Block Copolymer Membranes from Polystyrene-b-poly(solketal methacrylate) (PS-b-PSMA) and Amphiphilic Polystyrene-b-poly(glyceryl methacrylate) (PS-b-PGMA)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Synthesis of (PS-b-PSMA) and Chemical Modification into (PS-b-PGMA)
2.4. Membrane Formation
3. Results and Discussion
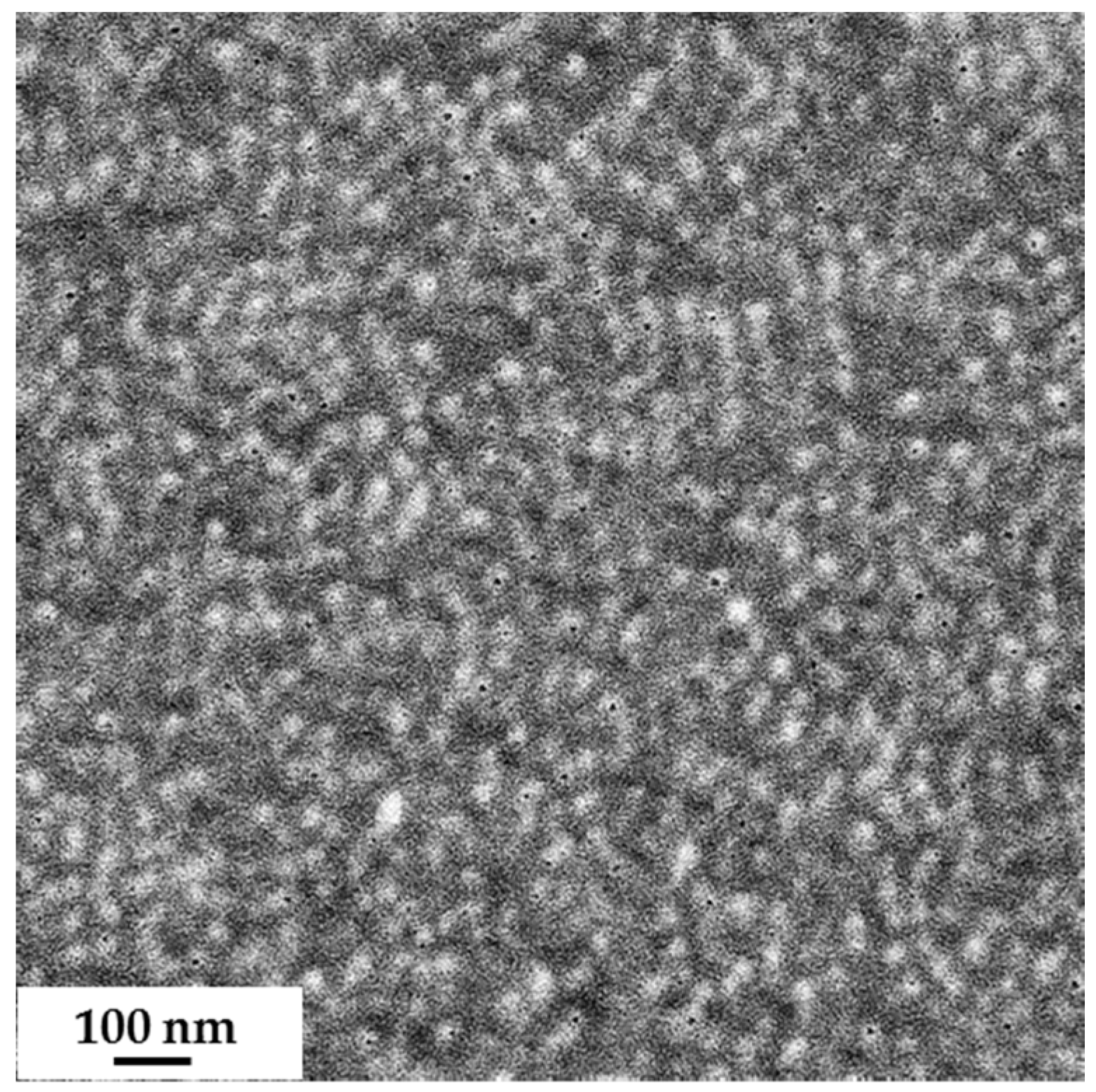
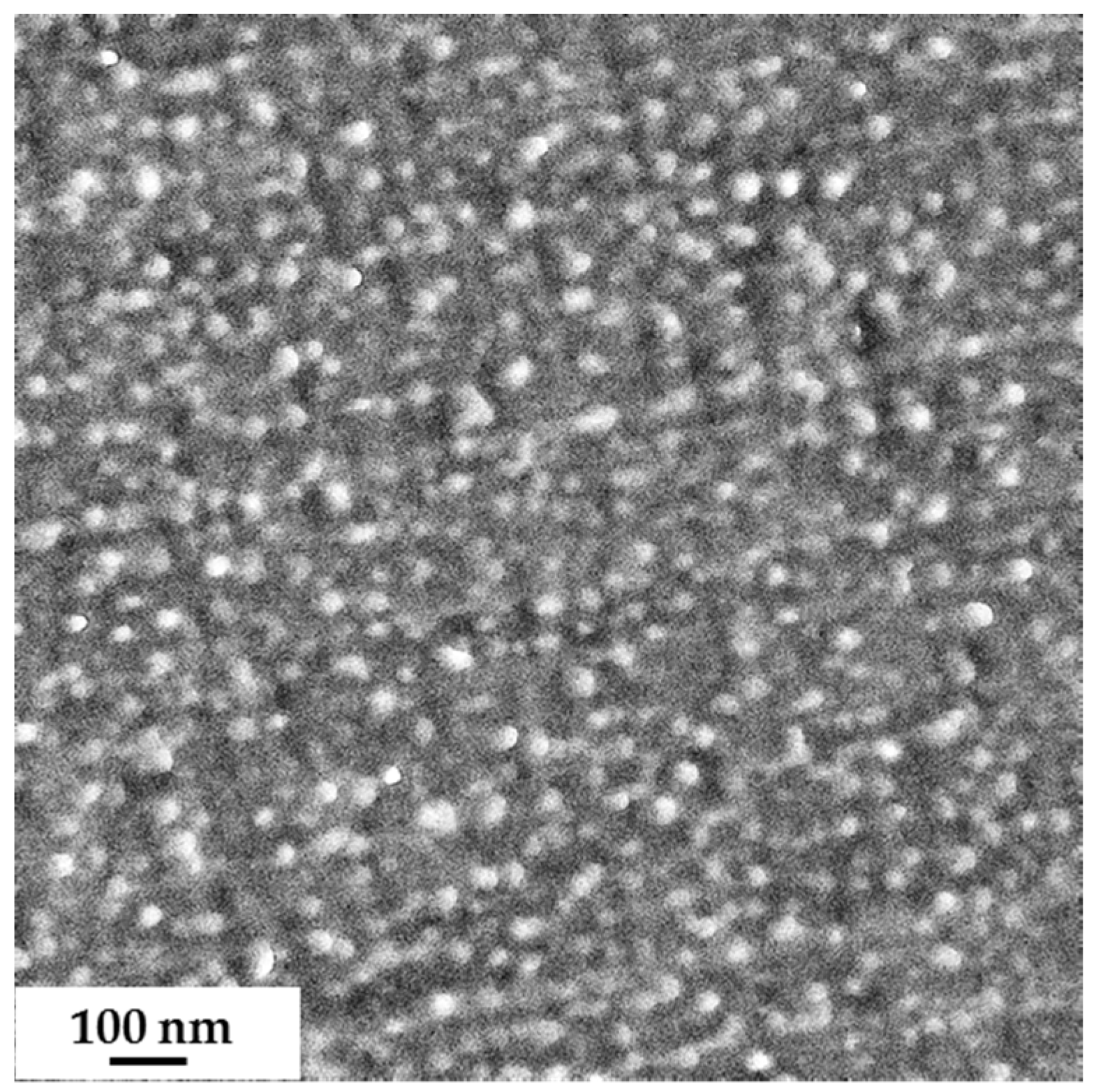
3.1. Synthesis of Diblock Copolymers (PS-b-PSMA) (PS-b-PGMA)
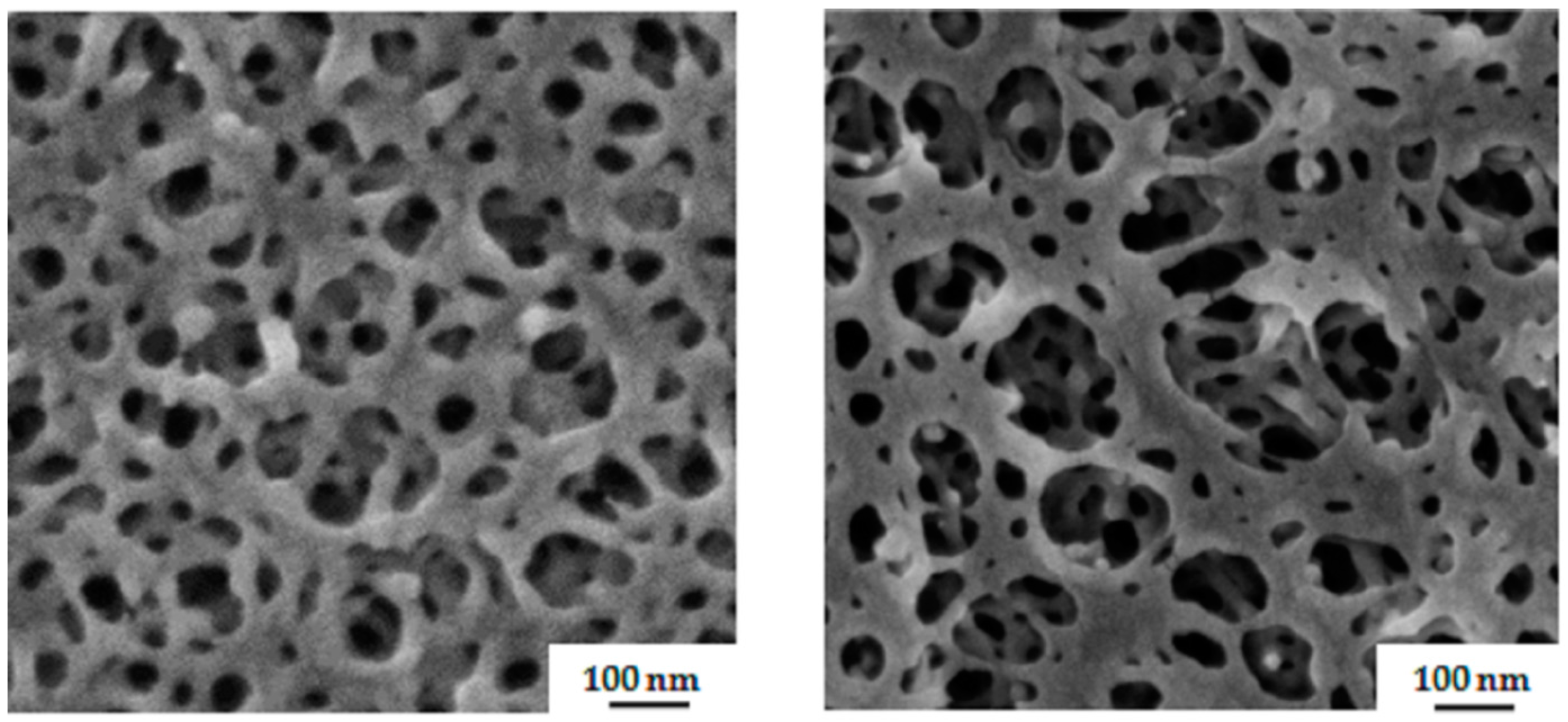
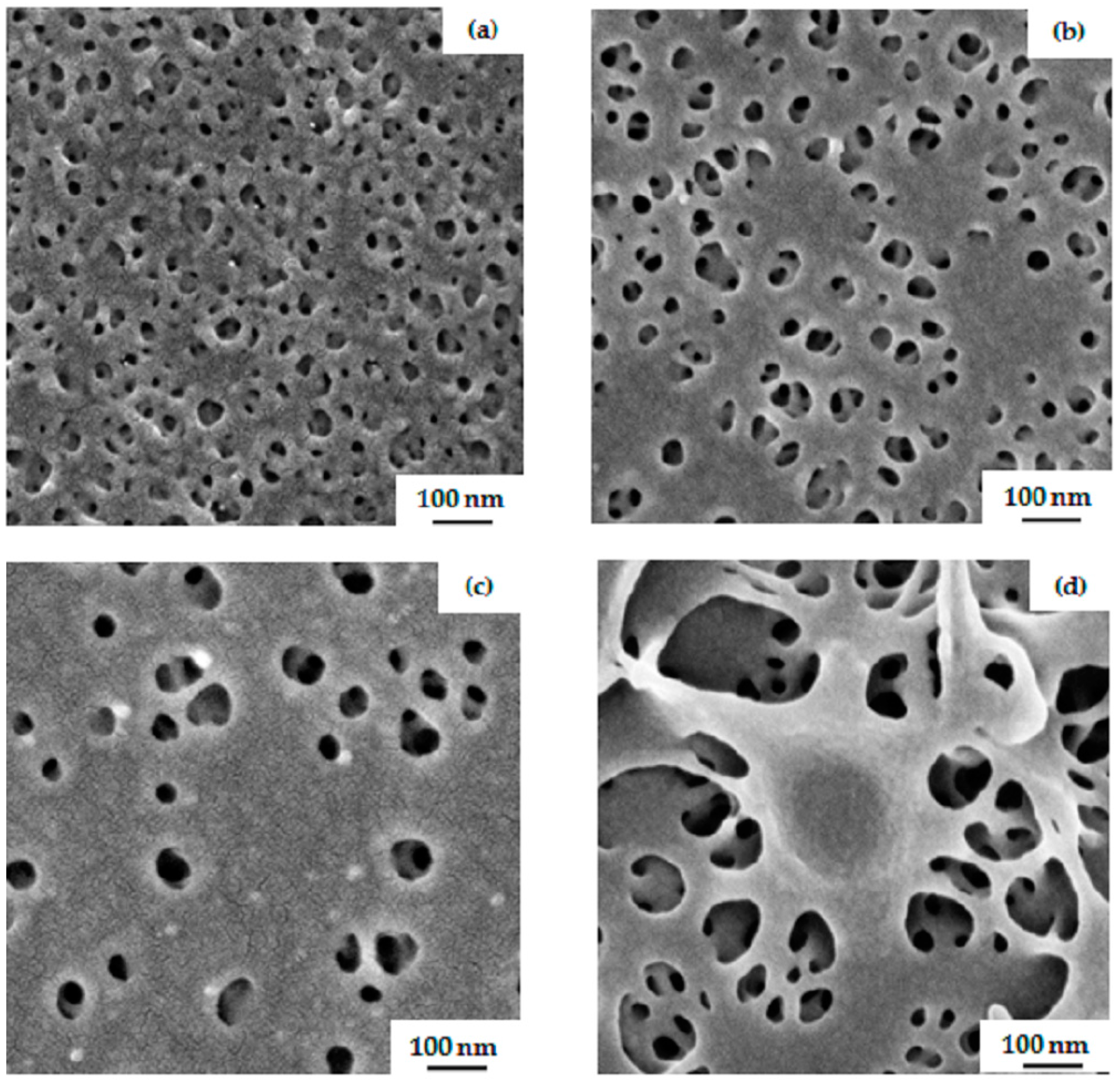
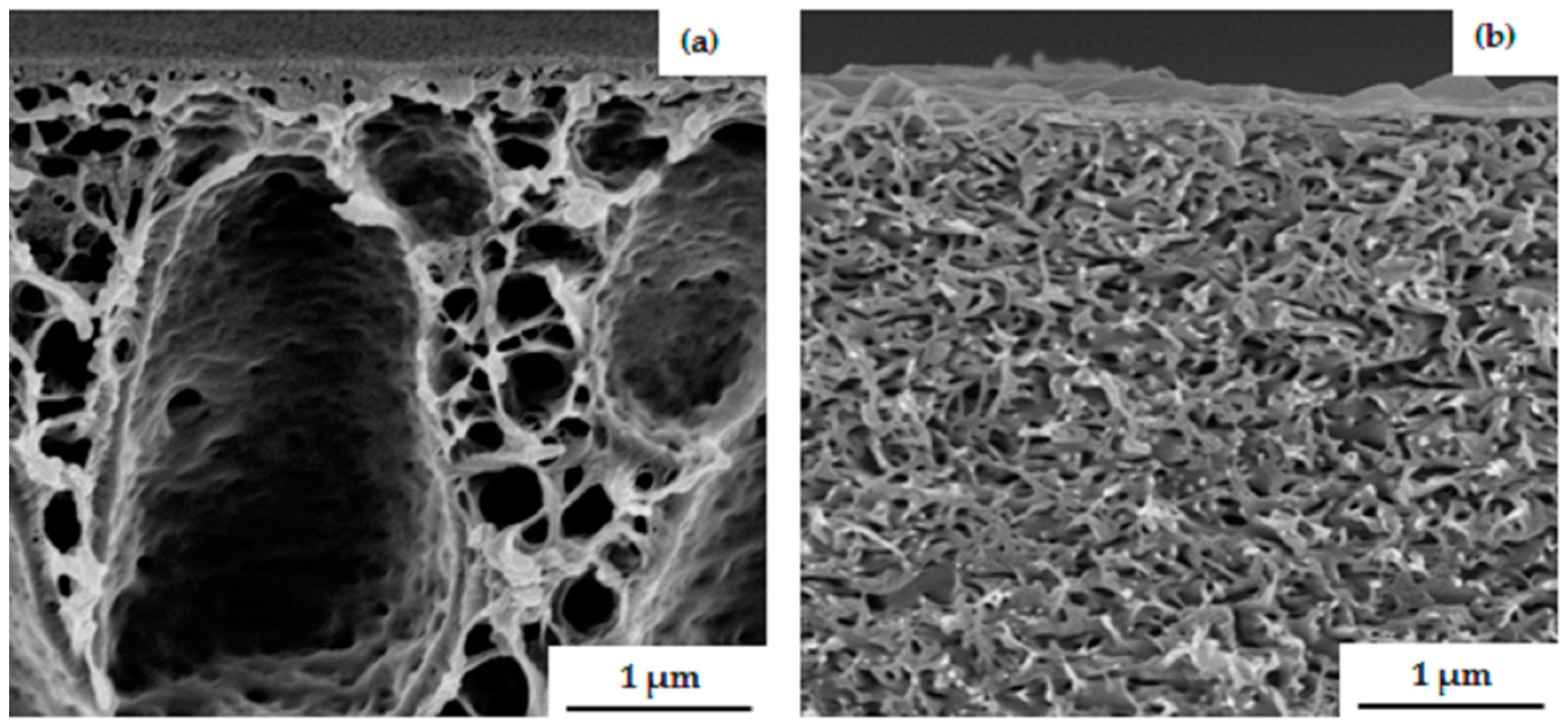
3.2. Membrane Formation via SNIPS
3.3. Dynamic Contact Angle Measurement
3.4. Water Flux Measurement
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jackson, E.A.; Hillmyer, M.A. Nanoporous Membranes Derived from Block Copolymers: From Drug Delivery to Water Filtration. ACS Nano 2010, 4, 3548–3553. [Google Scholar] [CrossRef] [PubMed]
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef] [PubMed]
- Schacher, F.; Ulbricht, M.; Müller, A.H.E. Self-Supporting, Double Stimuli-Responsive Porous Membranes From Polystyrene-block-poly(N,N-dimethylaminoethyl methacrylate) Diblock Copolymers. Adv. Funct. Mater. 2009, 19, 1040–1045. [Google Scholar] [CrossRef]
- Szwarc, M. ‘Living’ Polymers. Nature 1956, 178, 1168–1169. [Google Scholar] [CrossRef]
- Mori, H.; Hirao, A.; Nakahama, S. Synthesis and Surface Characterization of Hydrophilic-Hydrophobic Block Copolymers Containing Poly(2,3-dihydroxypropyl methacrylate). Macromolecules 1994, 27, 4093–4100. [Google Scholar] [CrossRef]
- Kishimura, A.; Koide, A.; Osada, K.; Yamasaki, Y.; Kataoka, K. Encapsulation of Myoglobin in PEGylated Polyion Complex Vesicles Made from a Pair of Oppositely Charges Block Ionomers: A physiologically Available Oxygen Carrier. Angew. Chem. Int. Ed. 2007, 46, 6085–6088. [Google Scholar] [CrossRef] [PubMed]
- Zarka, M.T.; Nuyken, O.; Weberskirch, R. Amphiphilic polymer supports for the asymmetric hydrogenation of amino acid precursors in water. Chem. Eur. J. 2003, 9, 3228–3234. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Muller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materilas. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Schacher, F.H.; Rupar, P.A.; Manners, I. Functional Block Copolymers: Naostructured Materials with Emerging Applications. Angew. Chem. Int. Ed. 2012, 51, 7898–7921. [Google Scholar] [CrossRef] [PubMed]
- Sourirajan, S. Mechanism of Demineralization of Aqueous Sodium Chloride Solutions by Flow, under Pressure, through Porous Membranes. Ind. Eng. Chem. Fund. 1963, 2, 51–55. [Google Scholar] [CrossRef]
- Peinemann, K.-V.; Abetz, V.; Simon, P.F.W. Asymmetric superstructure formed in a block copolymer via phase separation. Nat. Mater. 2007, 6, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Clodt, J.I.; Rangou, S.; Schröder, A.; Buhr, K.; Hahn, J.; Jung, A.; Filiz, V.; Abetz, V. Carbohydrates as Additives for the Formation of Isoporous PS-b-P4VP Diblock Copolymer Membranes. Macromol. Rapid Commun. 2013, 34, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Gallei, M.; Rangou, S.; Filiz, V.; Buhr, K.; Bolmer, S.; Abetz, C.; Abetz, V. The Influence of Magnesium Acetate on the Structure Formation of Polystyrene-block-poly(4-vinylpyridine)-Based Integral Asymmetric Membranes. Macromol. Chem. Phys. 2013, 214, 1037–1046. [Google Scholar] [CrossRef]
- Nunes, S.P.; Sougrat, R.; Hooghan, B.; Anjum, D.H.; Behzad, A.R.; Zhao, L.; Pradeep, N.; Pinnau, I.; Vainio, U.; Peinemann, K.-V. Ultraporous Films with Uniform Nanochannels by Block Copolymer Micelles Assembly. Macromolecules 2010, 43, 8079–8085. [Google Scholar] [CrossRef]
- Clodt, J.I.; Filiz, V.; Rangou, S.; Buhr, K.; Abetz, C.; Höche, D.; Hahn, J.; Jung, A.; Abetz, V. Double Stimuli-Responsive Isoporous Membranes via Post-Modification of pH-Sensitive Self-Assembled Diblock Copolymer membranes. Adv. Funct. Mater. 2013, 23, 731–738. [Google Scholar] [CrossRef]
- Jung, A.; Filiz, V.; Rangou, S.; Buhr, K.; Merten, P.; Hahn, J.; Clodt, J.; Abetz, C.; Abetz, V. Formation of Integral Asymmetric Membranes of AB Diblock and ABC Triblock Copolymers by Phase Inversion. Macromol. Rapid Commun. 2013, 34, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.; Filiz, V.; Rangou, S.; Clodt, J.; Jung, A.; Buhr, K.; Abetz, C.; Abetz, V. Structure Formation of Integral-Asymmetric Membranes of Polystyrene-block-Poly(ethylene oxide). J. Polym. Sci. Part B Polym. Phys. 2013, 51, 281–290. [Google Scholar] [CrossRef]
- Radjabian, M.; Abetz, V. Tailored Pore Sizes in Integral Asymmetric membranes Formed by Blends of Block Copolymers. Adv. Mater. 2015, 27, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Radjabian, M.; Abetz, C.; Fischer, B.; Meyer, A.; Abetz, V. Influence of solvent on the Structure of an Amphiphilic Block Copolymer in Solution and in Formation of an Integral Asymmetric Membrane. ACS Appl. Mater. Inter. 2017. [Google Scholar] [CrossRef] [PubMed]
- Abetz, V. Isoporous Block Copolymer Membranes. Macromol. Rapid Commun. 2015, 36, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.M.; Hong, P.; Guo, H.; Liu, W.-T. Biofilm Formation Characteristics of Bacterial Isolates Retrieved from a Reverse Osmosis Membrane. Environ. Sci. Technol. 2005, 39, 7541–7550. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Hakim, L.F.; Bowman, C.N.; Davis, R.H. Factors affecting membrane fouling reduction by surface modification and back pulsing. J. Membr. Sci. 2001, 189, 255–270. [Google Scholar] [CrossRef]
- Burguière, C.; Chassenieux, C.; Charleux, B. Characterization of aqueous micellar solutions of amphiphilic block copolymers of poly(acrylic acid) and polystyrene prepared via ATRP. Toward the control of the number of particles in emulsion polymerization. Polymer 2003, 44, 509–518. [Google Scholar] [CrossRef]
- Schöttner, S.; Schaffrath, H.-J.; Gallei, M. Poly(2-hydroxyethyl methacrylate)-Based Amphiphilic Block Copolymers for High Water Flux Membranes and Ceramic Templates. Macromolecules 2016, 49, 7286–7295. [Google Scholar] [CrossRef]
- Mori, H.; Hirao, A.; Nakahama, S.; Senshu, K. Synthesis and Surface Characterization of Hydrophilic-Hydrophobic Block Copolymers Containing Poly(2,3-dihydroxypropyl methacrylate). Macromolecules 1994, 27, 4093–4100. [Google Scholar] [CrossRef]
- Zhang, H.; Ruckenstein, E. A Novel Successive Route to Well-Defined Water-Soluble Poly(2,3-dihydroxypropyl methacrylate) and Amphiphilic Block Copolymers Based on an osmylation Reaction. Macromolecules 2000, 33, 4738–4744. [Google Scholar] [CrossRef]
- Hahn, J.; Clodt, J.I.; Abetz, C.; Filiz, V.; Abetz, V. Thin Isoporous Block Copolymer membranes: It Is All about the Process. ACS Appl. Mater. Inter. 2015, 7, 21130–21137. [Google Scholar] [CrossRef] [PubMed]
- Wolf, F.K.; Hofmann, A.M.; Frey, H. Poly(isoglycerol methacrylate)-b-poly(d or l-Lactide) Copolymers: A Novel Hydrophilic Methacrylate as Building Block for Supramolecular Aggregates. Macromolecules 2010, 43, 3314–3324. [Google Scholar] [CrossRef]
- Ishizone, T.; Mochizuki, A.; Hirao, A.; Nakahama, S. Protection and Polymerization of Functional Monomers. 24. Anionic Living Polymerization of 5-Vinyl- and 4-Vinyl-1,3-benzodioxoles. Macromolecules 1995, 28, 3787–3793. [Google Scholar] [CrossRef]
- Van Krevelen, D.W.; Nijenhuis, K.T. Cohesive Properties and Solubility. In Properties of Polymers, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2009; p. 189. [Google Scholar]
- Zhang, Q.; Li, Y.M.; Gu, Y.; Dorin, R.M.; Wiesner, U. Tuning substructure and properties of supported asymmetric triblock terpolymer membranes. Polymer 2016, 107, 398–405. [Google Scholar] [CrossRef]
- Sutisna, B.; Polymeropoulos, G.; Mygiakis, E.; Musteata, V.; Peinemann, K.V.; Smilgies, D.M.; Hadjichristidis, N.; Nunes, S.P. Artificial membranes with selective nanochannels for protein transport. Polym. Chem. 2016, 7, 6189–6201. [Google Scholar] [CrossRef]






| Before Hydrolysis | After Hydrolysis | ||||
|---|---|---|---|---|---|
| Polymer | Mn (kg/mol) | PDI | Polymer | Mn (kg/mol) | PDI |
| PS76-PSMA24135 | 135 | 1.03 | PS81-PGMA19128 | 128 | 1.06 |
| PS81-PSMA19170 | 170 | 1.04 | PS85-PGMA15158 | 158 | 1.07 |
| PS76-PSMA24200 | 200 | 1.03 | PS81-PGMA19190 | 190 | 1.05 |
| Polymer | δ (MPa0.5) | Solvents | δ (MPa0.5) |
|---|---|---|---|
| PS | 18.6 | DMF | 24.8 |
| PSMA | 19.98 | THF | 20.3 |
| PGMA | 25.8 | Dioxane | 20.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, S.; Rangou, S.; Abetz, C.; Lademann, B.; Filiz, V.; Abetz, V. Block Copolymer Membranes from Polystyrene-b-poly(solketal methacrylate) (PS-b-PSMA) and Amphiphilic Polystyrene-b-poly(glyceryl methacrylate) (PS-b-PGMA). Polymers 2017, 9, 216. https://doi.org/10.3390/polym9060216
Saleem S, Rangou S, Abetz C, Lademann B, Filiz V, Abetz V. Block Copolymer Membranes from Polystyrene-b-poly(solketal methacrylate) (PS-b-PSMA) and Amphiphilic Polystyrene-b-poly(glyceryl methacrylate) (PS-b-PGMA). Polymers. 2017; 9(6):216. https://doi.org/10.3390/polym9060216
Chicago/Turabian StyleSaleem, Sarah, Sofia Rangou, Clarissa Abetz, Brigitte Lademann, Volkan Filiz, and Volker Abetz. 2017. "Block Copolymer Membranes from Polystyrene-b-poly(solketal methacrylate) (PS-b-PSMA) and Amphiphilic Polystyrene-b-poly(glyceryl methacrylate) (PS-b-PGMA)" Polymers 9, no. 6: 216. https://doi.org/10.3390/polym9060216







