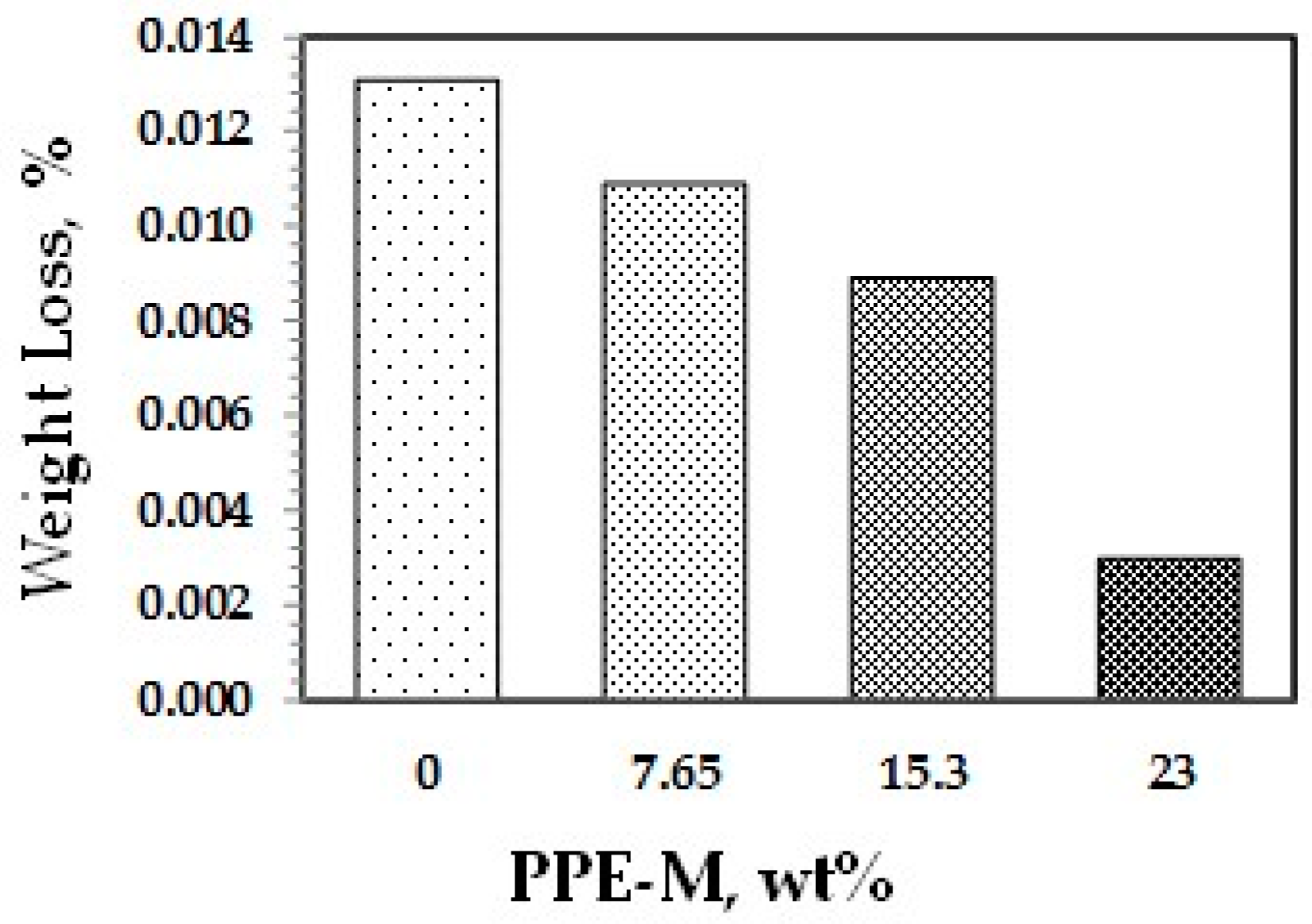
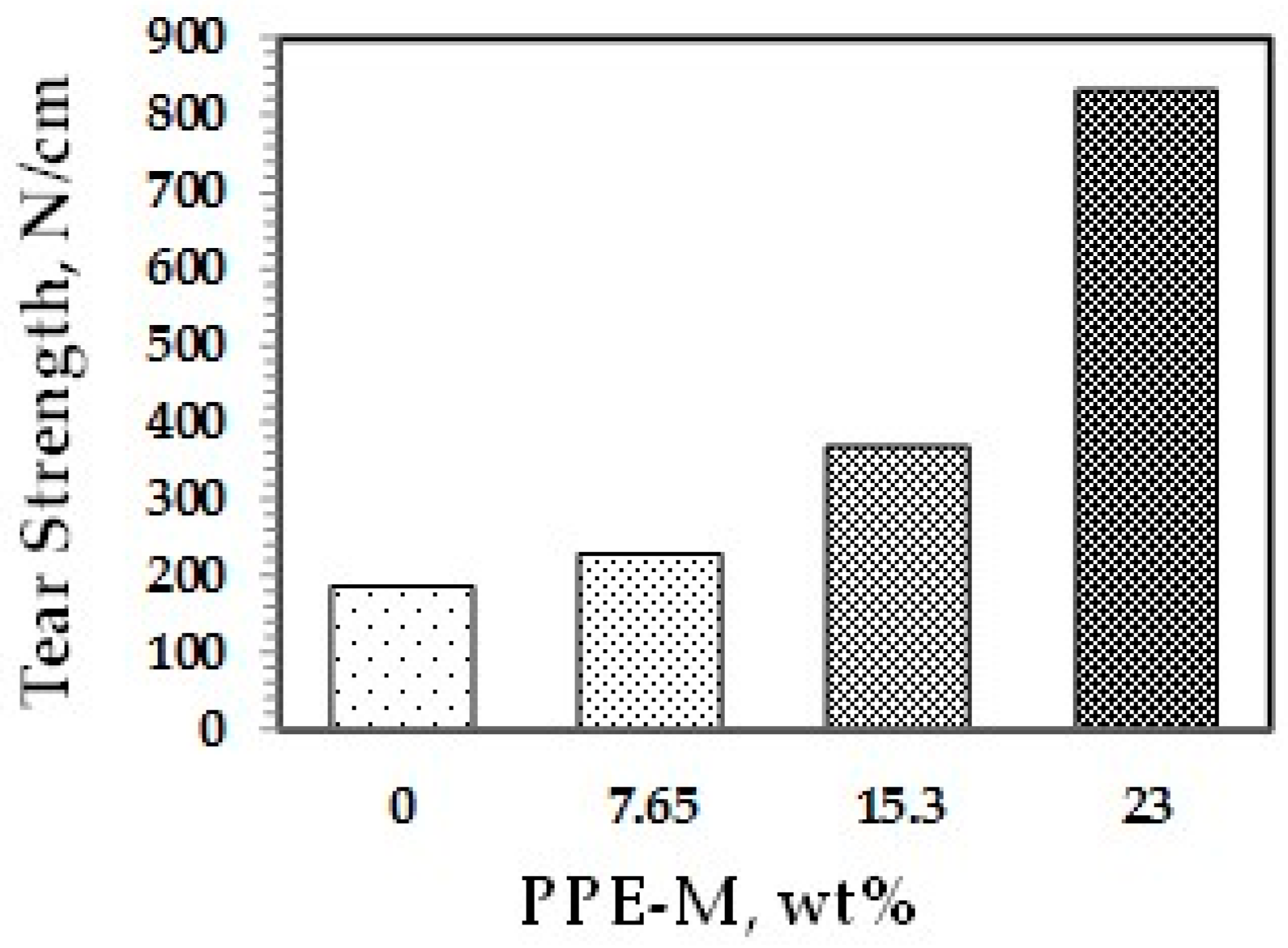
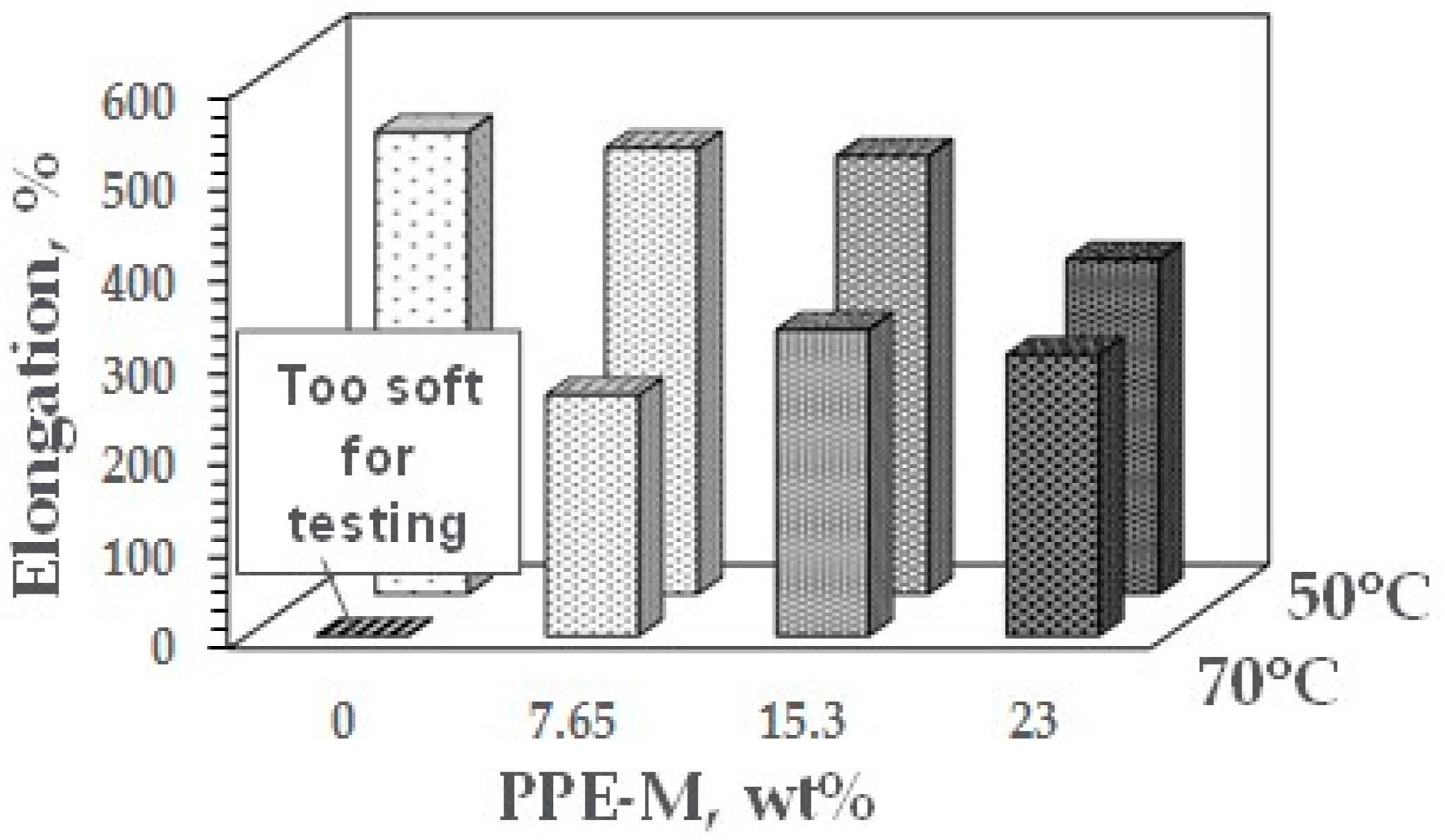
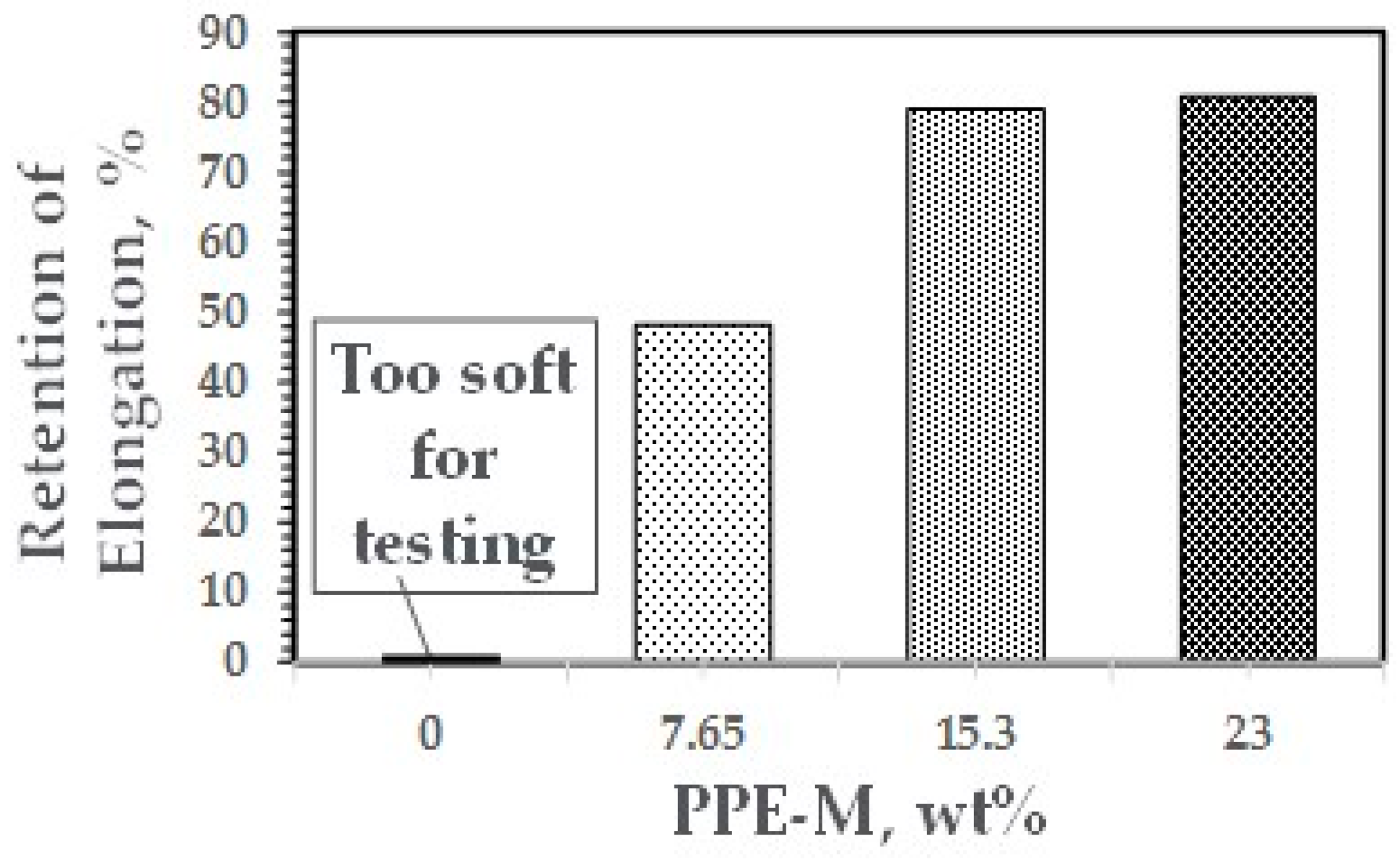
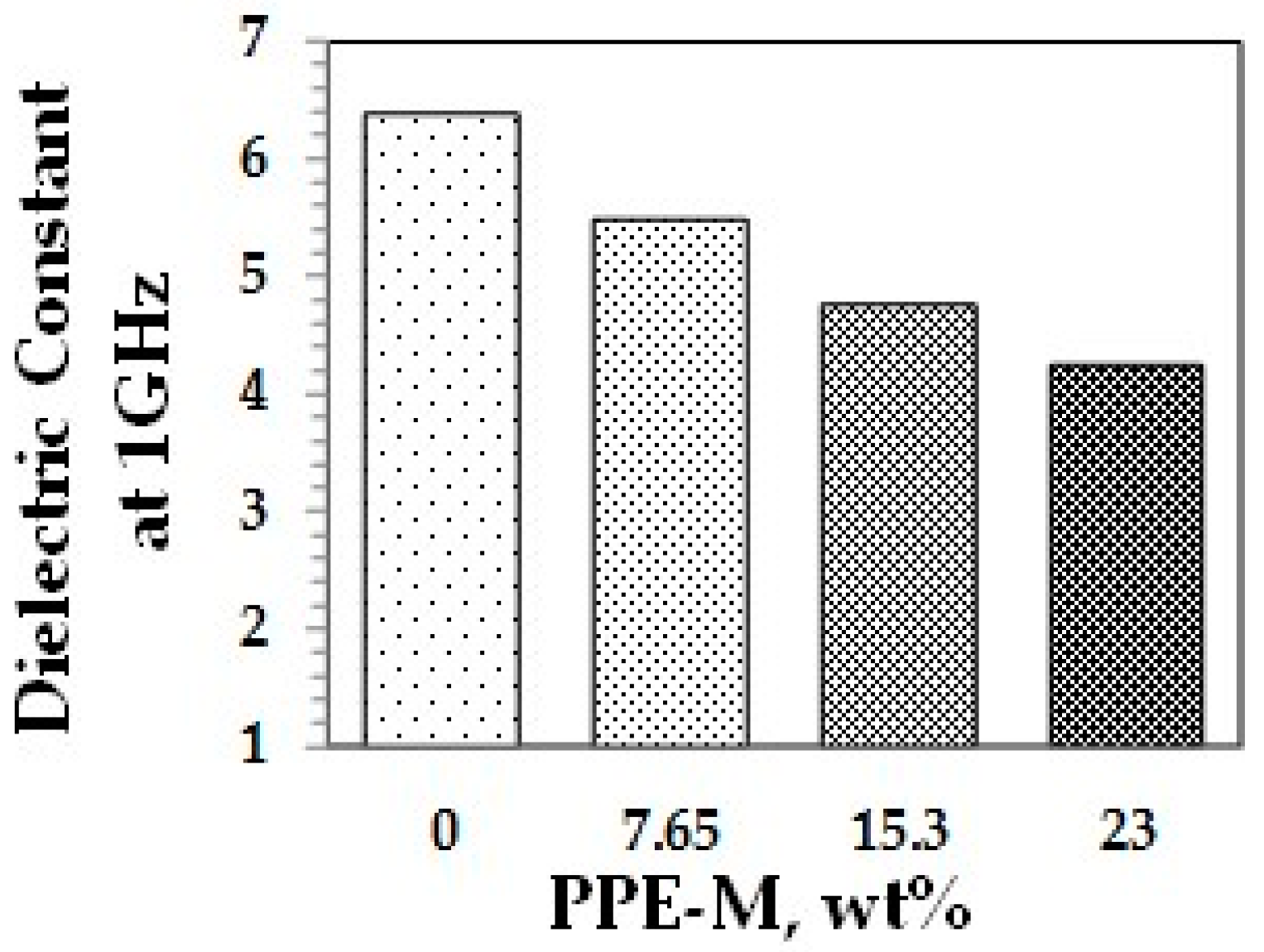
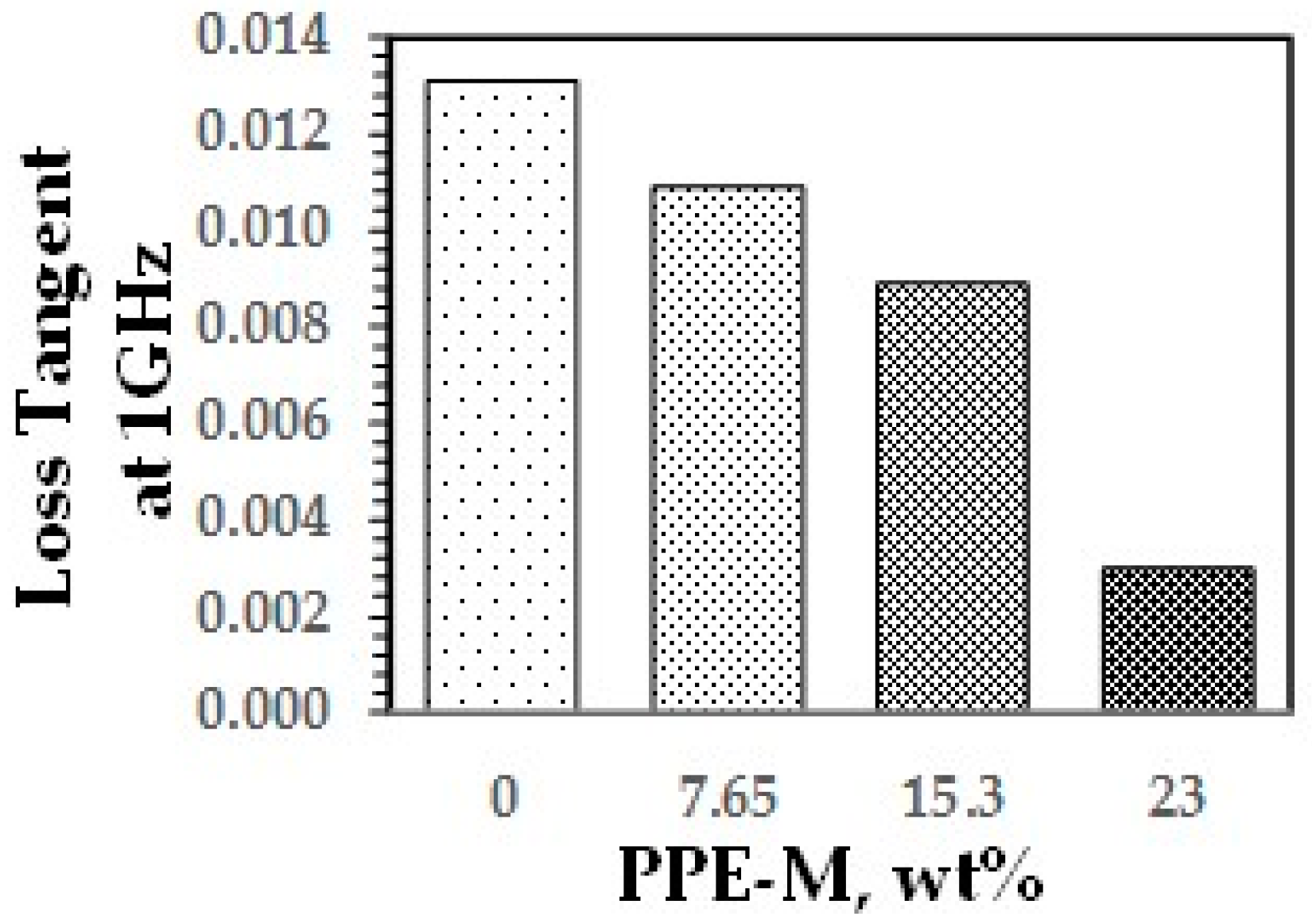
3.3. Thermoplastic Polyurethanes
4. Conclusions
Disclaimer
Conflicts of Interest
References
- Peters, E.N. Polyphenylene Ether Blends and Alloys. In Engineering Plastics Handbook Thermoplastics, Properties, and Applications; Margolis, J., Ed.; McGraw-Hill: New York, NY, USA, 2006; pp. 181–220. [Google Scholar]
- Peters, E.N.; Arisman, R.K. Engineering Thermoplastics. In Applied Polymer Science—21st Century; Craver, C.D., Carraher, C.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; pp. 177–196. [Google Scholar]
- Peters, E.N. Plastics: Thermoplastics, Thermosets, and Elastomers. In Handbook of Materials Selection; Kutz, M., Ed.; Wiley-Interscience: New York, NY, USA, 2002; pp. 335–355. [Google Scholar]
- Peters, E.N. Poly(2,6-dimethyl-1,4-phenylene oxide). In Polymer Data Handbook, 2nd ed.; Mark, J.E., Ed.; Oxford University Press: Oxford, UK, 2009; pp. 534–538. [Google Scholar]
- Peters, E.N. Engineering Thermoplastics—Materials, Properties, Trends. In Applied Plastics Engineering Handbook, 2nd ed.; Kutz, M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 3–26. [Google Scholar]
- Peters, E.N.; Kruglov, A.; Delsman, E.; Guo, H.; Carrillo, A.; Rocha, G. Polyphenylene Ether Macromonomers. I. Property Enhancements in Thermoset Resins via Novel Telechelic Oligomers. In Proceedings of the 65th Annual Technical Conference & Exhibition, Society of Plastics Engineers, Cincinnati, OH, USA, 6–11 May 2007; pp. 2125–2128. [Google Scholar]
- Peters, E.N.; Fisher, S.M.; Guo, H. Polyphenylene Ether Macromonomers. III. Enhancement of Dielectric Materials. In Proceedings of the IPC Printed Circuits EXPO & APEX, Las Vegas, NV, USA, 31 March–2 April 2009. [Google Scholar]
- Peters, E.N.; Fisher, S.M.; Jestel, N.; Pietrafesa, M.; Guo, H. Polyphenylene Ether Macromonomers. II. Property Enhancements in Cyanate Ester Resins. In Proceedings of the 67th Annual Technical Conference & Exhibition, Society of Plastics Engineers, Chicago, IL, USA, 22–24 June 2009; pp. 611–614. [Google Scholar]
- Peters, E.N.; Fisher, S.M.; Guo, H.; Degonzague, C.; Howe, R. Polyphenylene Ether Macromolecules. VII. Performance in t-Butyl Styrene/Divinyl Benzene Resin System. In Proceedings of the 68th Annual Technical Conference & Exhibition, Society of Plastics Engineers, Orlando, FL, USA, 16–20 May 2010; pp. 1858–1861. [Google Scholar]
- Peters, E.N. PPE Macromonomers. XIV. Structure-Property Relationships in Epoxy Resins. In Proceedings of the Society of Plastics Engineers EUROTEC Conference, Lyon, France, 4–5 July 2013. [Google Scholar]
- Peters, E.N.; Tarkin-Tas, E. PPE Macromonomers—Use in Anhydride cured epoxy. In Proceedings of the 72nd Annual Technical Conference & Exhibition, Society of Plastics Engineers, Las Vegas, NV, USA, 28–30 April 2014. [Google Scholar]
- Peters, E.N.; Flanagan, J.; Denniston, M. Polyphenylene Ether Macromonomers: XIV. Preparation of Polyhydroxyether Block Copolymers. In Proceedings of the 70th Annual Technical Conference & Exhibition, Society of Plastics Engineers, Orlando, FL, USA, 2–4 April 2012. [Google Scholar]
- DeKok, R.; Delsman, E.R.; Freshour, A.R.; Toublan, J.-J.F.; McCloskey, P.J.; Peters, E.N. Poly(arylene ether) Block Copolymer Compositions, Methods, and Articles. U.S. Patent Application 20080275185, 6 November 2008. [Google Scholar]
- Peters, E.N. Polyurethane Foam and Associated Method and Article. U.S. Patent 9,266,997, 23 February 2016. [Google Scholar]
- Peters, E.N. Rigid Foam and Associated Article. U.S. Patent 9,169,368, 27 October 2016. [Google Scholar]
- Peters, E.N. Thermoplastic Polyurethane and Associated Method and Article. U.S. Patent 9,422,394, 23 August 2016. [Google Scholar]
- Peters, E.N. Triblock Copolymer, Method for Its Formation, and Compatibilized Compositions Comprising It. U.S. Patent 8,865,823, 21 October 2014. [Google Scholar]
- Reinking, N.H.; Barnabeo, A.E.; Hale, W.F. Polyhydroxyethers. I. Effect of Structure on Properties of High Molecular Weight Polymers from Dihydric Phenols and Epichlorohydrin. J. Appl. Polym. Sci. 1963, 7, 2135–2144. [Google Scholar] [CrossRef]
- Batzer, H.; Zahir, S.A. Studies in the molecular weight distribution of epoxide resins. III. Gel permeation chromatography of epoxide resins subject to post glycidylation. J. Appl. Polym. Sci. 1975, 19, 609–617. [Google Scholar]
- Burchard, W.; Bantle, S.; Zahir, S.A. Branching in high molecular weight polyhydroxyethers based on bisphenol A. Makromol. Chem. 1981, 182, 145–163. [Google Scholar] [CrossRef]
- Senger, J.S.; Subramanian, R.; Ward, T.C.; McGrath, J.E. Synthesis and Determination of Chain Branching in Linear High Molecular Weight Polyhydroxyethers. In Polymer Preprints, Division of Polymer Chemistry. Am. Chem. Soc. 1986, 27, 144–146. [Google Scholar]
- ASTM International Voluntary Organization D2572-97. Standard Test Method for Isocyanate Groups in Urethane Materials or Prepolymers; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar]
- Peters, E.N.; Flanagan, J.; Guise, O. Amphiphilic PPE-PHE Block Copolymers as Compatibilizers for Polyester Blends. In Proceedings of the 72nd Annual Technical Conference & Exhibition, Society of Plastics Engineer, Las Vegas, NV, USA, 28–30 April 2014. [Google Scholar]
- Peters, E.N.; Bajaj, P.; Flanagan, J.; Pecak, W. Polyphenylene Ether Macromonomers: XV. Enhancement of PS/GF Composites by PPE-PHE Block Copolymers. In Proceedings of the 72nd Annual Technical Conference & Exhibition, Society of Plastics Engineers, Las Vegas, NV, USA, 28–30 April 2014. [Google Scholar]
- Peters, E.N. Introduction to Polymer Characterization. In Comprehensive Desk Reference of Polymer Characterization and Analysis; Brady, R.F., Jr., Ed.; Oxford University Press: Oxford, UK, 2003; pp. 3–29. [Google Scholar]
- Potschke, P.; Wallheinke, K.; Fritsche, H.; Stutz, H. Morphology and properties of blends with different thermoplastic polyurethanes and polyolefines. J. Appl. Polym. Sci. 1997, 64, 749–762. [Google Scholar] [CrossRef]
- Shonaike, G.O.; Simon, G.P. Polymer Blends and Alloys; Society of Plastics Engineers: Brookfield, CT, USA, 1999. [Google Scholar]
- Olabisi, O.; Robeson, L.M.; Shaw, M.T. Polymer-Polymer Miscibility; Academic Press: Cambridge, MA, USA, 1979. [Google Scholar]
- Solc, K. Polymer Compatibility and Incompatibility: Principles and Practice; Harwood Academic Publishers GmbH: New York, NY, USA, 1982. [Google Scholar]
- Peters, E.N. Compatibilized Composition, Method for the Formation Thereof, and Article Comprising Same. U.S. Patent 8552105 B2, 8 October 2013. [Google Scholar]
- Melton, G.; Peters, E.N.; Arisman, R.K. Engineering Thermoplastics. In Applied Plastics Engineering Handbook; Kutz, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 7–21. [Google Scholar]
- Robeson, L.M. Polymer Blends: A Comprehensive Review; Hanser: Cincinnati, OH, USA, 2007. [Google Scholar]
- Harris, J.E.; Goh, S.H.; Paul, D.R.; Barlow, J.W. Miscible binary blends containing the polyhydroxy ether of bisphenol-A and various aliphatic polyesters. J. Appl. Polym. Sci. 1982, 27, 839–855. [Google Scholar] [CrossRef]
- Robeson, L.M.; Furtek, A.B. Miscible Blends of poly(butylene terephthalate) and the Polyhydroxyether of Bisphenol A. J. Appl. Polym. Sci. 1979, 23, 645–659. [Google Scholar] [CrossRef]
- Christiansen, W.H.; Paul, D.R.; Barlow, J.W. The phase behavior of ternary blends containing polycarbonate, phenoxy, and polycaprolactone. J. Appl. Polym. Sci. 1987, 34, 537–548. [Google Scholar] [CrossRef]
- Uriarte, C.; Eguiazábal, J.I.; Llanos, M.; Iribarren, J.I.; Iruin, J.J. Miscibility and phase separation in poly(vinyl methyl ether)/poly(bisphenol A hydroxy ether) blends. Macromolecules 1987, 20, 3038–3042. [Google Scholar] [CrossRef]
- Snodgrass, H.E.; Lauchlan, R.L. Polyphenylene Oxide Resins Modified with Polyhydroxy Ethers. U.S. Patent 3,631,126, 28 December 1971. [Google Scholar]
- Moskala, E.J.; Coleman, M.M. FTIR Studies of Polyvinyl ether Blends with the poly(hydroxyl ether of Bisphenol A). Polym. Commun. 1983, 24, 206–208. [Google Scholar]
- Coleman, M.M.; Moskala, E.J. FTIR Studies of Polymer Blends Containing the poly(hydroxyl ether of bisphenol A) and Poly(ε-caprolactone). Polymer 1983, 24, 251–257. [Google Scholar] [CrossRef]
- Kim, H.C.; Nam, K.H.; Jo, W.H. The effect of a styrene-methyl methacrylate block copolymer on the morphological, rheological and mechanical properties of poly(2,6-dimethyl-1,4-phenylene ether) (PPE) and poly(hydroxy ether of bisphenol A) (Phenoxy) blends. Polymer 1993, 34, 4043–4051. [Google Scholar] [CrossRef]
- Gallucci, R.; Hamilton, D.G. The effects of molecular weight on polycarbonate-polybutylene terephthalate blends. J. Appl. Polym. Sci. 1993, 48, 2249–2252. [Google Scholar]
- Plueddemann, E.P. Silane Coupling Agents, 2nd ed.; Springer: New York, NY, USA, 1991. [Google Scholar]
- Fowkes, F.M. Quantitative characterization of the acid-base properties of solvents, polymers, and inorganic surfaces. J. Adhes. Sci. Technol. 1990, 4, 669–691. [Google Scholar] [CrossRef]
- Takemori, M.T. Towards an understanding of the heat distortion temperature of thermoplastics. Polym. Eng. Sci. 1979, 19, 1104–1109. [Google Scholar] [CrossRef]
- Takemori, M.T. Towards an Understanding of the Heat Distortion Temperature of Thermoplastics, Filled and Unfilled; General Electric: Boston, MA, USA, 1976. [Google Scholar]
- Chang, F.C.; Yang, M.Y. Mechanical fracture behavior of polyacetal and thermoplastic polyurethane elastomer toughened polyacetal. Polym. Eng. Sci. 1990, 30, 543–552. [Google Scholar] [CrossRef]
- Agrawal, R.K.; Drzal, L.T. Adhesion mechanisms of polyurethanes to glass surfaces. III. Investigation of possible physico-chemical interactions at the interphase. J. Adhes. 1996, 55, 221–243. [Google Scholar] [CrossRef]
- Houvenaghel, G.; Carmeliet, J. Dynamic Contact Angles, Wettability and Capillary Suction of Hydrophobic Porous Materials. In Proceedings of the Hydrophobe III—3rd International Conference on Surface Technology with Water Repellent Agents, Aedificatio Publishers, Freiburg, Germany, 25–26 September 2001; pp. 191–200. [Google Scholar]
- Förch, R.; Schönherr, H.; Tobias, A.; Jenkins, A. See Appendix C. In Surface Design: Applications in Bioscience and Nanotechnology; Wiley-VCH: Weinheim, Germany, 2009; p. 471. [Google Scholar]
- Frisch, K.C.; Sendijarevic, A.; Sendijarevic, V.; Yokelson, H.B.; Nubel, P.O. Polyurethane Elastomers Based Upon Novel Hydrocarbon-Based Diols. In Proceedings of the Conference UTECH 96, The Hague, The Netherlands, 26–28 March 1996; p. 42. [Google Scholar]
- Yokelson, H.B.; Nubel, P.O.; Sendijarevic, A.; Sendijarevic, V.; Frisch, K.C. Novel Hydrocarbon-Based Diols for Polyurethane Elastomers. In Proceedings of the SPI Polyurethanes 1995 Conference, Chicago, IL, USA, 26–29 September 1995. [Google Scholar]
- Adhikari, R.; Gunatillake, T.; Mccarthy, S.; Meijs, G.F. Mixed macrodiol-based siloxane polyurethanes: Effect of the comacrodiol structure on properties and morphology. J. Appl. Polym. Sci. 2000, 78, 1071–1082. [Google Scholar] [CrossRef]
- Trombetta, T.; Scicchitano, M.; Simeone, G.; Ajroldi, G. New fluorinated thermoplastic elastomers. J. Appl. Polym. Sci. 1996, 59, 311–327. [Google Scholar]
- Alves, P.; Pinto, S.; de Sousa, H.C.; Gil, M.H. Surface modification of a thermoplastic polyurethane by low-pressure plasma treatment to improve hydrophilicity. J. Appl. Polym. Sci. 2011, 122, 2302–2308. [Google Scholar] [CrossRef]
- Bolland, J.L.; Gee, G. Kinetic studies in the chemistry of rubber and related materials. II. The kinetics of oxidation of unconjugated olefins. Trans. Faraday Soc. 1946, 42, 236–243. [Google Scholar] [CrossRef]
- Gryn’ova, G.; Hodgsona, J.L.; Coote, M.L. Revising the mechanism of polymer autooxidation. Org. Biomol. Chem. 2011, 9, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Levchik, S.V.; Weil, E.D. Thermal decomposition, combustion and fire-retardency of polyurethanes—A review of the recent literature. Polym. Int. 2004, 53, 1585–1610. [Google Scholar] [CrossRef]
- Chambers, J.; Jiricny, J.; Reese, C.B. The thermal decomposition of polyurethanes and polyisocyanurates. Fire Mater. 1981, 5, 133–141. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Q.; He, J.; Mao, Z. Study on the Pyrolytic Behaviors and Kinetics of Rigid Polyurethane Foams. Procedia Eng. 2013, 52, 377–385. [Google Scholar] [CrossRef]
- Grassie, N.; Mendoza, G.A.P. Thermal degradation of polyether-urethanes: Part 1—Thermal degradation of poly(ethylene glycols) used in the preparation of polyurethanes. Polym. Degrad. Stab. 1984, 9, 155–165. [Google Scholar] [CrossRef]
- Jachowicz, J.; Kryszwski, M.; Kowlaski, P. Thermal degradation of Poly(2,6-dimethyl-1,4-phenylene oxide). I. The mechanism of degradation. J. Appl. Polym. Sci. 1978, 22, 2891–2899. [Google Scholar] [CrossRef]
- Factor, A. The high-temperature degradation of poly(2,6-dimethyl-1,4-phenylene ether). J. Polym. Sci. Part A 1969, 7, 363–377. [Google Scholar] [CrossRef]
- Murashko, E.A.; Levchik, G.F.; Levchik, S.V.; Bright, D.A.; Dashevsky, S. Fire Retardant Action of Resorcinol Bis(Diphenyl Phosphate) in a PPO/HIPS Blend. J. Fire Sci. 1998, 16, 233–249. [Google Scholar] [CrossRef]
- Weil, E.D.; Levchik, S. A Review of Current Flame Retardant Systems for Epoxy Resins. J. Fire Sci. 2004, 22, 25–40. [Google Scholar] [CrossRef]
- Cullis, C.F.; Hirschler, M.M. The Combustion of Organic Polymers; Clarendon Press: Oxford, UK, 1981. [Google Scholar]
- Van Krevelen, D.W. Some basic aspects of flame resistance of polymeric materials. Polymer 1975, 16, 615–620. [Google Scholar] [CrossRef]






| Property | PPE | PPE-M |
|---|---|---|
| Mn, g/mol | 15,000 | 1670 |
| HEW, g/equiv. | ~15,000 | 800 |
| Tg, °C | 215 | 150 |
| Solubility at 25 °C | ||
| 2-Butanone | <1% | >50% |
| Toluene | ~20% | >50% |
| Functionality | ~1 | ~2 |
| PBT, wt % | PPE, wt % | SEBS, wt % | PHE, wt % | ABC-36, wt % | |
|---|---|---|---|---|---|
| Blend | 40 | 48 | 12 | - | - |
| Blend with PHE | 40 | 40 | 10 | 10 | - |
| Blend with ABC-36 | 40 | 40 | 10 | - | 10 |
| Designation | PS, wt % | GF, wt % | PHE, wt % | ABC-36, wt % |
|---|---|---|---|---|
| PS/GF | 80 | 20 | 0 | 0 |
| PS/GF/PHE | 75 | 20 | 5 | 0 |
| PS/GF/ABC-36 | 75 | 20 | 0 | 5 |
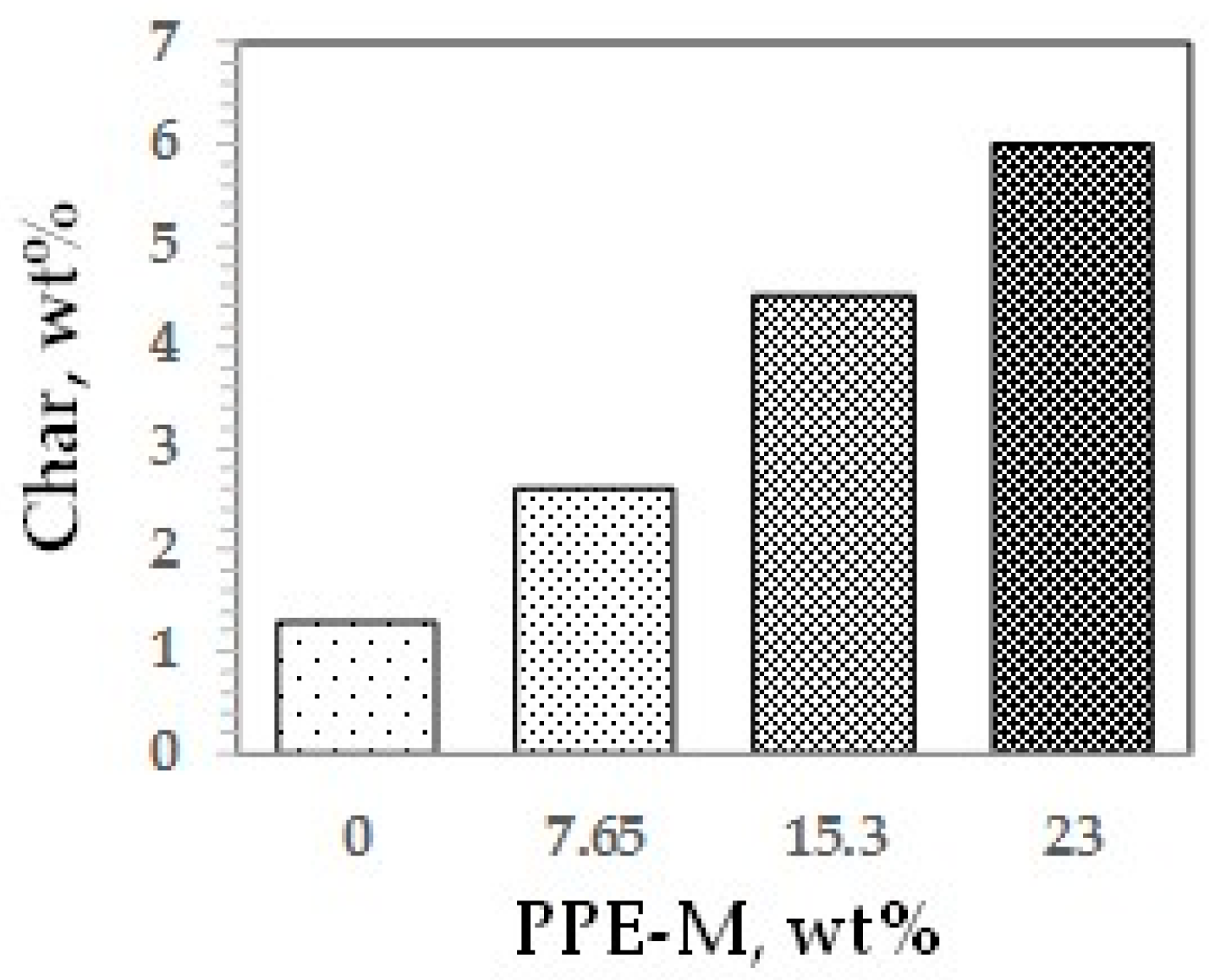
| PPE-M, wt % | EO-PO, wt % | 4,4′-MDI, wt % | BD, wt % | DBTDL, phr |
|---|---|---|---|---|
| 0 | 76.70 | 19.99 | 3.31 | 0.0089 |
| 7.65 | 68.89 | 19.95 | 3.51 | 0.0038 |
| 15.29 | 61.17 | 20.02 | 3.52 | 0.0013 |
| 23.00 | 53.67 | 20.34 | 2.99 | 0.0027 |
| Chemical | PPE-M Content, wt % | |
|---|---|---|
| 0 | 23 | |
| Toluene | 290 | 150 |
| Oil | 0.34 | 0.18 |
| Hydrochloric acid, pH 1 | 84 | 19 |
| Sodium hydroxide, pH 13 | 70 | 29 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peters, E.N. Poly(phenylene ether) Based Amphiphilic Block Copolymers. Polymers 2017, 9, 433. https://doi.org/10.3390/polym9090433
Peters EN. Poly(phenylene ether) Based Amphiphilic Block Copolymers. Polymers. 2017; 9(9):433. https://doi.org/10.3390/polym9090433
Chicago/Turabian StylePeters, Edward N. 2017. "Poly(phenylene ether) Based Amphiphilic Block Copolymers" Polymers 9, no. 9: 433. https://doi.org/10.3390/polym9090433




