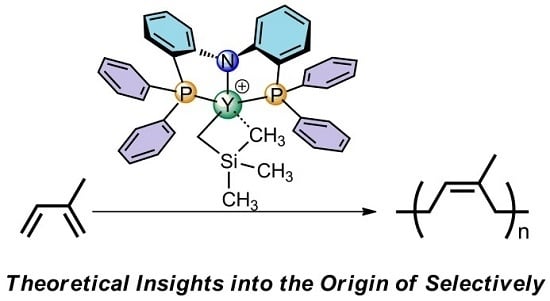
DFT Studies on cis-1,4-Polymerization of Dienes Catalyzed by a Cationic Rare-Earth Metal Complex Bearing an Ancillary PNP Ligand
Abstract
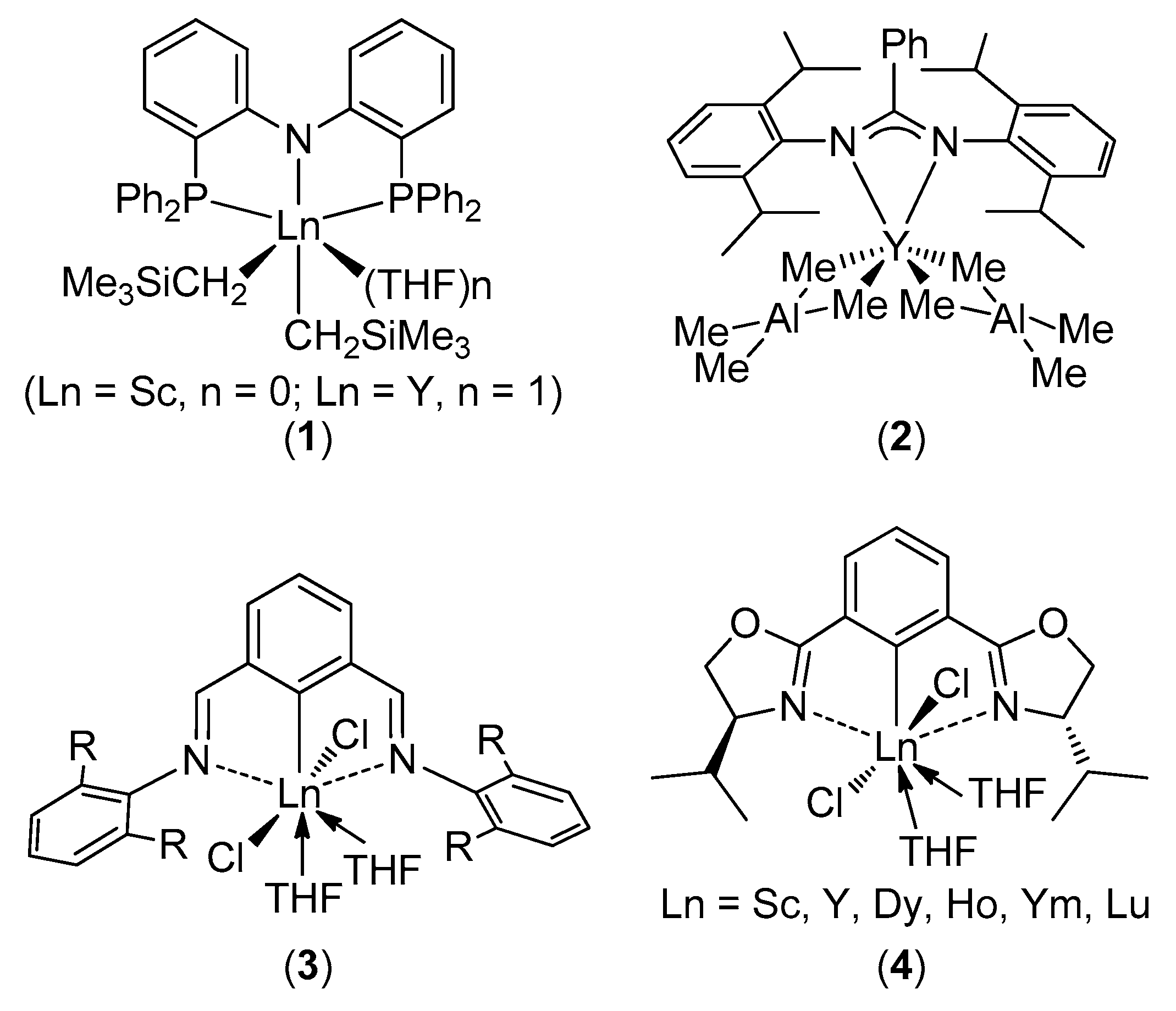
:1. Introduction
2. Computational Details
3. Results and Discussion
3.1. Cationic Species
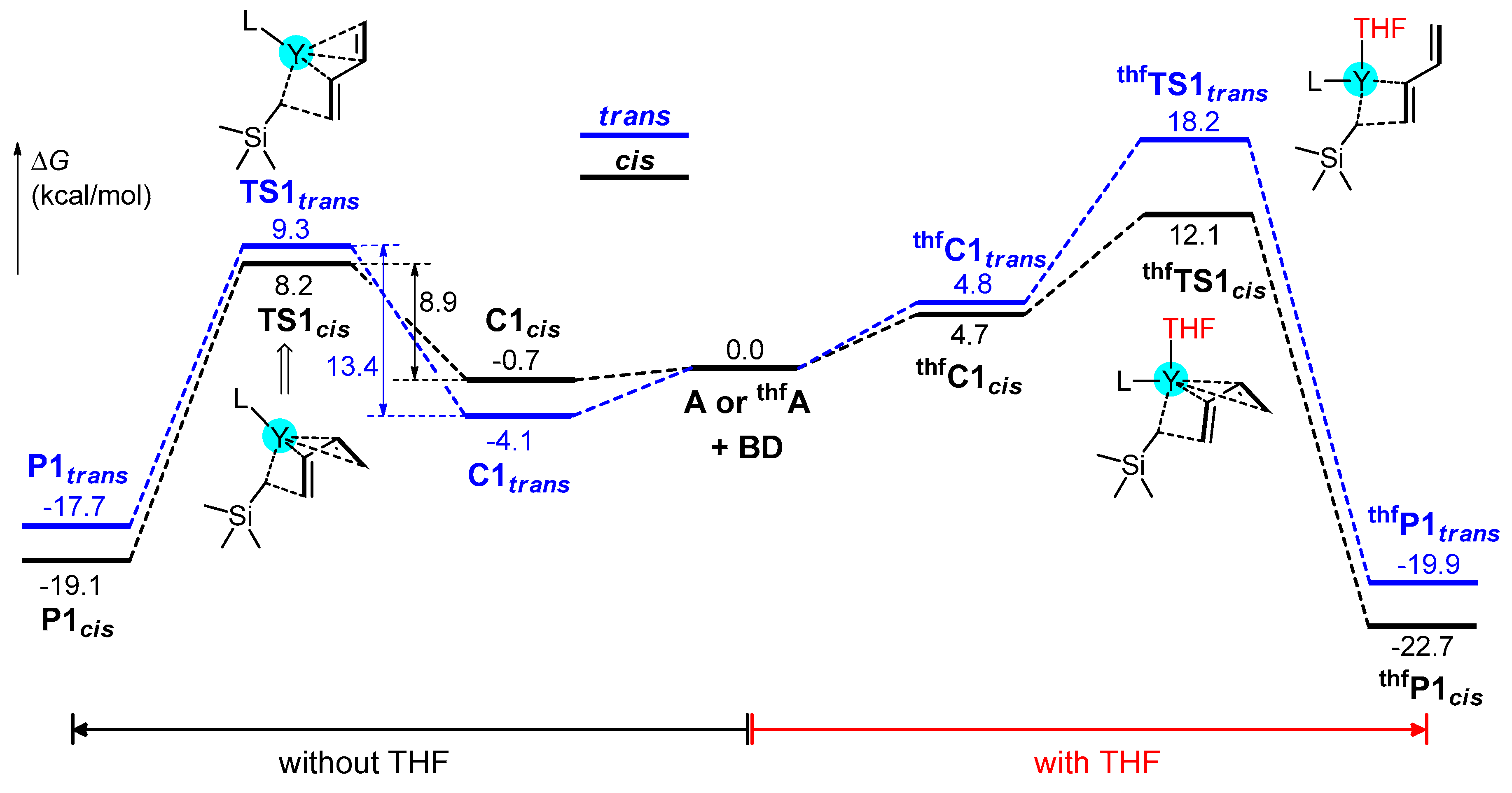
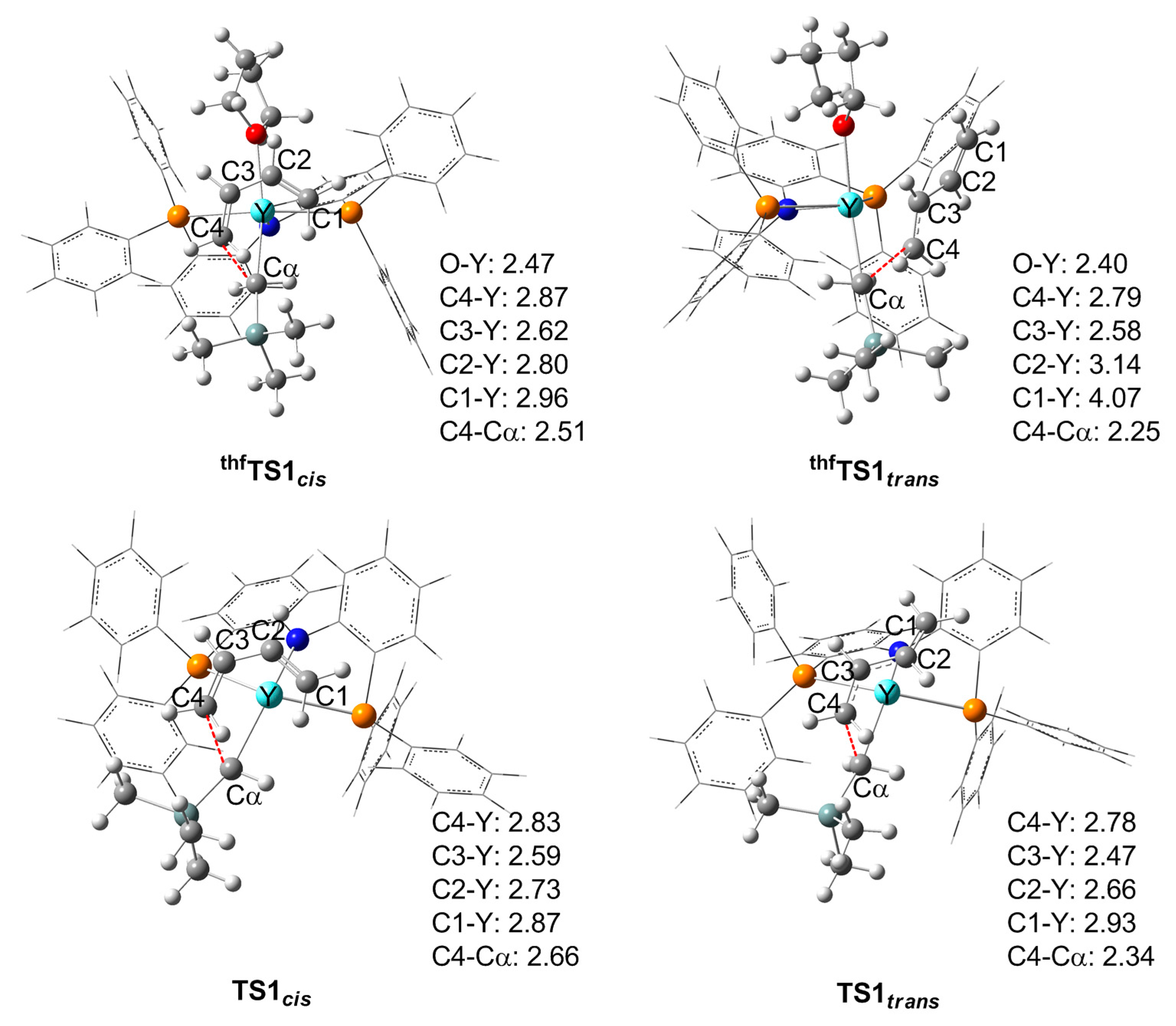
3.2. Chain Initiation Stage
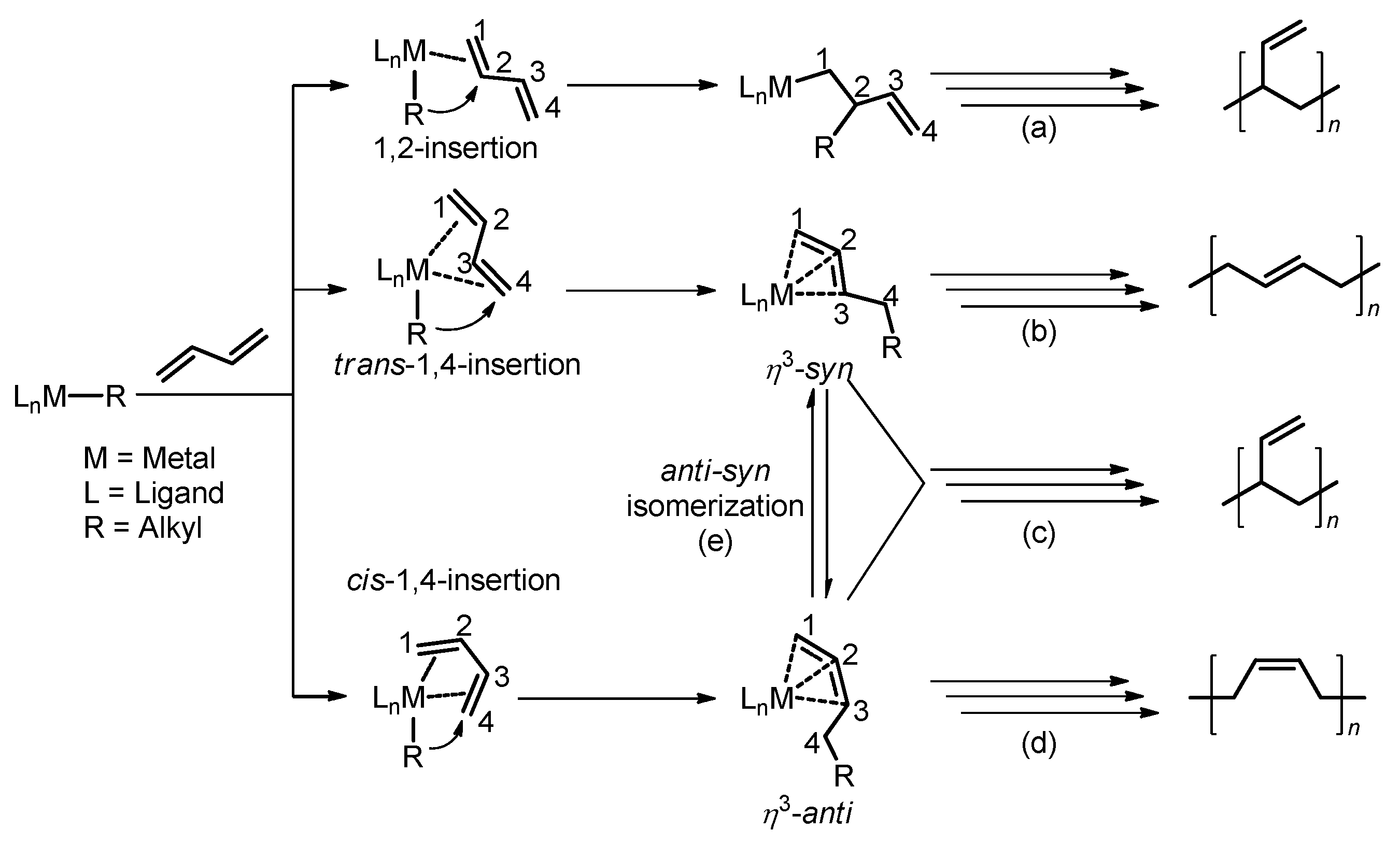
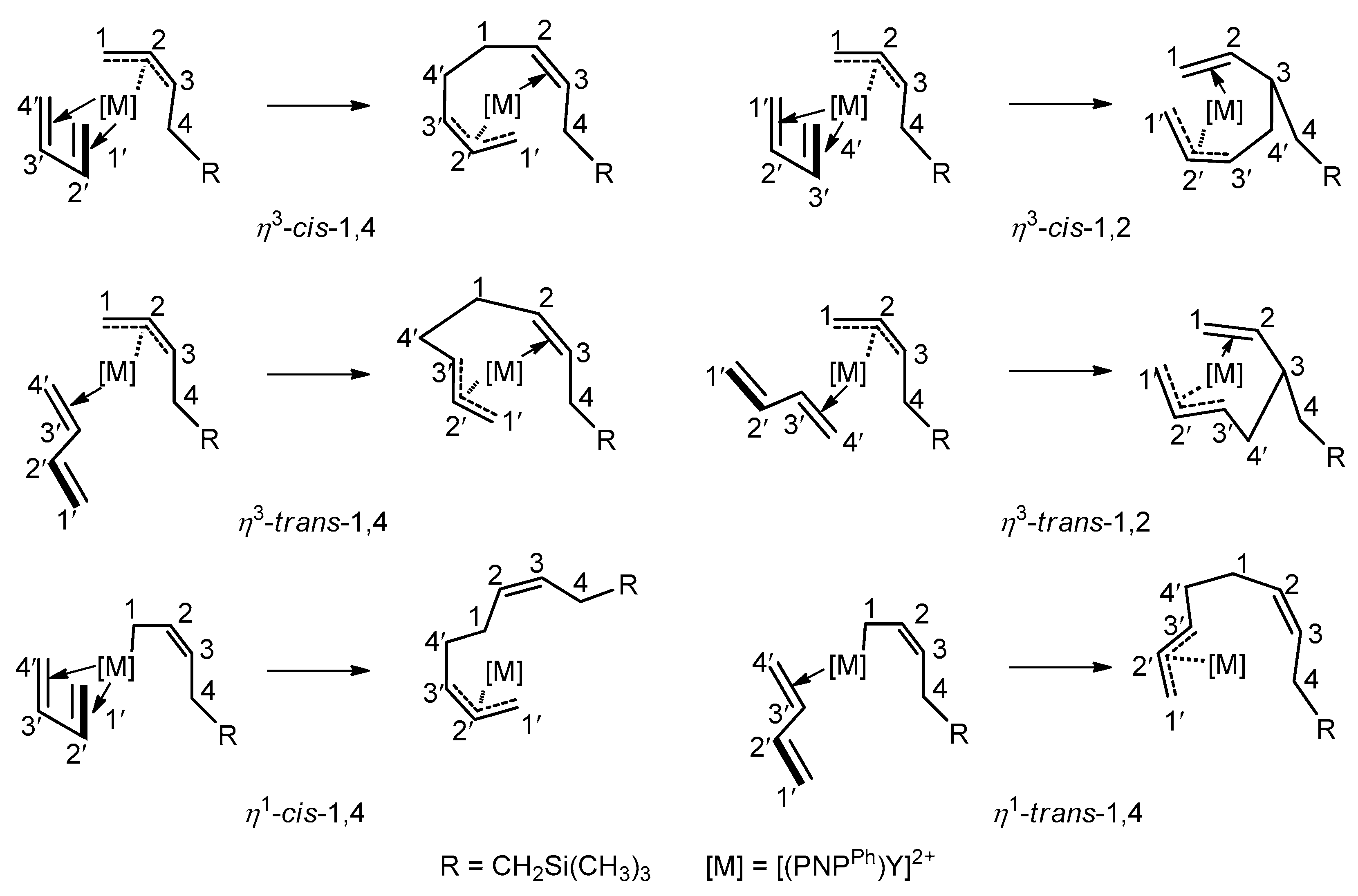
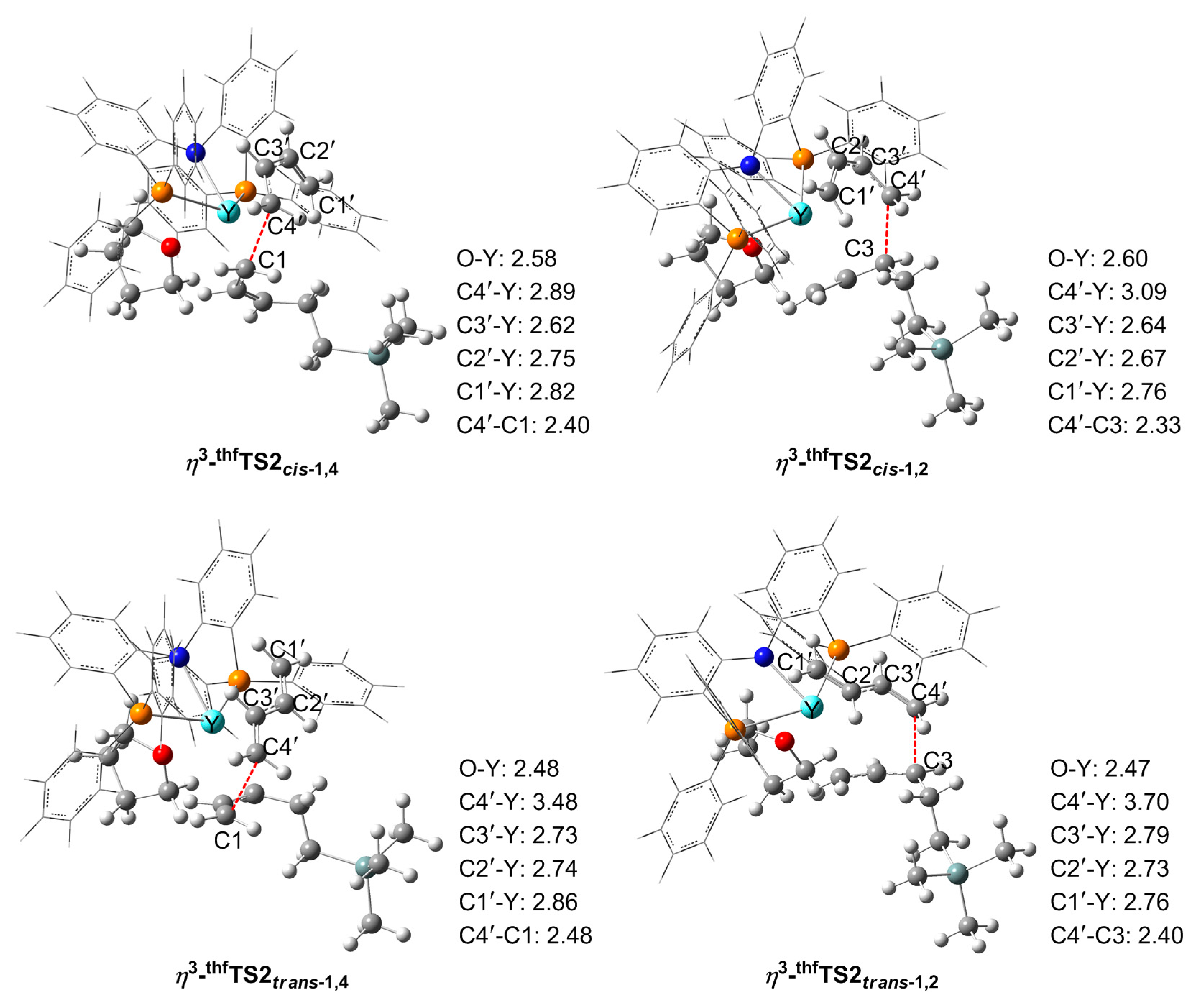
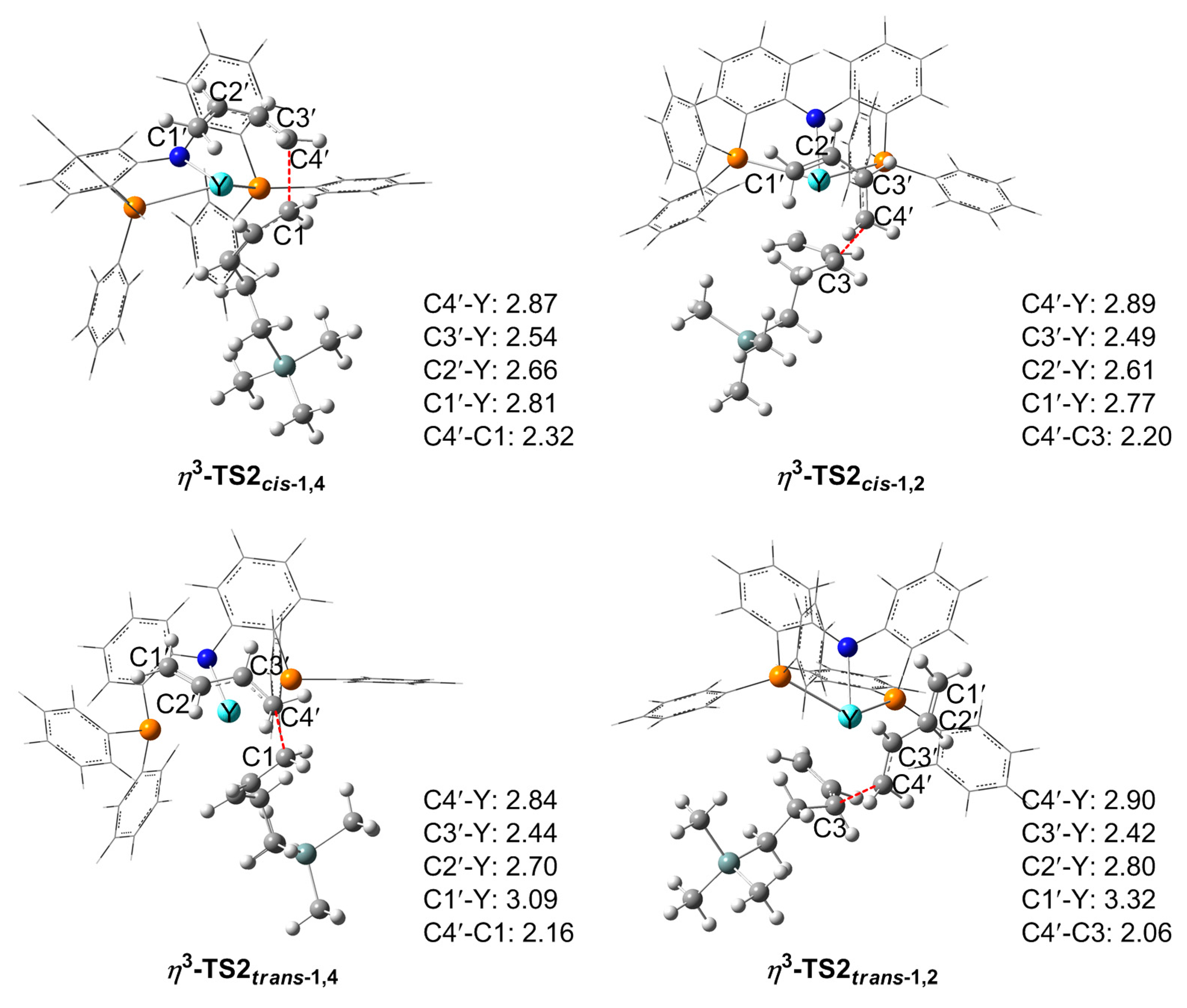
3.3. Chain Propagation Stage
3.3.1. With THF
3.3.2. Without THF
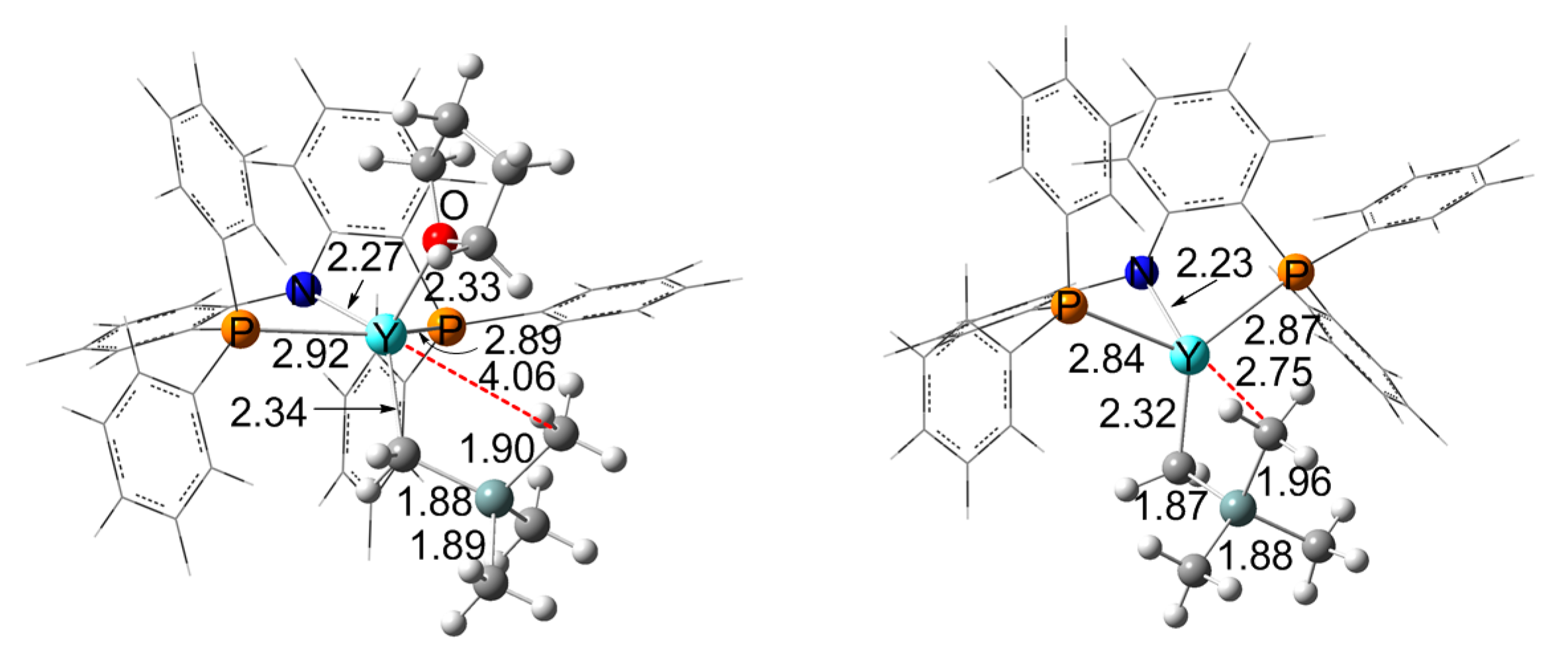
3.4. Effect of THF
3.5. The Origin of the Selectivity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kuran, W. Principle of Coordination Polymerization; John Wiley and Sons Ltd.: New York, NY, USA, 2001; pp. 275–321. [Google Scholar]
- Wolpers, J. 3,4-Polyisorene Containing Rubber Blend Mixtures for Tire Treads. U.S. Patent US5104941, 14 April 1992. [Google Scholar]
- Tsukada, G.; Tokuda, M.; Torii, M. Temperature Triggered Shape Memory Effect of Transpolyisoprene-Based Polymer. J. Endod. 2014, 40, 1658–1662. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Wakatsuki, Y. Recent Developments in Organolanthanide Polymerization Catalysts. Coord. Chem. Rev. 2002, 231, 1–22. [Google Scholar] [CrossRef]
- Wilson, D. Recent Advances in the Neodymium Catalysed Polymerisation of 1,3-Dienes. Makromol. Chem. Symp. 1993, 66, 273–288. [Google Scholar] [CrossRef]
- Zhao, J.; Ghebremeskel, G.N. A Review of Some of the Factors Affecting Fracture and Fatigue in SBR and BR Vulcanizates. Rubber Chem. Technol. 2001, 74, 409–427. [Google Scholar] [CrossRef]
- Gao, W.; Cui, D. Highly cis-1,4 Selective Polymerization of Dienes with Homogeneous Ziegler–Natta Catalysts Based on NCN-Pincer Rare Earth Metal Dichloride Precursors. J. Am. Chem. Soc. 2008, 130, 4984–4991. [Google Scholar] [CrossRef] [PubMed]
- Coates, G.W. Precise Control of Polyolefin Stereochemistry Using Single-Site Metal Catalysts. Chem. Rev. 2000, 100, 1223–1252. [Google Scholar] [CrossRef] [PubMed]
- Gibson, V.C.; Spitzmesser, S.K. Advances in Non-Metallocene Olefin Polymerization Catalysis. Chem. Rev. 2003, 103, 283–316. [Google Scholar] [CrossRef] [PubMed]
- Ricci, G.; Sommazzi, A.; Masi, F.; Ricci, M.; Boglia, A.; Leone, G. Well-Defined Transition Metal Complexes with Phosphorus and Nitrogen Ligands for 1,3-Dienes Polymerization. Coord. Chem. Rev. 2010, 254, 661–676. [Google Scholar] [CrossRef]
- Nishiura, M.; Guo, F.; Hou, Z. Half-Sandwich Rare-Earth-Catalyzed Olefin Polymerization, Carbometalation, and Hydroarylation. Acc. Chem. Res. 2015, 48, 2209–2220. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Nishiura, M.; Hu, L.; Mori, K.; Hou, Z. Alternating and Random Copolymerization of Isoprene and Ethylene Catalyzed by Cationic Half-Sandwich Scandium Alkyls. J. Am. Chem. Soc. 2009, 131, 13870–13882. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Cui, D. Regioselective Chain Shuttling Polymerization of Isoprene: An Approach To Access New Materials from Single Monomer. Macromolecules 2016, 49, 6226–6231. [Google Scholar] [CrossRef]
- Liu, B.; Wang, X.; Pan, Y.; Lin, F.; Wu, C.; Qu, J.; Luo, Y.; Cui, D. Unprecedented 3,4-Isoprene and cis-1,4-Butadiene Copolymers with Controlled Sequence Distribution by Single Yttrium Cationic Species. Macromolecules 2014, 47, 8524–8530. [Google Scholar] [CrossRef]
- Wang, B.; Cui, D.; Lv, K. Highly 3,4-Selective Living Polymerization of Isoprene with Rare Earth Metal Fluorenyl N-Heterocyclic Carbene Precursors. Macromolecules 2008, 41, 1983–1988. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, K.; Nishiura, M.; Hou, Z. Chain-Shuttling Polymerization at Two Different Scandium Sites: Regio- and Stereospecific “One-Pot” Block Copolymerization of Styrene, Isoprene, and Butadiene. Angew. Chem. Int. Ed. 2011, 50, 12012–12015. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Törnroos, K.W.; Anwander, R. Cationic Rare-Earth-Metal Half-Sandwich Complexes for the Living trans-1,4-Isoprene Polymerization. Angew. Chem. Int. Ed. 2008, 47, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Suzuki, T.; Luo, Y.; Nishiura, M.; Hou, Z. Cationic Alkyl Rare-Earth Metal Complexes Bearing an Ancillary Bis(phosphinophenyl)amido Ligand: A Catalytic System for Living cis-1,4-Polymerization and Copolymerization of Isoprene and Butadiene. Angew. Chem. 2007, 119, 1941–1945. [Google Scholar] [CrossRef]
- Zhang, L.; Nishiura, M.; Yuki, M.; Luo, Y.; Hou, Z. Isoprene Polymerization with Yttrium Amidinate Catalysts: Switching the Regio- and Stereoselectivity by Addition of AlMe3. Angew. Chem. Int. Ed. 2008, 47, 2642–2645. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Xu, T.; Yang, G.-W.; Jin, K.; Lu, X.-B. Bis(oxazolinyl)phenyl-Ligated Rare-Earth-Metal Complexes: Highly Regioselective Catalysts for cis-1,4-Polymerization of Isoprene. Inorg. Chem. 2013, 52, 2802–2808. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cui, D.; Hou, Z.; Li, W.; Li, Y. Highly cis-1,4-Selective Living Polymerization of 1,3-Conjugated Dienes and Copolymerization with ε-Caprolactone by Bis(phosphino)carbazolide Rare-Earth-Metal Complexes. Organometallics 2011, 30, 760–767. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, B.; Lv, K.; Gao, W.; Cui, D.; Chen, X.; Jing, X. Pyrrolide-Supported Lanthanide Alkyl Complexes. Influence of Ligands on Molecular Structure and Catalytic Activity toward Isoprene Polymerization. Organometallics 2007, 26, 4575–4584. [Google Scholar] [CrossRef]
- Shi, L.; Su, Q.; Chen, J.; Li, X.; Luo, Y. Rare-Earth Metal Bis(silylamide) Complexes Supported by Mono-Dentate Arylamido Ligand: Synthesis, Reactivity, and Catalyst {recursors in Living cis-1,4-Selective Polymerization of Isoprene. Dalton Trans. 2016, 45, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Tolpygin, A.O.; Glukhova, T.A.; Cherkasov, A.V.; Fukin, G.K.; Aleksanyan, D.V.; Cui, D.; Trifonov, A.A. Bis(alkyl) Rare-Earth Complexes Supported by a New Tridentate Amidinate Ligand with a Pendant Diphenylphosphine Oxide Group. Synthesis, Structures and Catalytic Activity in Isoprene Polymerization. Dalton Trans. 2015, 44, 16465–16474. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Sun, G.; Li, S.; Liu, D.; Cui, D. Isoprene Polymerization with Iminophosphonamide Rare-Earth-Metal Alkyl Complexes: Influence of Metal Size on the Regio- and Stereoselectivity. Organometallics 2015, 34, 4063–4068. [Google Scholar] [CrossRef]
- Li, S.; Cui, D.; Li, D.; Hou, Z. Highly 3,4-Selective Polymerization of Isoprene with NPN Ligand Stabilized Rare-Earth Metal Bis(alkyl)s. Structures and Performances. Organometallics 2009, 28, 4814–4822. [Google Scholar] [CrossRef]
- Rong, W.; Liu, D.; Zuo, H.; Pan, Y.; Jian, Z.; Li, S.; Cui, D. Rare-Earth-Metal Complexes Bearing Phosphazene Ancillary Ligands: Structures and Catalysis toward Highly Trans-1,4-Selective (Co)Polymerizations of Conjugated Dienes. Organometallics 2013, 32, 1166–1175. [Google Scholar] [CrossRef]
- Tobisch, S. Mechanistic Insight into the Selective trans-1,4-Polymerization of Butadiene by Terpyridine–iron(II) Complexes—A Computational Study. Can. J. Chem. 2009, 87, 1392–1405. [Google Scholar] [CrossRef]
- Perrin, L.; Bonnet, F.; Visseaux, M.; Maron, L. A DFT Study of Conjugated Dienes Polymerisation Catalyzed by [Cp*ScR]+: Insights into the Propensity for cis-1,4 Insertion. Chem. Commun. 2010, 46, 2965–2967. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Song, Y.; Luo, Y.; Li, G.; Hou, Z.; Qu, J. Computational Studies on Isospecific Polymerization of 1-Hexene Catalyzed by Cationic Rare Earth Metal Alkyl Complex Bearing a C3iPr-trisox Ligand. Macromolecules 2012, 45, 640–651. [Google Scholar] [CrossRef]
- Tobisch, S. Mechanism of the Chain Termination of the Allylnickel(II)-Catalyzed Polymerization of 1,3-Butadiene. A Density Functional Investigation for the Cationic [NiII(RC3H4)(cis-C4H6)L]+ Active Catalyst. Macromolecules 2003, 36, 6235–6244. [Google Scholar] [CrossRef]
- Luo, Y.; Luo, Y.; Qu, J.; Hou, Z. QM/MM Studies on Scandium-Catalyzed Syndiospecific Copolymerization of Styrene and Ethylene. Organometallics 2011, 30, 2908–2919. [Google Scholar] [CrossRef]
- Tobisch, S.; Taube, R. Mechanistic Studies of the 1,4-Polymerization of Butadiene According to the π-Allyl-Insertion Mechanism. 3. Density Functional Study of the C–C Bond Formation Reaction in Cationic “Ligand-Free” (η3:η2-Heptadienyl)(η2-/η4-butadiene)nickel(II) Complexes [Ni(C7H11)(C4H6)]+. Organometallics 1999, 18, 3045–3060. [Google Scholar]
- Woo, T.K.; Fan, L.; Ziegler, T. Density Functional Study of the Insertion Step in Olefin Polymerization by Metallocene and Constrained-Geometry Catalysts. Organometallics 1994, 13, 432–433. [Google Scholar] [CrossRef]
- Kang, X.; Luo, Y.; Zhou, G.; Wang, X.; Yu, X.; Hou, Z.; Qu, J. Theoretical Mechanistic Studies on the trans-1,4-Specific Polymerization of Isoprene Catalyzed by a Cationic La–Al Binuclear Complex. Macromolecules 2014, 47, 4596–4606. [Google Scholar] [CrossRef]
- Kang, X.; Yamamoto, A.; Nishiura, M.; Luo, Y.; Hou, Z. Computational Analyses of the Effect of Lewis Bases on Styrene Polymerization Catalyzed by Cationic Scandium Half-Sandwich Complexes. Organometallics 2015, 34, 5540–5548. [Google Scholar] [CrossRef]
- Kang, X.; Zhou, G.; Wang, X.; Qu, J.; Hou, Z.; Luo, Y. Alkyl Effects on the Chain Initiation Efficiency of Olefin Polymerization by Cationic Half-Sandwich Scandium Catalysts: A DFT Study. Organometallics 2016, 35, 913–920. [Google Scholar] [CrossRef]
- Perrin, L.; Kirillov, E.; Carpentier, J.-F.; Maron, L. DFT Investigation of the Tacticity Control during Styrene Polymerization Catalyzed by Single-Component Allyl ansa-Lanthanidocenes {(C5H4CMe2(9-C13H8)}Ln(C3H5). Macromolecules 2010, 43, 6330–6336. [Google Scholar] [CrossRef]
- Perrin, L.; Bonnet, F.; Chenal, T.; Visseaux, M.; Maron, L. A Joint Experimental/Theoretical Investigation of the Statistical Olefin/Conjugated Diene Copolymerization Catalyzed by a Hemi-Lanthanidocene [(Cp*)(BH4)LnR]. Chem. Eur. J. 2010, 16, 11376–11385. [Google Scholar] [CrossRef] [PubMed]
- Perrin, L.; Sarazin, Y.; Kirillov, E.; Carpentier, J.-F.; Maron, L. On the Initiation Mechanism of Syndiospecific Styrene Polymerization Catalyzed by Single-Component ansa-Lanthanidocenes. Chem. Eur. J. 2009, 15, 3773–3783. [Google Scholar] [CrossRef] [PubMed]
- Nsiri, H.; Belaid, I.; Larini, P.; Thuilliez, J.; Boisson, C.; Perrin, L. Ethylene–Butadiene Copolymerization by Neodymocene Complexes: A Ligand Structure/Activity/Polymer Microstructure Relationship Based on DFT Calculations. ACS Catal. 2015, 5, 1028–1036. [Google Scholar] [CrossRef]
- Wang, X.; Lin, F.; Qu, J.; Hou, Z.; Luo, Y. DFT Studies on Styrene Polymerization Catalyzed by Cationic Rare-Earth-Metal Complexes: Origin of Ligand-Dependent Activities. Organometallics 2016, 35, 3205–3214. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; heeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Perdew, J.P.; Wang, Y. Accurate and Simple Analytic Representation of the Electron-Gas Correlation Energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Benchmark Energetic Data in a Model System for Grubbs II Metathesis Catalysis and Their Use for the Development, Assessment, and Validation of Electronic Structure Methods. J. Chem. Theory Comput. 2009, 5, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Scalmani, G.; Frisch, M.J. Continuous Surface Charge Polarizable Continuum Models of Solvation. I. General Formalism. J.Chem. Phys. 2010, 132, 114110. [Google Scholar] [CrossRef] [PubMed]
- Tobisch, S. Reaction Mechanism in the Stereospecific 1,4-Polymerization of Butadiene with Ziegler–Natta Type Catalysts of Early Transition Metals: Comprehensive Density Functional Investigation for the Cationic [TiIIICp(polybutadienyl)(butadiene)]+ Active Catalyst. Organometallics 2003, 22, 2729–2740. [Google Scholar] [CrossRef]
- Castro, L.; Kirillov, E.; Miserque, O.; Welle, A.; Haspeslagh, L.; Carpentier, J.-F.; Maron, L. Are Solvent and Dispersion Effects Crucial in Olefin Polymerization DFT Calculations? Some Insights from Propylene Coordination and Insertion Reactions with Group 3 and 4 Metallocenes. ACS Catal. 2015, 5, 416–425. [Google Scholar] [CrossRef]
- Arlman, E.J.; Cossée, P. Ziegler-Natta Catalysis II. Surface Structure of Layer-Lattice Transition Metal Chlorides. J. Catal. 1964, 3, 89–98. [Google Scholar] [CrossRef]
- Arlman, E.J. Ziegler-natta catalysis: IV. The Stereospecificity in the Polymerization of Olefins and Conjugated Dienes in Relation to the Crystal Structure of TiCl3(α,β). J. Catal. 1966, 5, 178–189. [Google Scholar] [CrossRef]
- Taube, R.; Gehrke, J.P.; Radeglia, R. Komplexkatalyse: XXV. 31P-NMR-Spektroskopische Charakterisierung der anti- und syn-struktur von C3-Substituierten η3-Allylbis(triarylphosphit)-Nickel(II)-Hexafluorophosphat-Komplexen und der Mechanismus der Durch [C3H5Ni(P(OPh)3)2]PF6 Katalysierten 1,4-trans-Polymerisation des Butadiens. J. Organomet. Chem. 1985, 291, 101–115. [Google Scholar]
- Tobisch, S.; Bögel, H.; Taube, R. Mechanistic Studies of the 1,4-cis Polymerization of Butadiene According to the π-Allyl Insertion Mechanism. 1. Density Functional Study of the C–C Bond Formation Reaction in Cationic (η3-Allyl)(η2-/η4-butadiene)nickel(II) Complexes [Ni(C3H5)(C4H6)]+ and [Ni(C3H5)(C4H6)(C2H4)]+. Organometallics 1996, 15, 3563–3571. [Google Scholar]
- Schaper, F.; Geyer, A.; Brintzinger, H.H. Displacement of H3CB(C6F5)3− Anions from Zirconocene Methyl Cations by Neutral Ligand Molecules: Equilibria, Kinetics, and Mechanisms. Organometallics 2002, 21, 473–483. [Google Scholar] [CrossRef]
- Kitaura, K.; Morokuma, K. A new energy decomposition scheme for molecular interactions within the Hartree-Fock approximation. Int. J. Quantum Chem. 1976, 10, 325–340. [Google Scholar] [CrossRef]
- Liu, B.; Li, L.; Sun, G.; Liu, J.; Wang, M.; Li, S.; Cui, D. 3,4-Polymerization of Isoprene by Using NSN- and NPN-Ligated Rare Earth Metal Precursors: Switching of Stereo Selectivity and Mechanism. Macromolecules 2014, 47, 4971–4978. [Google Scholar] [CrossRef]

| Mechanism | Insertion Fashions | thfC2 | thfTS2 | thfP2 | ∆G‡ |
|---|---|---|---|---|---|
| π-allyl-insertion | η3-cis-1,4 | 13.0 | 28.6 | −4.9 | 28.6 |
| η3-cis-1,4 b | 15.4 | 37.6 | 1.2 | 37.5 | |
| η3-cis-1,2 | 11.8 | 30.8 | −2.7 | 30.8 | |
| η3-trans-1,4 | 9.2 | 31.8 | −3.3 | 31.8 | |
| η3-trans-1,2 | 14.3 | 30.2 | −2.3 | 30.2 | |
| σ-allyl-insertion | η1-cis-1,4 | 21.4 | 29.2 | −6.3 | 29.2 |
| η1-trans-1,4 | n.a. c | 35.1 | −3.2 | 35.1 |
| Mechanism | Insertion fashions | C2 | TS2 | P2 | ∆G‡ |
|---|---|---|---|---|---|
| π-allyl-insertion | η3-cis-1,4 | −0.4 | 6.5 | −16.5 | 6.9 |
| η3-cis-1,4 b | 3.5 | 11.8 | −12.5 | 11.8 | |
| η3-cis-1,2 | 3.5 | 10.5 | −8.6 | 10.5 | |
| η3-trans-1,4 | −5.4 | 9.4 | −5.3 | 14.8 | |
| η3-trans-1,2 | −2.1 | 19.1 | −14.0 | 21.2 | |
| σ-allyl-insertion | η1-cis-1,4 | n.a. c | 19.1 | −2.9 | ≥19.1 |
| η1-trans-1,4 | −1.8 | 22.2 | −4.0 | 24.0 |
| η3-TS2 | ∆Eint | ∆Edef (PNP) | ∆Edef (Bu) | ∆Edef (Chain) | ∆Edef a | ∆ETS |
|---|---|---|---|---|---|---|
| η3-TS2cis-1,4 | −4.4 | 2.2 | 1.2 | 1.2 | 4.6 | 0.2 |
| η3-TS2cis-1,2 | −5.0 | 1.0 | 6.3 | 0.0 | 7.3 | 2.3 |
| η3-TS2trans-1,4 | 0.0 | 0.0 | 0.0 | 2.8 | 2.8 | 2.8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Kang, X.; Zhou, G.; Qu, J.; Hou, Z.; Luo, Y. DFT Studies on cis-1,4-Polymerization of Dienes Catalyzed by a Cationic Rare-Earth Metal Complex Bearing an Ancillary PNP Ligand. Polymers 2017, 9, 53. https://doi.org/10.3390/polym9020053
Wang X, Kang X, Zhou G, Qu J, Hou Z, Luo Y. DFT Studies on cis-1,4-Polymerization of Dienes Catalyzed by a Cationic Rare-Earth Metal Complex Bearing an Ancillary PNP Ligand. Polymers. 2017; 9(2):53. https://doi.org/10.3390/polym9020053
Chicago/Turabian StyleWang, Xingbao, Xiaohui Kang, Guangli Zhou, Jingping Qu, Zhaomin Hou, and Yi Luo. 2017. "DFT Studies on cis-1,4-Polymerization of Dienes Catalyzed by a Cationic Rare-Earth Metal Complex Bearing an Ancillary PNP Ligand" Polymers 9, no. 2: 53. https://doi.org/10.3390/polym9020053
APA StyleWang, X., Kang, X., Zhou, G., Qu, J., Hou, Z., & Luo, Y. (2017). DFT Studies on cis-1,4-Polymerization of Dienes Catalyzed by a Cationic Rare-Earth Metal Complex Bearing an Ancillary PNP Ligand. Polymers, 9(2), 53. https://doi.org/10.3390/polym9020053