From Insulating PMMA Polymer to Conjugated Double Bond Behavior: Green Chemistry as a Novel Approach to Fabricate Small Band Gap Polymers
Abstract
:1. Introduction
2. Experimental
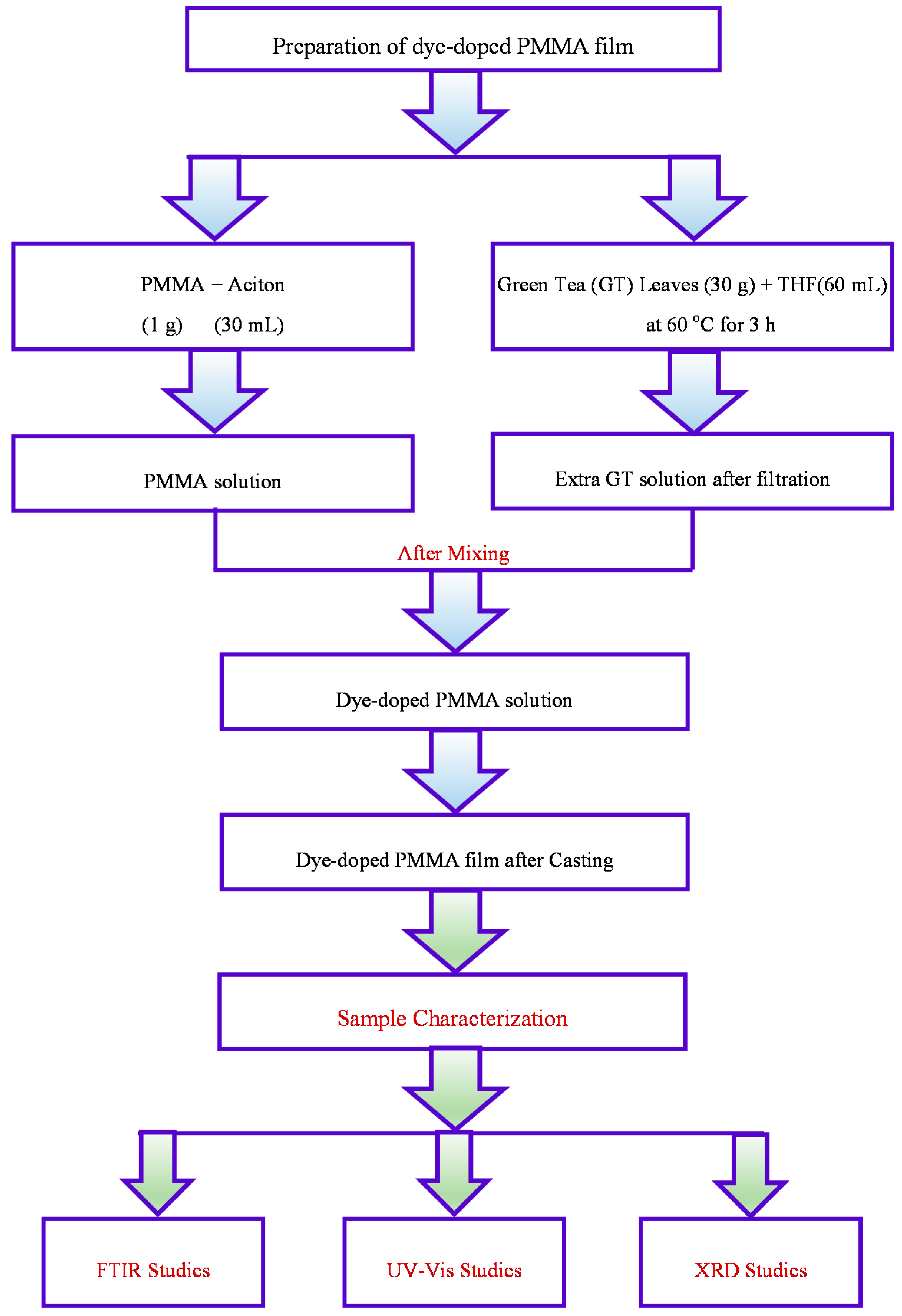
2.1. Preparation of Dye-Doped PMMA Solid Polymeric Films
2.2. UV–VIS Measurement
2.3. FTIR and X-ray Diffraction Analysis
3. Results and Discussion
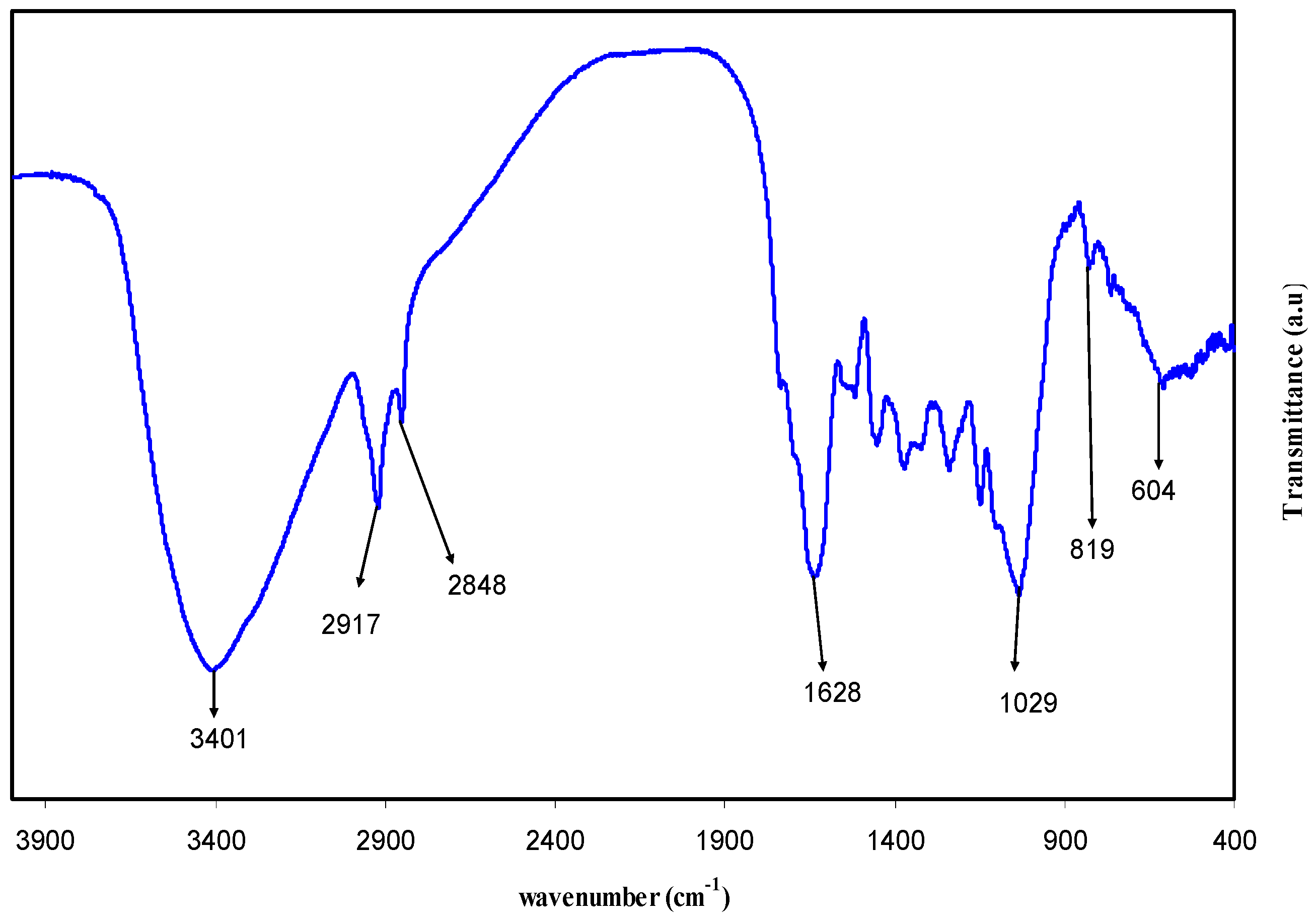
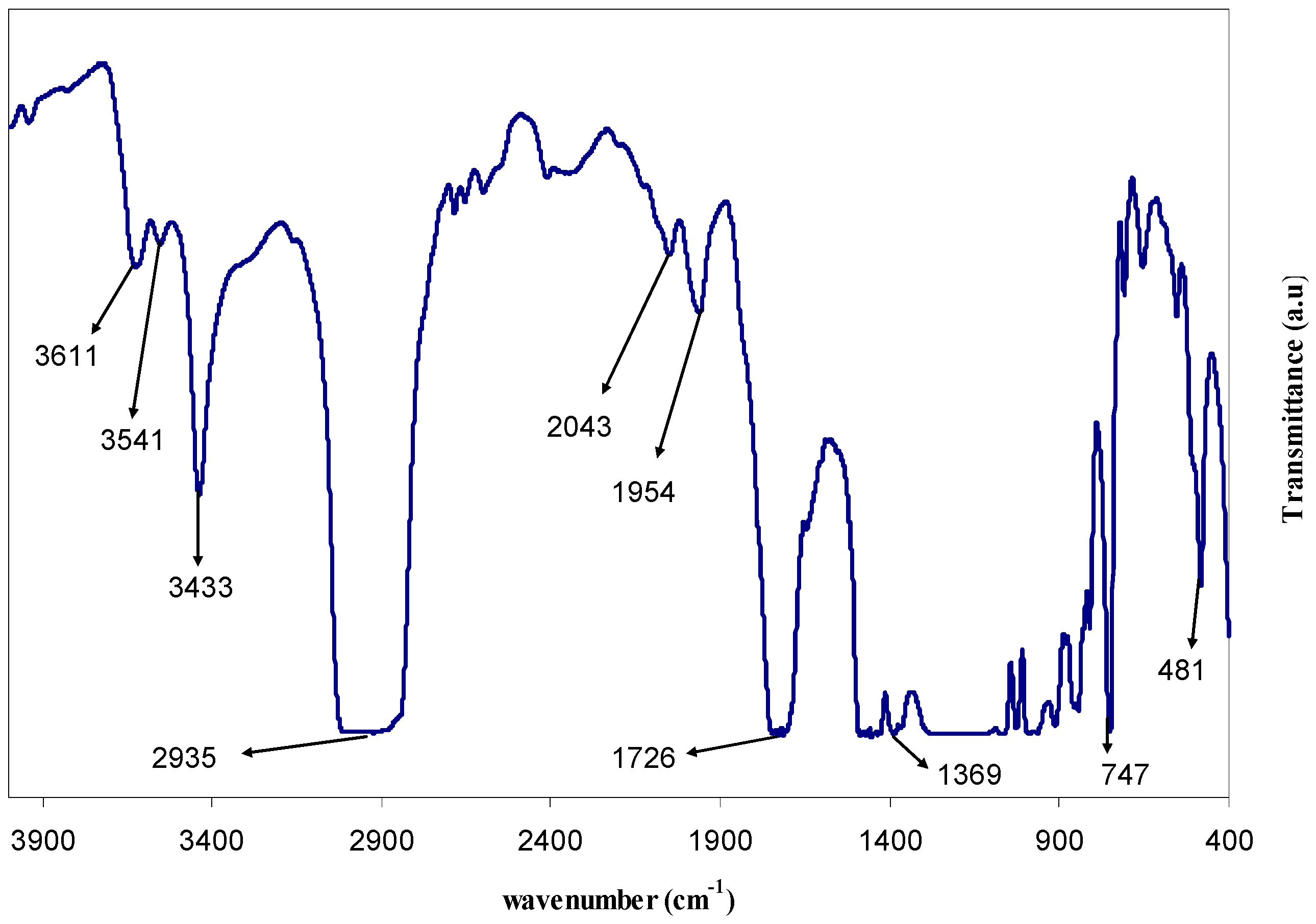
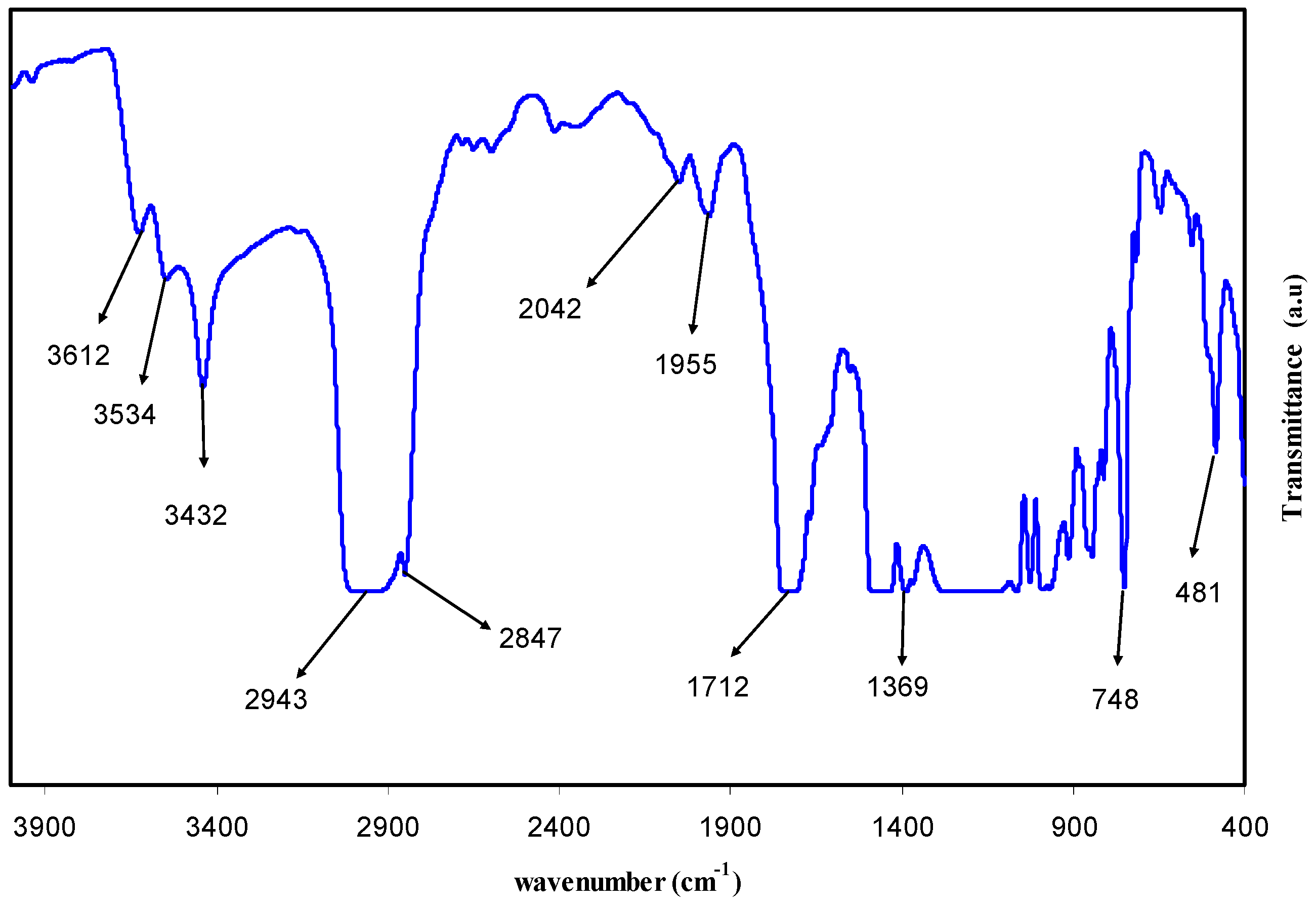
3.1. FTIR Study
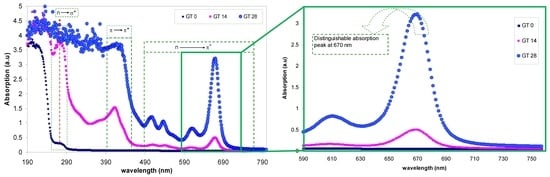
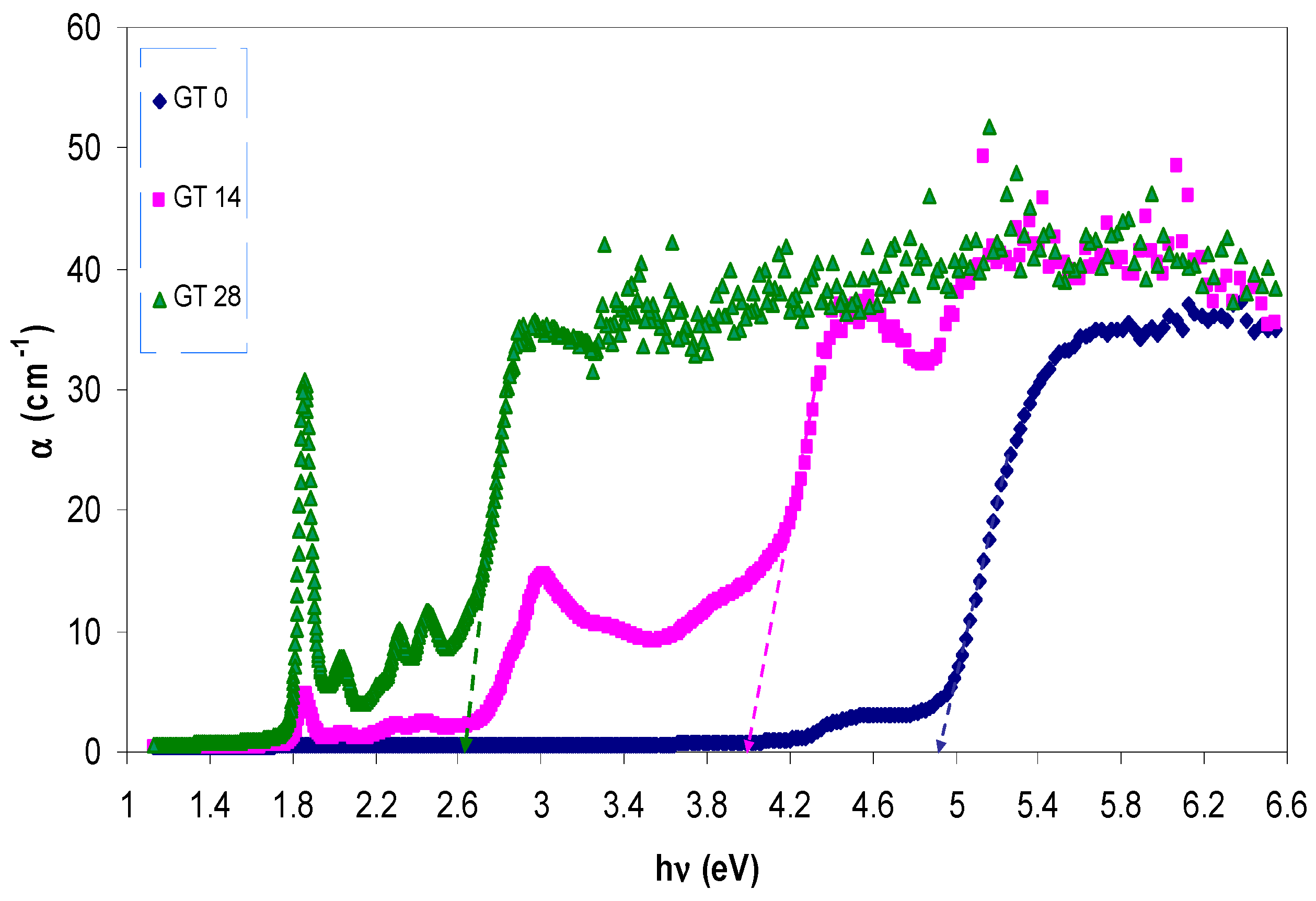
3.2. Absorption and Absorption Coefficient Study
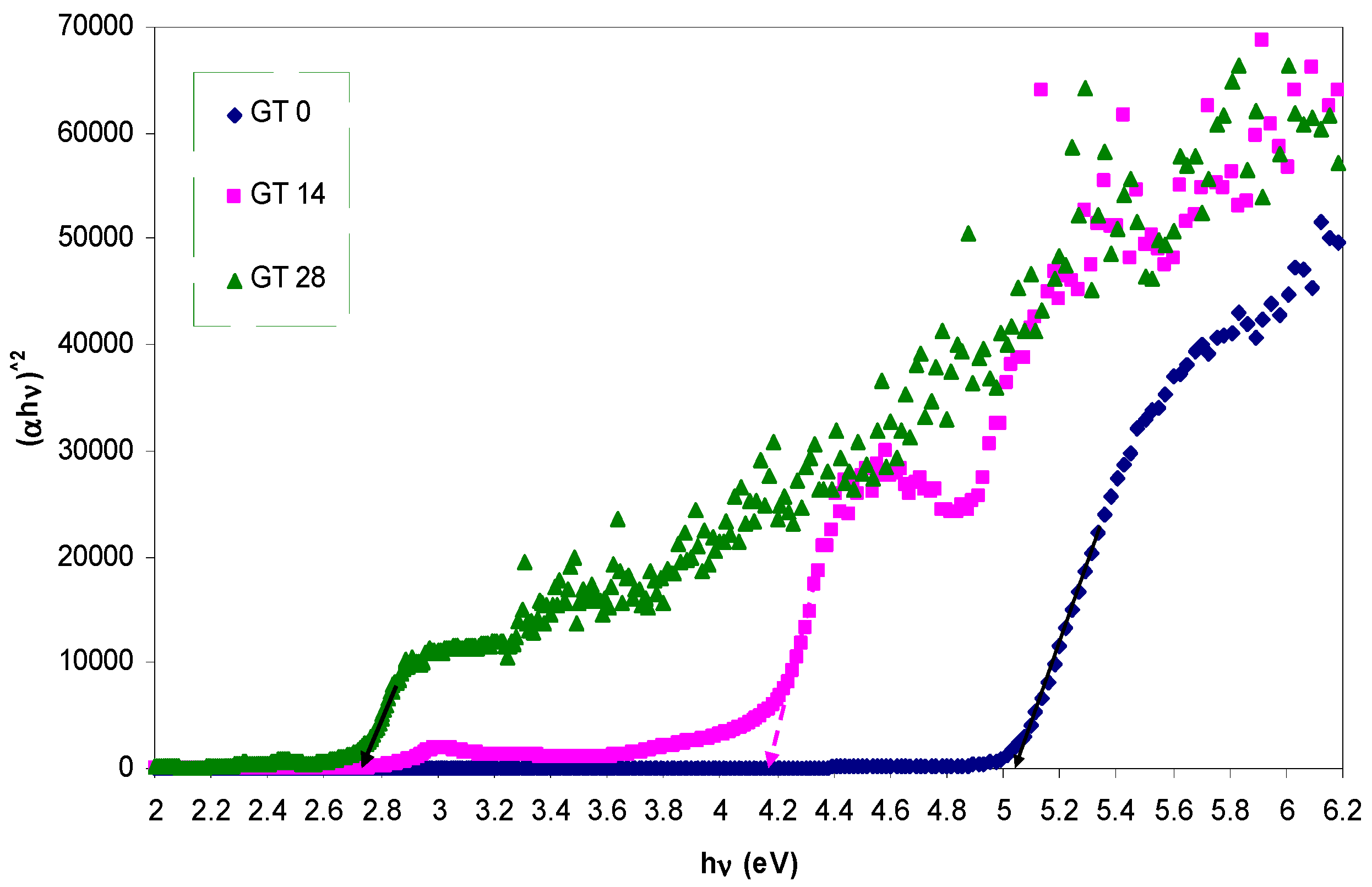
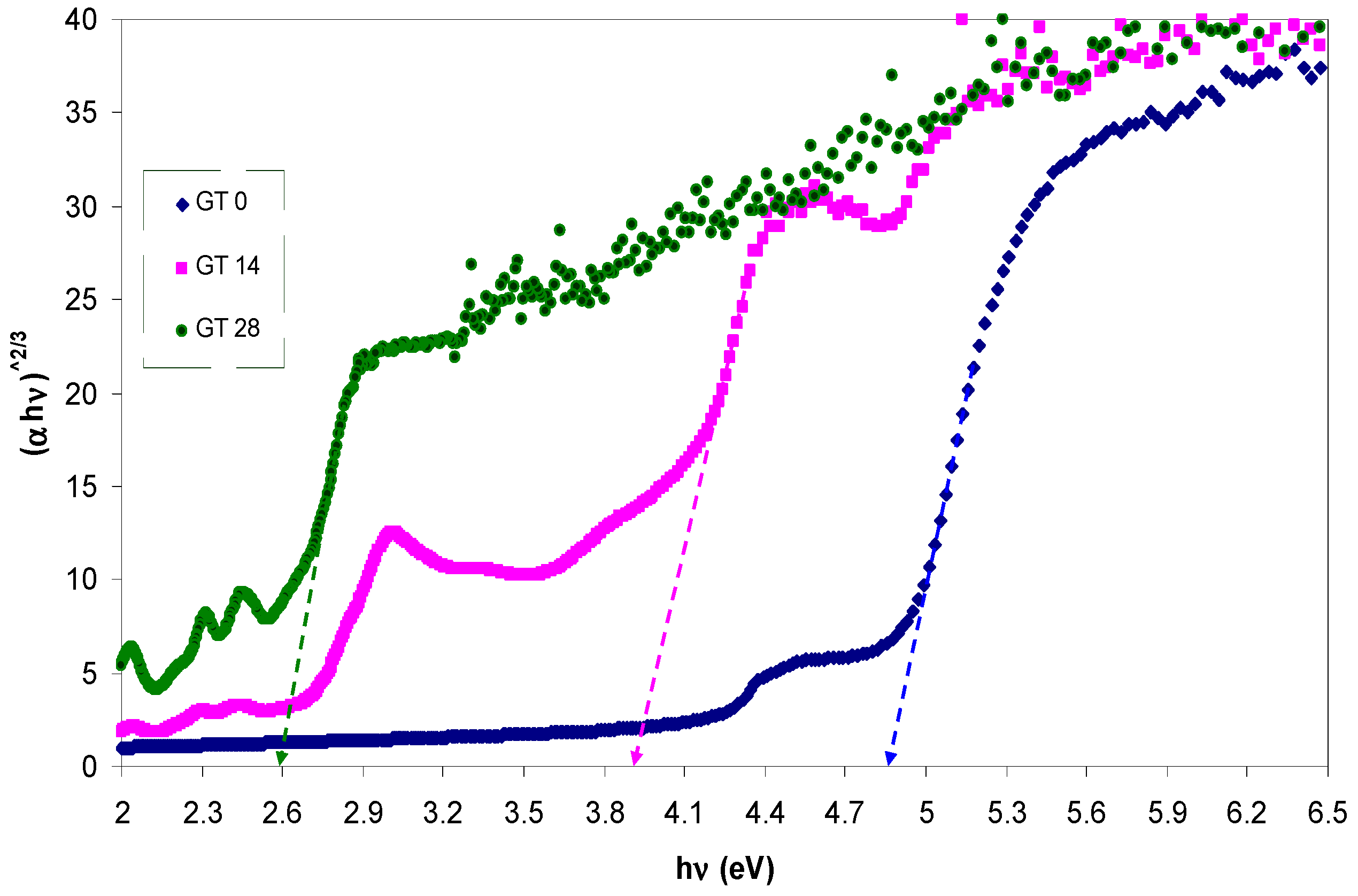
3.3. Band Gap Study
3.4. Urbach Energy and Materials Structure
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gaur, S.S.; Ghawana, K.; Tripathi, K.N. Fabrication and characterization of dye-doped polymeric mode filter. Opt. Quantum Electron. 2005, 37, 805–812. [Google Scholar] [CrossRef]
- Chandrahalim, H.; Fan, X. Reconfigurable solid-state dye-doped polymer ring resonator lasers. Sci. Rep. 2015, 5, 18310. [Google Scholar] [CrossRef] [PubMed]
- Ishchenko, A.A. Photonics and molecular design of dye-doped polymers for modern light-sensitive materials. Pure Appl. Chem. 2008, 80, 1525–1538. [Google Scholar] [CrossRef]
- Maji, P.; Choudhary, R.B.; Majhi, M. Structural, optical and dielectric properties of ZrO2 reinforced polymeric nanocomposite films of polymethylmethacrylate (PMMA). Optik 2016, 127, 4848–4853. [Google Scholar] [CrossRef]
- Alsawafta, M.; Badilescu, S.; Paneri, A.; Truong, V.V.; Packirisamy, M. Gold-poly(methyl methacrylate) nanocomposite films for plasmonic biosensing applications. Polymers 2011, 3, 1833–1848. [Google Scholar] [CrossRef]
- Hamdy, M.S.; AlFaify, S.; Al-Hajry, A.; Yahia, I.S. Optical constants, photo-stability and photo-degradation of MB/PMMA thin films for UV sensors. Optik 2016, 127, 4959–4963. [Google Scholar] [CrossRef]
- Ebnalwaleda, A.A.; Thabet, A. Controlling the optical constants of PVC nanocomposite films for optoelectronic applications. Synth. Met. 2016, 220, 374–383. [Google Scholar] [CrossRef]
- Hassan, H.E.; Refat, M.S.; Sharshar, T. Optical and positron annihilation spectroscopic studies on PMMA polymer doped by rhodamine B/chloranilic acid charge transfer complex: Special relevance to the effect of γ-rays irradiation. Spectrochim. Acta A 2016, 159, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Enculescu, M.; Matei, E. Influence of metallic and semiconducting nanostructures on the optical properties of dye-doped polymer thin films. Thin Solid Films 2016, 614, 31–35. [Google Scholar] [CrossRef]
- Fleischmann, C.; Lievenbrück, M.; Ritter, H. Polymers and dyes: Developments and applications. Polymers 2015, 7, 717–746. [Google Scholar] [CrossRef]
- Ishchenko, A. Molecular engineering of dye-doped polymers for optoelectronics. Polym. Adv. Technol. 2002, 13, 744–752. [Google Scholar] [CrossRef]
- Sun, X.; Chang, F.; Gai, K. Optoelectronic fast response properties of PQ/PMMA polymer. Mater. Today Proc. 2016, 3, 632–634. [Google Scholar] [CrossRef]
- Lee, L.S.; Kim, S.H.; Kim, Y.B.; Kim, Y.C. Quantitative analysis of major constituents in green tea with different plucking periods and their antioxidant activity. Molecules 2014, 19, 9173–9186. [Google Scholar] [CrossRef] [PubMed]
- Reto, M.; Figueira, M.E.; Filipe, H.M.; Almeida, C.M. Chemical composition of green tea (Camellia sinensis) infusions commercialized in Portugal. Plant Foods Hum. Nutr. 2007, 62, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Hwang, E.K.; Kim, H.D. Colorimetric assay and antibacterial activity of cotton, silk, and wool fabrics dyed with peony, pomegranate, clove, coptischinenis and gallnut extracts. Materials 2009, 2, 10–21. [Google Scholar] [CrossRef] [Green Version]
- Hwang, E.K.; Lee, Y.H.; Kim, H.D. Dyeing, fastness, and deodorizing properties of cotton, silk, and wool fabrics dyed with gardenia, coffee sludge, Cassia tora. L., and pomegranate extracts. Fibers Polym. 2008, 9, 334–340. [Google Scholar] [CrossRef]
- Loo, Y.Y.; Chieng, B.W.; Nishibuchi, M.; Radu, S. Synthesis of silver nanoparticles by using tea leaf extract from Camellia sinensis. Int. J. Nanomed. 2012, 7, 4263–4267. [Google Scholar] [CrossRef]
- Senthilkumar, S.R.; Sivakumar, T. Green tea (Camellia sinensis) mediated synthesis of zinc oxide (ZnO) nanoparticles and studies on their antimicrobial activities. Int. J. Pharm. Pharm. Sci. 2014, 6, 461–465. [Google Scholar]
- Dubey, S.P.; Sillanpaa, M.; Varma, R.S. Reduction of hexavalent chromium using Sorbaria sorbifolia aqueous leaf extract. Appl. Sci. 2017, 7, 715. [Google Scholar] [CrossRef]
- Huang, L.; Weng, X.; Chen, Z.; Megharaj, M.; Naidu, R. Synthesis of iron-based nanoparticles using oolong tea extract for thedegradation of malachite green. Spectrochim. Acta A 2014, 117, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Huang, L.; Chen, Z.; Megharaj, M.; Naidu, R. Synthesis of iron-based nanoparticles by green tea extract and theirdegradation of malachite. Ind. Crop. Prod. 2013, 51, 342–347. [Google Scholar] [CrossRef]
- Ahmed, R.M. Optical study on poly(methyl methacrylate)/poly(vinyl acetate) blends. Int. J. Photoenergy 2009, 2009, 7. [Google Scholar] [CrossRef]
- Kalsi, P.S. Spectroscopy of Organic Compounds, 6th ed.; New Age International, Pvt Ltd. Publishers: Delhi, India, 2004. [Google Scholar]
- Balamurugan, A.; Kannan, S.; Selvaraj, V.; Rajeswari, S. Development and spectral characterization of poly(methyl methacrylate)/hydroxyapatite composite for biomedical applications. Trends Biomater. Artif. Organs 2004, 18, 41–45. [Google Scholar]
- Aziz, S.B.; Abidin, Z.H.Z. Electrical conduction mechanism in solid polymer electrolytes: New concepts to Arrhenius equation. J. Soft Matter 2013, 2013, 8. [Google Scholar] [CrossRef]
- Wei, D.; Sun, W.; Qian, W.; Ye, Y.; Ma, X. The synthesis of chitosan-based silver nanoparticles and their antibacterial activity. Carbohydr. Res. 2009, 344, 2375–2382. [Google Scholar] [CrossRef] [PubMed]
- Soman, V.V.; Kelkar, D.S. FTIR studies of doped PMMA—PVC blend system. Macromol. Symp. 2009, 277, 152–161. [Google Scholar] [CrossRef]
- Kumar, R.; Ali, S.A.; Mahur, A.K.; Virk, H.S.; Singh, F.; Khan, S.A.; Avasthi, D.K.; Prasad, R. Study of optical band gap and carbonaceous clusters in swift heavy ion irradiated polymers with UV–Vis spectroscopy. Nucl. Instrum. Methods Phys. Res. B 2008, 266, 1788–1792. [Google Scholar] [CrossRef]
- Ingle, J.D.; Crouch, S.R. Spectrochemical Analysis; Prentice Hall: Englewood Cliffs, NJ, USA, 1988. [Google Scholar]
- Srivastava, A.; Singh, V.; Aggarwal, P.; Schneeweiss, F.; Scherer, U.W.; Friedrich, W. Optical studies of insulating polymers for radiation dose monitoring. Indian J. Pure Appl. Phys. 2010, 48, 782–786. [Google Scholar]
- Marzuki, A.; Suryanti, V.; Virgynia, A. Spectroscopic study of green tea (Camellia sinensis) leaves extraction. IOP Conf. Ser. Mater. Sci. Eng. 2017, 193. [Google Scholar] [CrossRef]
- Kumar, K.R.P.; Murali, M.G.; Udayakumar, D. Synthesis and study of optical properties of linear and hyperbranched conjugated polymers containing thiophene and riphenylamine units. Des. Monomers Polym. 2014, 17, 7–18. [Google Scholar] [CrossRef]
- Koyuncu, F.B.; Sefer, E.; Koyuncu, S.; Ozdemir, E. A new low band gap electrochromic polymer containing 2,5-bis-dithienyl-1H-pyrrole and 2,1,3-benzoselenadiazole moiety with high contrast ratio. Polymer 2011, 52, 5772–5779. [Google Scholar] [CrossRef]
- Noelia, L.G.; Roberto, R.G.; Patricia, P.B.; Vidal, J.L.M.; Frenich, A.G. Identification and quantification of phytochemicals in nutraceutical products from green tea by UHPLC–Orbitrap-MS. Food Chem. 2015, 173, 607–618. [Google Scholar] [CrossRef]
- Pasrija, D.; Anandharamakrishnan, C. Techniques for extraction of green tea polyphenols: A review. Food Bioprocess Technol. 2015, 8, 935–950. [Google Scholar] [CrossRef]
- Aziz, S.B. Modifying poly(vinyl alcohol) (PVA) from insulator to small band gap polymer: A novel approach for organic solar cells and optoelectronic devices. J. Electron. Mater. 2016, 45, 736–745. [Google Scholar] [CrossRef]
- Arslan, M.; Atak, F.B.; Yakuphanoglu, F. Synthesis and refractive index dispersion properties of the N,N′,N′′-trinaphthylmethyl melamine–DDQ complex thin film. Opt. Mater. 2007, 29, 516–520. [Google Scholar] [CrossRef]
- Calvert, P. Optical Properties of Polymer Composites. In Wiley Encyclopedia of Composites; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 1–15. [Google Scholar] [CrossRef]
- Yakuphanoglu, F.; Sekerci, M.; Ozturk, O.F. The determination of the optical constants of Cu(II) compound having 1-chloro-2,3-o-cyclohexylidinepropane thin film. Opt. Commun. 2004, 239, 275–280. [Google Scholar] [CrossRef]
- Yakuphanoglu, F.; Cukurovali, A.; Yilmaz, I. Refractive index and optical absorption properties of the complexes of a cyclobutane containing thiazolylhydrazone ligand. Opt. Mater. 2005, 27, 1363–1368. [Google Scholar] [CrossRef]
- Yakuphanoglu, F.; Viswanathan, C. Electrical conductivity and single oscillator model properties of amorphous CuSe semiconductor thin film. J. Non-Cryst. Solids 2007, 353, 2934–2937. [Google Scholar] [CrossRef]
- Bhajantri, R.F.; Ravindrachary, V.; Harisha, A.; Crasta, V.; Nayak, S.P.; Poojary, B. Microstructural studies on BaCl2 doped poly(vinyl alcohol). Polymer 2006, 47, 3591–3598. [Google Scholar] [CrossRef]
- Yakuphanoglu, F.; Arslan, M. Determination of electrical conduction mechanism and optical band gap of a new charge transfer complex: TCNQ-PANT. Solid State Commun. 2004, 132, 229–234. [Google Scholar] [CrossRef]
- Abdullah, O.G.; Aziz, S.B.; Omer, K.M.; Salih, Y.M. Reducing the optical band gap of polyvinyl alcohol (PVA) based nanocomposite. J. Mater. Sci. Mater. Electron. 2015, 26, 5303–5309. [Google Scholar] [CrossRef]
- Mohan, V.M.; Bhargav, P.B.; Raja, V.; Sharma, A.K.; Rao, V.V.R.N. Optical and electrical properties of pure and doped PEO polymer electrolyte films. Soft Mater. 2007, 5, 33–46. [Google Scholar] [CrossRef]
- Kumar, K.K.; Ravi, M.; Pavani, Y.; Bhavani, S.; Sharma, A.K.; Rao, V.V.R.N. Investigations on the effect of complexation of NaF salt with polymer blend (PEO/PVP) electrolytes on ionic conductivity and optical energy band gaps. Phys. B Condens. Matter 2011, 406, 1706–1712. [Google Scholar] [CrossRef]
- Yakuphanoglu, F.; Arslan, M. The fundamental absorption edge and optical constants of some charge transfer compounds. Opt. Mater. 2004, 27, 29–37. [Google Scholar] [CrossRef]
- Kittel, C. Introduction to Solid State Physics, 8th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; Volume 429, Chapter 15. [Google Scholar]
- Aziz, S.B.; Rasheed, M.A.; Ahmed, H.M. Synthesis of polymer nanocomposites based on [methyl cellulose](1-x):(CuS)x (0.02 M ≤ x ≤ 0.08 M) with desired optical band gaps. Polymers 2017, 9, 194. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, O.G.; Rasheed, M.A. A Novel polymer composite with a small optical bandgap: New approaches for photonics and optoelectronics. J. Appl. Polym. Sci. 2017, 134, 44847. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, O.G.; Hussein, A.M.; Abdulwahid, R.T.; Rasheed, M.A.; Ahmed, H.M.; Abdalqadir, S.W.; Mohammed, A.R. Optical properties of pure and doped PVA:PEO based solid polymer blend electrolytes: Two methods for band gap study. J. Mater. Sci. Mater. Electron. 2017, 28, 7473–7479. [Google Scholar] [CrossRef]
- Patterson, J.D.; Bailey, B.C. Solid State Physics: Introduction to the Theory; Springer: Berlin, Germany, 2007; Volume 568, Chapter 10. [Google Scholar]
- El-Nahass, M.M.; Farid, A.M.; Atta, A.A. Structural and optical properties of Tris(8-hydroxyquinoline) aluminum (III) (Alq3) thermal evaporated thin films. J. Alloys Compd. 2010, 507, 112–119. [Google Scholar] [CrossRef]
- Ahmad, F.; Sheha, E. Preparation and physical properties of (PVA)0.7(NaBr)0.3(H3PO4)xM solid acid membrane for phosphoric acid—Fuel cells. J. Adv. Res. 2013, 4, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Sheha, E.; Khoder, H.; Shanap, T.S.; El-Shaarawy, M.G.; El Mansy, M.K. Structure, dielectric and optical properties of p-type (PVA/CuI) nanocomposite polymer electrolyte for photovoltaic cells. Optik 2012, 123, 1161–1166. [Google Scholar] [CrossRef]
- Aziz, S.B.; Ahmed, H.M.; Hussein, A.M.; Fathulla, A.B.; Wsw, R.M.; Hussein, R.T. Tuning the absorption of ultraviolet spectra and optical parameters of aluminum doped PVA based solid polymer composites. J. Mater. Sci. Mater. Electron. 2015, 26, 8022–8028. [Google Scholar] [CrossRef]
- Prasher, S.; Kumar, M.; Singh, S. Electrical and optical properties of O6+ion beam–irradiated polymers. Int. J. Polym. Anal. Charact. 2014, 19, 204–211. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdulwahid, R.T.; Rasul, H.A.; Ahmed, H.M. In situ synthesis of CuS nanoparticle with a distinguishable SPR peak in NIR region. J. Mater. Sci. Mater. Electron. 2016, 27, 4163–4171. [Google Scholar] [CrossRef]



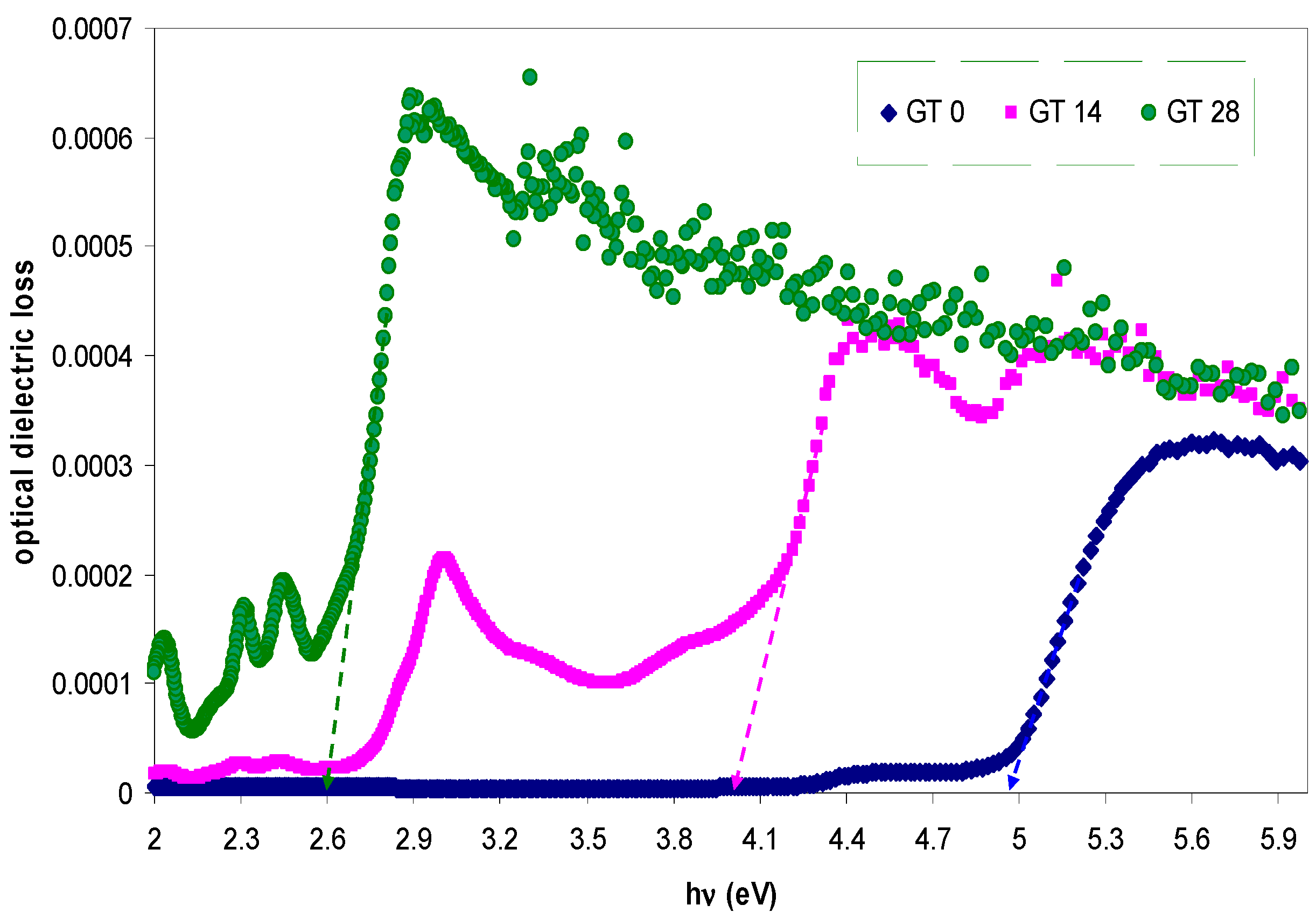
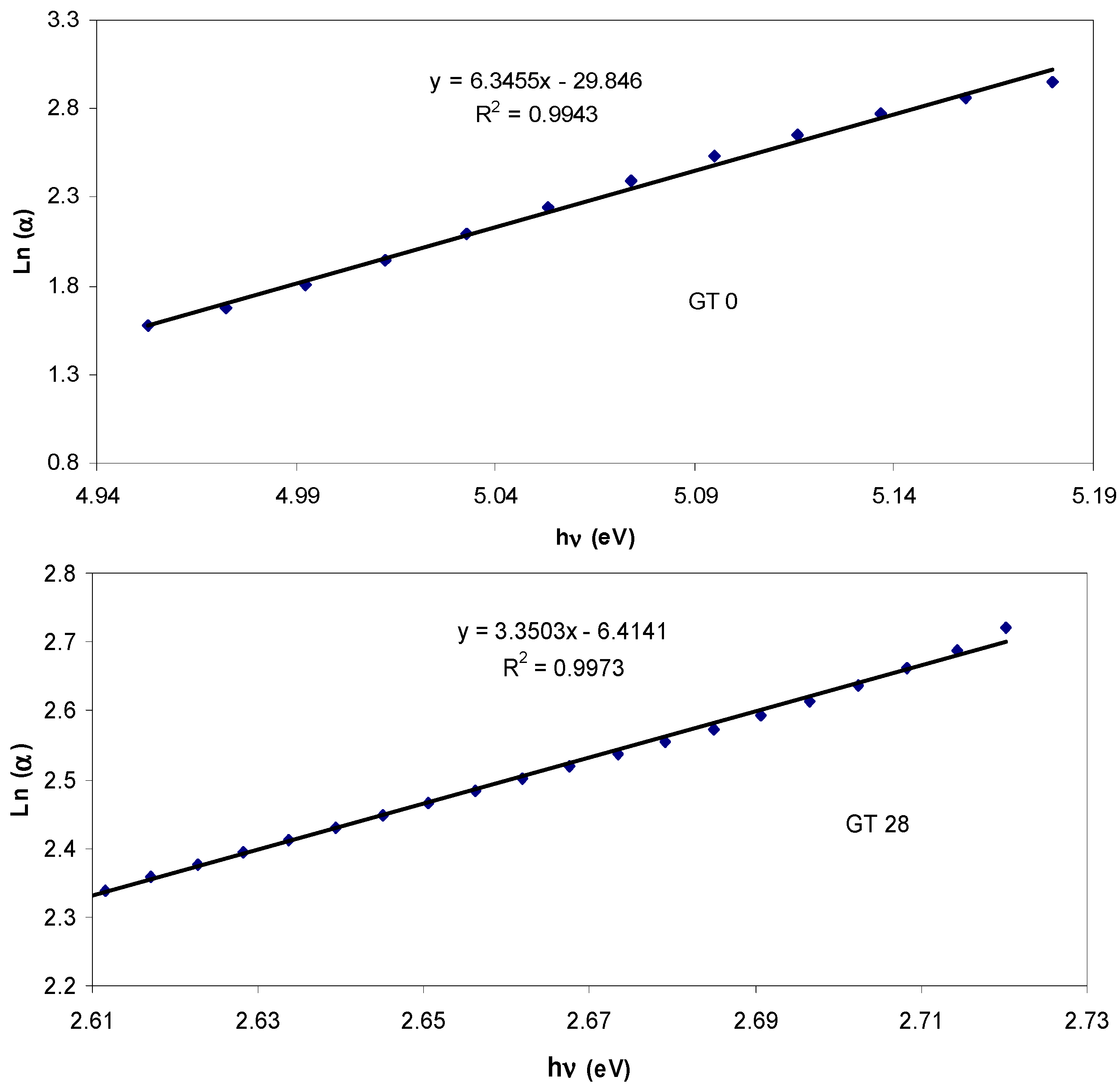
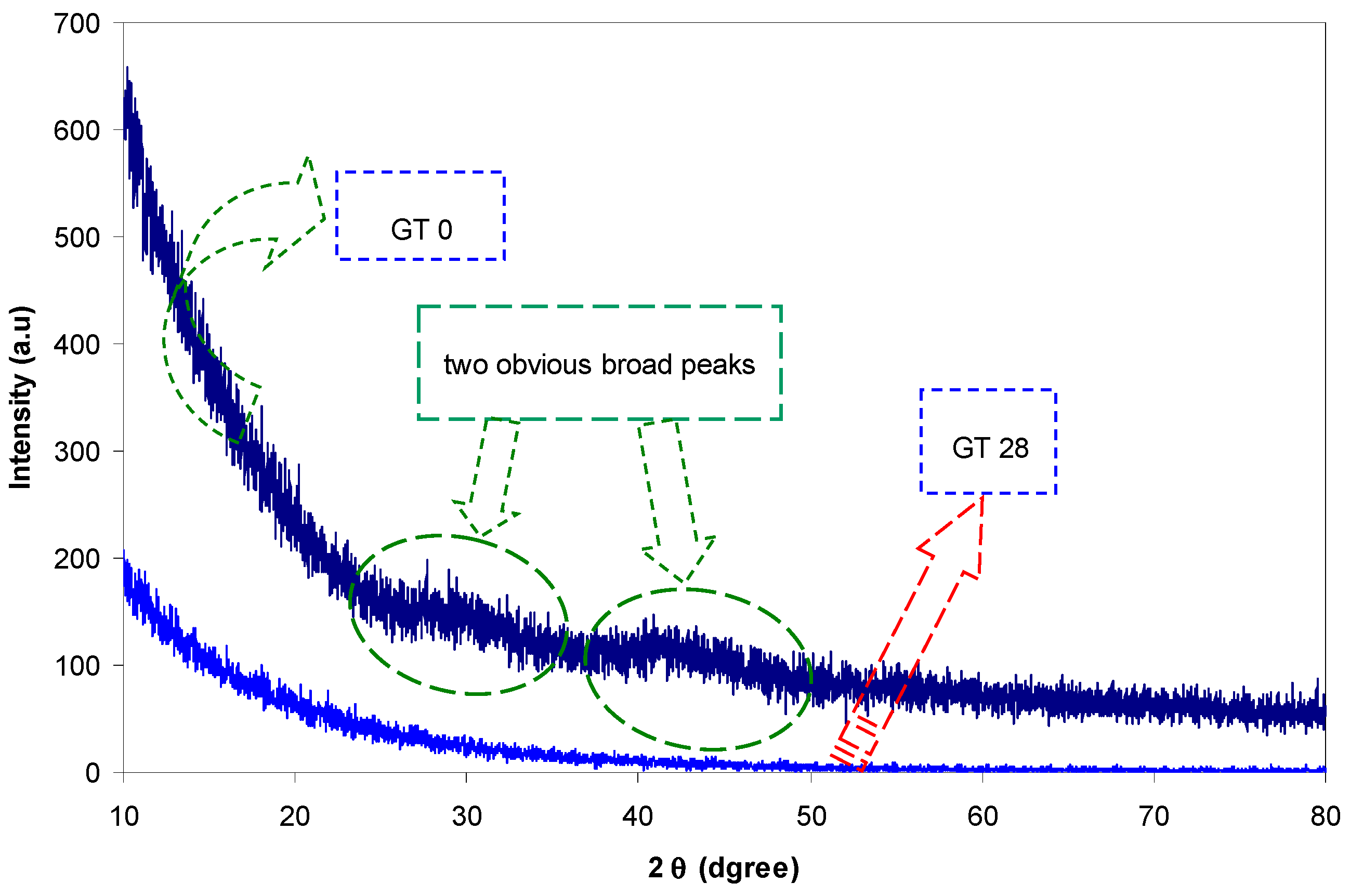
| Sample designation | Absorption edge (eV) | Optical bandgap from Tauc’s model (eV) | Optical bandgap from ε″ vs. hυ |
|---|---|---|---|
| GT 0 | 4.9 | 5.04, γ = 1/2 | 4.97 |
| GT 14 | 3.97 | 3.94, γ = 3/2 | 3.97 |
| GT 28 | 2.66 | 2.6, γ = 3/2 | 2.6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aziz, S.B.; Abdullah, O.G.; Hussein, A.M.; Ahmed, H.M. From Insulating PMMA Polymer to Conjugated Double Bond Behavior: Green Chemistry as a Novel Approach to Fabricate Small Band Gap Polymers. Polymers 2017, 9, 626. https://doi.org/10.3390/polym9110626
Aziz SB, Abdullah OG, Hussein AM, Ahmed HM. From Insulating PMMA Polymer to Conjugated Double Bond Behavior: Green Chemistry as a Novel Approach to Fabricate Small Band Gap Polymers. Polymers. 2017; 9(11):626. https://doi.org/10.3390/polym9110626
Chicago/Turabian StyleAziz, Shujahadeen B., Omed Gh. Abdullah, Ahang M. Hussein, and Hameed M. Ahmed. 2017. "From Insulating PMMA Polymer to Conjugated Double Bond Behavior: Green Chemistry as a Novel Approach to Fabricate Small Band Gap Polymers" Polymers 9, no. 11: 626. https://doi.org/10.3390/polym9110626