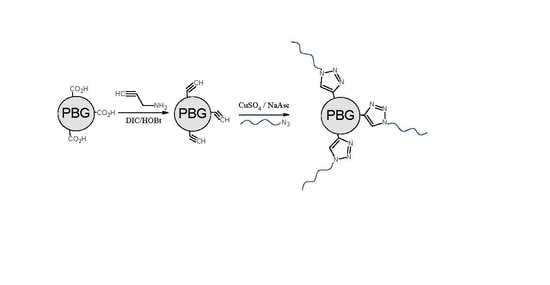
Arborescent Unimolecular Micelles: Poly(γ-Benzyl l-Glutamate) Core Grafted with a Hydrophilic Shell by Copper(I)-Catalyzed Azide–Alkyne Cycloaddition Coupling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization and Sample Preparation
2.2. Solvent and Reagent Purification
2.3. Synthesis of Linear Polymers with a Terminal Azide Functionality
2.3.1. Synthesis of α-Azido PGly
2.3.2. Synthesis of ω-Azido PEO
2.3.3. Synthesis of ω-Azido P(HEA-TMS)
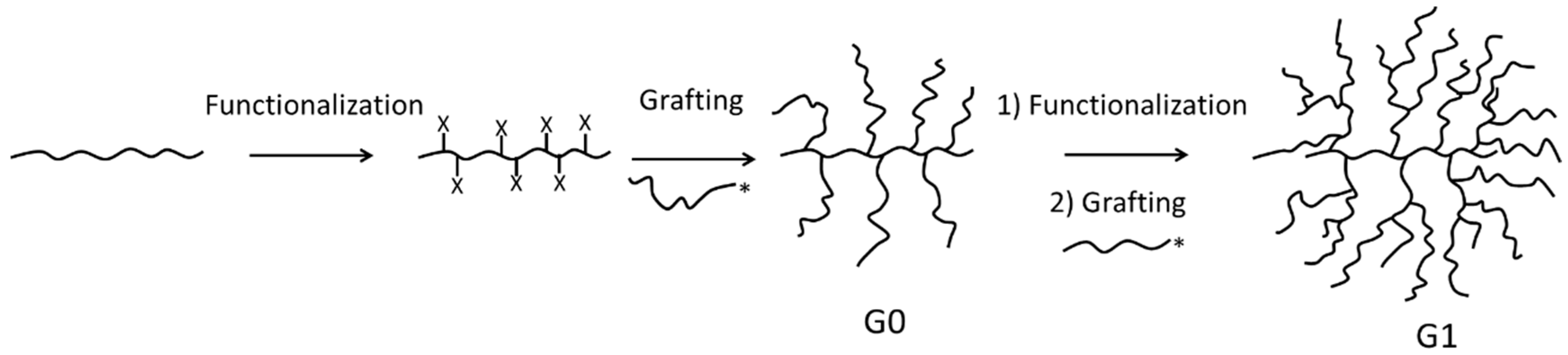
2.4. Synthesis of CuAAC-Grafted Arborescent Copolymers
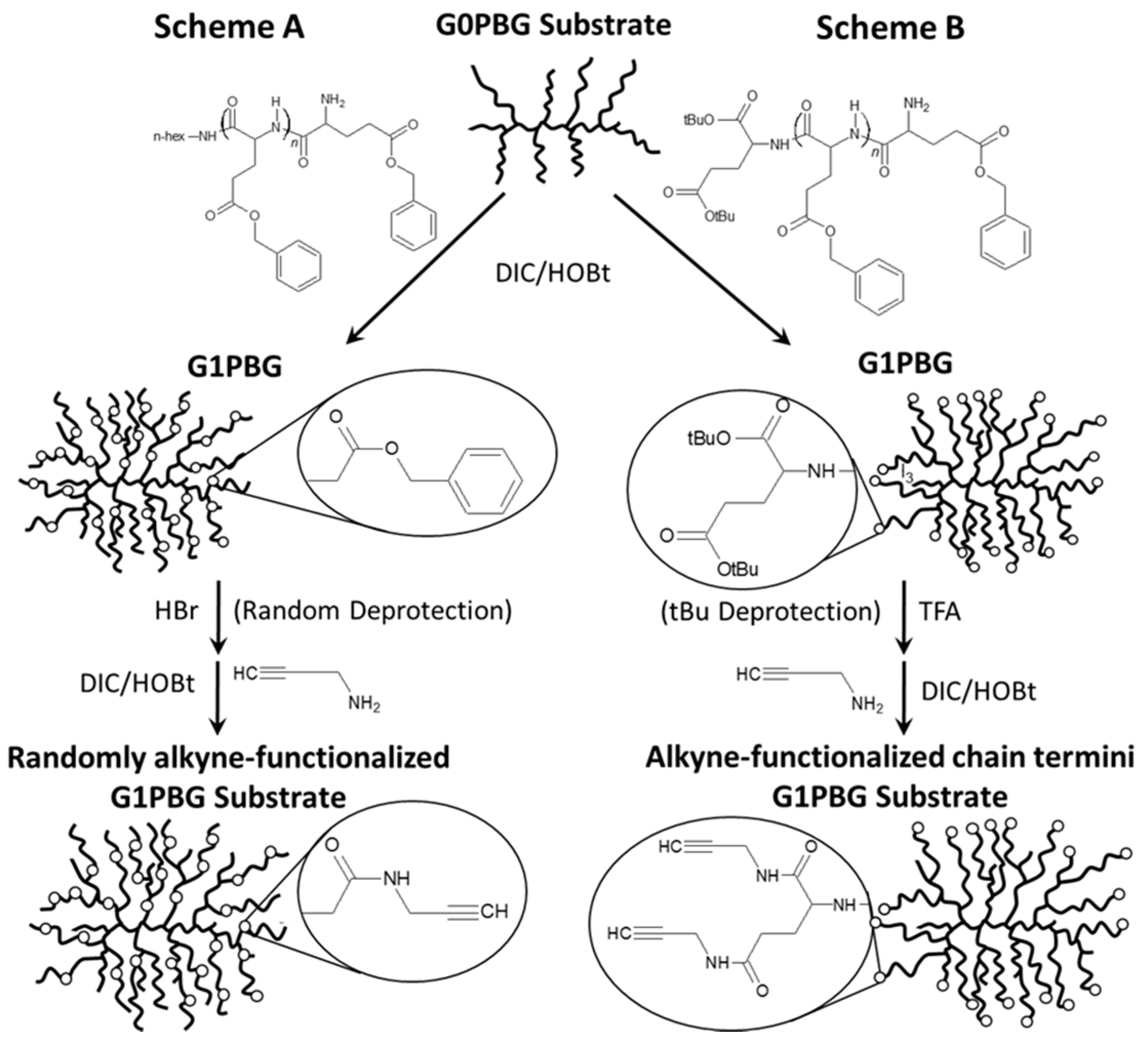
2.4.1. Synthesis of Alkyne-Functionalized Arborescent PBG Cores
2.4.2. Synthesis of CuAAC-Grafted Arborescent Copolymers
3. Results and Discussion
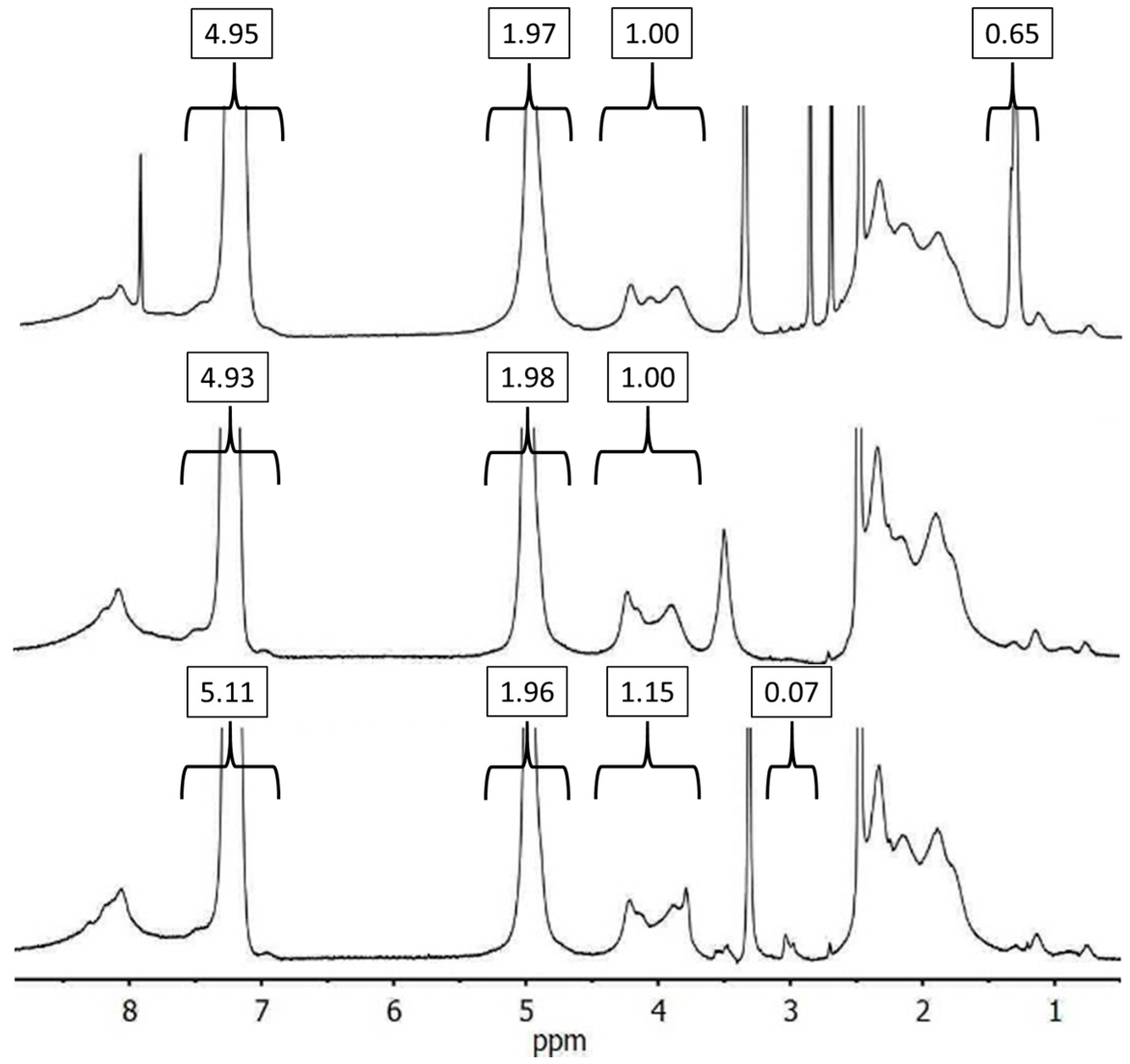
3.1. Synthesis of Linear Polymers with a Terminal Azide Functionality
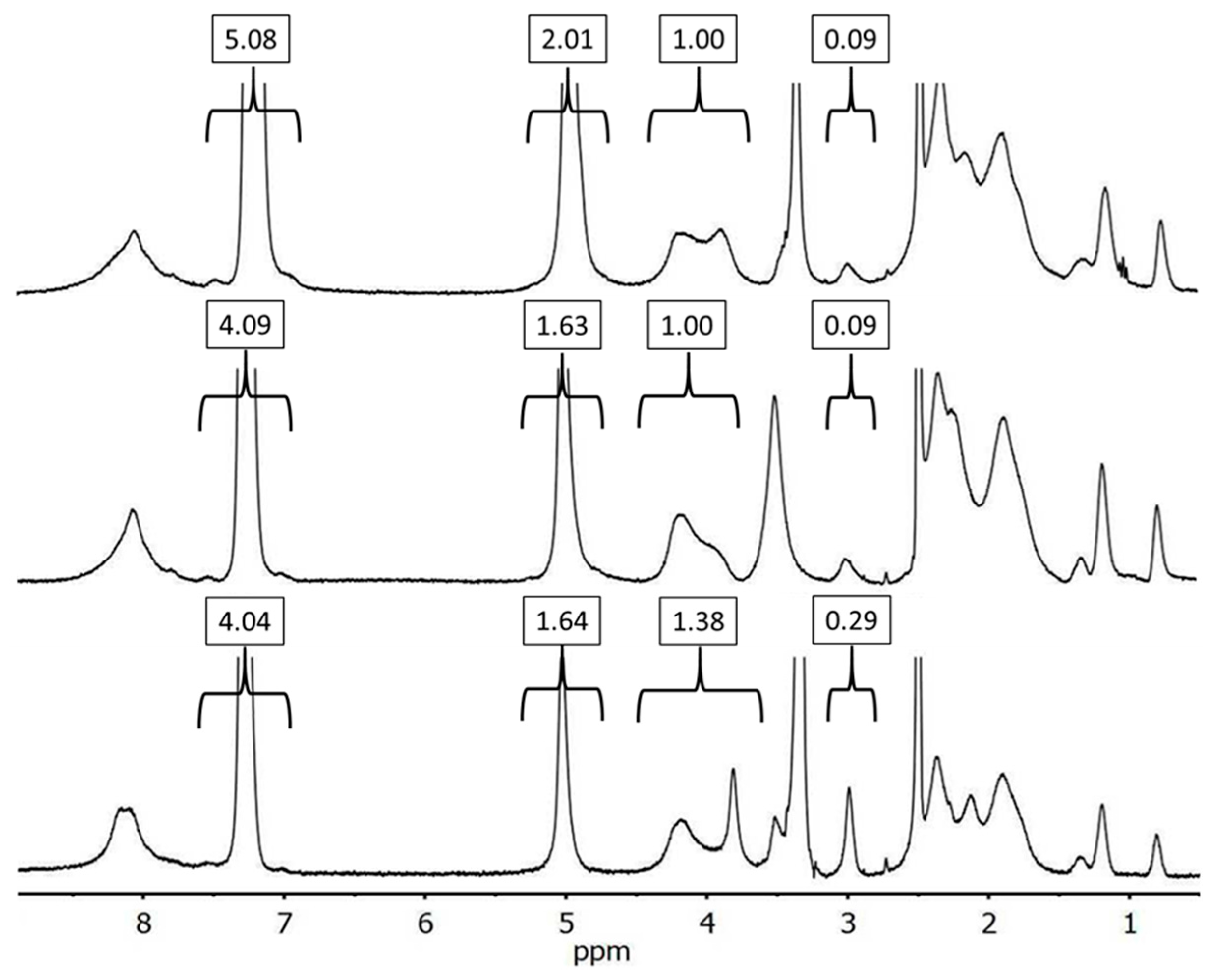
3.2. Synthesis of Alkyne-Functionalized Arborescent PBG Cores
3.3. Optimization of CuAAC Reactions with PBG and Synthesis of G0 Copolymers
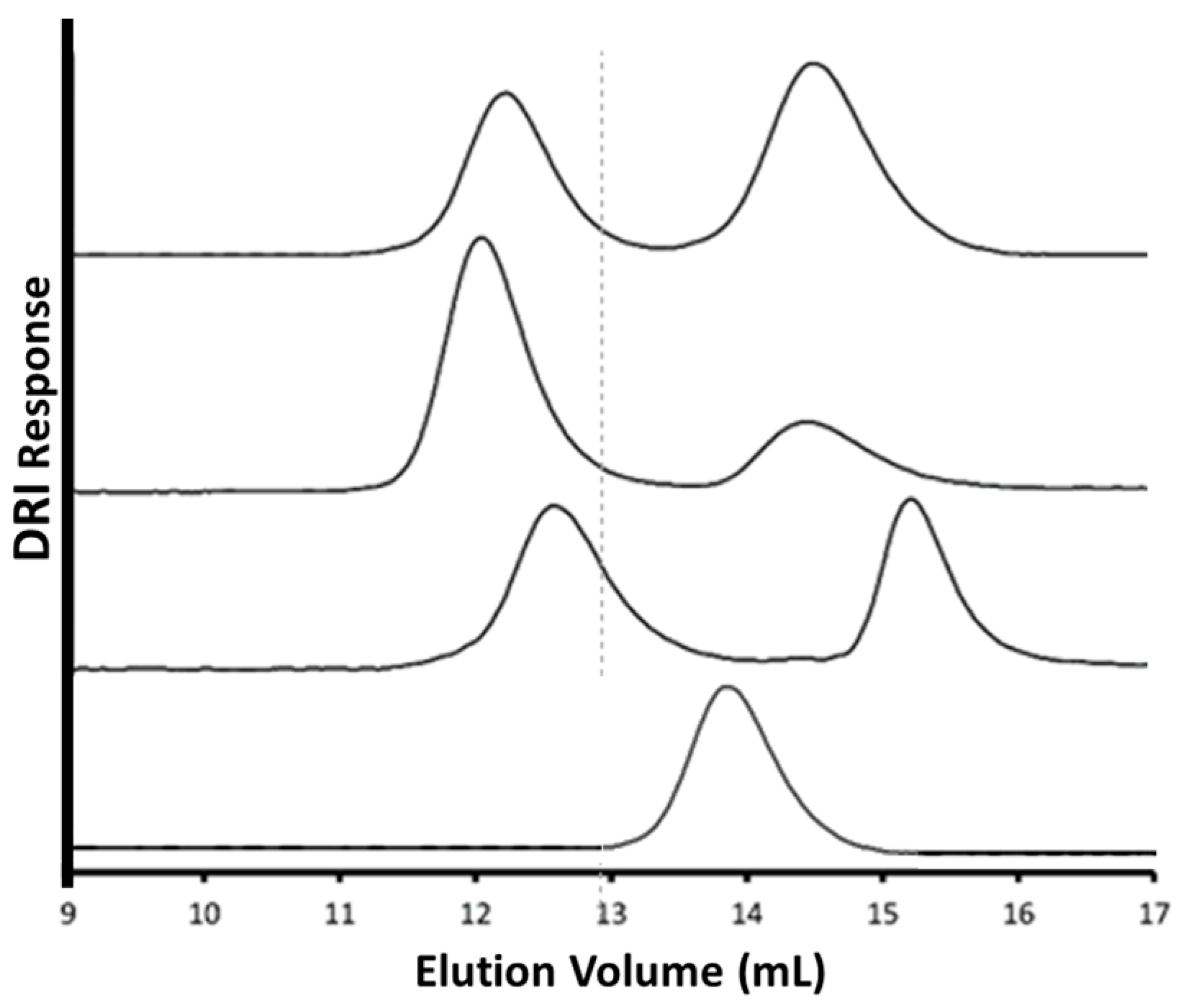
3.4. Randomly CuAAC-Grafted Arborescent Copolymers from G1 and G2 Substrates
Dynamic Light Scattering Measurements
3.5. Chain End CuAAC-Grafted Arborescent Copolymers
Dynamic Light Scattering Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gauthier, M.; Möller, M. Uniform highly branched polymers by anionic grafting: Arborescent graft polymers. Macromolecules 1991, 24, 4548–4553. [Google Scholar] [CrossRef]
- Gauthier, M. Arborescent polymers and other dendrigraft polymers: A journey into structural diversity. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 3803–3810. [Google Scholar] [CrossRef]
- Li, J.; Gauthier, M.; Teertstra, S.J.; Xu, H.; Sheiko, S.S. Synthesis of arborescent polystyrene-graft-polyisoprene copolymers using acetylated substrates. Macromolecules 2004, 37, 795–802. [Google Scholar] [CrossRef]
- Yuan, Z.; Gauthier, M. Synthesis of arborescent isoprene homopolymers. Macromolecules 2005, 38, 4124–4132. [Google Scholar] [CrossRef]
- Whitton, G.; Gauthier, M. Arborescent polypeptides from γ-benzyl l-glutamic acid. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 5270–5279. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, H.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-Triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Wu, P.; Feldman, A.K.; Nugent, A.K.; Hawker, C.J.; Sheel, A.; Voit, B.; Pyun, J.; Fréchet, J.M.; Sharpless, K.B.; Fokin, V.V. Efficiency and fidelity in a click-chemistry route to triazole dendrimers by the copper(I)-catalyzed ligation of azides and alkynes. Angew. Chem. Int. Ed. 2004, 43, 3928–3932. [Google Scholar] [CrossRef] [PubMed]
- Fournier, D.; Hoogenboom, R.; Schubert, U.S. Clicking polymers: A straightforward approach to novel macromolecular architectures. Chem. Soc. Rev. 2007, 36, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Kempe, K.; Krieg, A.; Becer, C.R.; Schubert, U.S. “Clicking” on/with polymers: A rapidly expanding field for the straightforward preparation of novel macromolecular architectures. Chem. Soc. Rev. 2012, 41, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Agut, W.; Taton, D.; Lecommandoux, S. A versatile synthetic approach to polypeptide based rod−coil block copolymers by click chemistry. Macromolecules 2007, 40, 5653–5661. [Google Scholar] [CrossRef]
- Agut, W.; Agnaou, R.; Lecommandoux, S.; Taton, D. Synthesis of block copolypeptides by click chemistry. Macromol. Rapid Commun. 2008, 29, 1147–1155. [Google Scholar] [CrossRef]
- Schatz, C.; Louguet, S.; Le Meins, J.-F.; Lecommandoux, S. Polysaccharide-block-polypeptide copolymer vesicles: Towards synthetic viral capsids. Angew. Chem. Int. Ed. 2009, 48, 2572–2575. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.C.; Lee, H.I.; Hammond, P.T. Highly efficient “grafting onto” a polypeptide backbone using click chemistry. Angew. Chem. Int. Ed. 2009, 48, 9334–9338. [Google Scholar] [CrossRef] [PubMed]
- Whitton, G.; Gauthier, M. Arborescent micelles: Dendritic poly(γ-benzyl l-glutamate) cores grafted with hydrophilic chain segments. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 1197–1209. [Google Scholar] [CrossRef]
- Fitton, A.O.; Hill, J.; Jane, D.E.; Millar, R. Synthesis of simple oxetanes carrying reactive 2-substituents. Synthesis 1987, 1987, 1140–1142. [Google Scholar] [CrossRef]
- Gervais, M.; Labbé, A.; Carlotti, S.; Deffieux, A. Direct synthesis of α-azido, ω-hydroxypolyethers by monomer-activated anionic polymerization. Macromolecules 2009, 42, 2395–2400. [Google Scholar] [CrossRef]
- Mendrek, S.; Mendrek, A.; Adler, H.-J.; Walach, W.; Dworak, A.; Kuckling, D. Synthesis of poly(glycidol)-block-poly(N-isopropylacrylamide) copolymers using new hydrophilic poly(glycidol) macroinitiator. J. Polym. Sci. Polym. Chem. Ed. 2008, 46, 2488–2499. [Google Scholar] [CrossRef]
- Mühlebach, A.; Gaynor, S.G.; Matyjaszewski, K. Synthesis of amphiphilic block copolymers by atom transfer radical polymerization (ATRP). Macromolecules 1998, 31, 6046–6052. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Xia, J. Atom transfer radical polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef] [PubMed]
- Meldal, M.; Tornøe, C.W. Cu-catalyzed azide−alkyne cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Burchard, W.; Ford, N.C. Quasielastic light scattering: An experimental study of polydispersity. Macromolecules 1978, 11, 452–454. [Google Scholar] [CrossRef]
- Ruf, H.; Gould, B.J.; Haase, W. The effect of nonrandom errors on the results from regularized inversions of dynamic light scattering data. Langmuir 2000, 16, 471–480. [Google Scholar] [CrossRef]
- Finsy, R. Particle sizing by quasi-elastic light scattering. Adv. Colloid Interface Sci. 1994, 52, 79–143. [Google Scholar] [CrossRef]


| Polymer | SEC a | 1H NMR | ||
|---|---|---|---|---|
| Mn (g/mol) | Mw/Mn | Xn b | Mn (g/mol) c | |
| PGlyAc | 14,100 | 1.06 | - | - |
| Mnapp (g/mol) | Mw/Mnapp | |||
| PGly | 10,200 | 1.14 | - | - |
| PEO | 5900 | 1.08 | 11.3 | 5000 |
| P(HEA-TMS) | 9500 | 1.21 | 57.3 | 10,800 |
| Copolymer a | PBG Substrate | Gy d | Graft Copolymer | ||||
|---|---|---|---|---|---|---|---|
| Mn (g/mol) b | % Alkyne c | Mn (g/mol) b | Mw/Mn b | fn e | % Shell f | ||
| G0PBG-g-P(HEA-TMS) | 51,000 | 18 | 57 | 324,000 | 1.17 | 25 | 84 |
| G0PBG-g-PGly | 51,000 | 18 | 93 | 331,000 | 1.02 | 40 | 85 |
| G0PBG-g-PEO | 51,000 | 18 | 54 | 172,000 | 1.09 | 24 | 70 |
| Copolymer a | PBG Substrate | Gy d | Graft Copolymer | ||||
|---|---|---|---|---|---|---|---|
| Mn (g/mol) b | % Alkyne c | Mn (g/mol) b | Mw/Mn b | fn e | % Shell f | ||
| G1PBG-g-P(HEA-TMS) | 322,000 | 19 | 27 | 1.2 × 106 | 1.14 | 80 | 73 |
| G2PBG-g-P(HEA-TMS) | 1.1 × 106 | 32 | Reaction Failed | ||||
| G1PBG-g-PGly | 322,000 | 19 | 20 | 734,000 | 1.09 | 58 | 56 |
| G2PBG-g-PGly | 1.1 × 106 | 32 | 13 | 2.8 × 106 | 1.03 | 234 | 60 |
| G1PBG-g-PEO | 322,000 | 19 | 57 | 1.2 × 106 | 1.13 | 166 | 72 |
| G2PBG-g-PEO | 1.1 × 106 | 32 | 8 | 1.8 × 106 | 1.08 | 140 | 38 |
| Copolymer | PBG Core (DMF) a | Graft Copolymer (DMF) a | Graft Copolymer (PBS) b | |||
|---|---|---|---|---|---|---|
| dh1 (nm) c | dh2 (nm) d | dh1 (nm) c | dh2 (nm) d | dh1 (nm) c | dh2 (nm) d | |
| G1PBG-g-PHEA | 11.7 ± 1 | 10.4 ± 1 | 61.8 ± 1 | 47.6 ± 1 | 128 ± 1 | 104 ± 1 |
| G1PBG-g-PGly | 11.7 ± 1 | 10.4 ± 1 | 78.5 ± 1 | 65.8 ± 1 | 221 ± 1 | 199 ± 1 |
| G2PBG-g-PGly | 18.7 ± 1 | 17.5 ± 1 | 39.0 ± 1 | 35.1 ± 1 | 94.9 ± 1 | 67.4 ± 1 |
| G1PBG-g-PEO | 11.7 ± 1 | 11.4 ± 1 | 24.2 ± 1 | 20.5 ± 1 | insoluble | |
| G2PBG-g-PEO | 18.7 ± 1 | 17.5 ± 1 | 30.4 ± 1 | 27.8 ± 1 | insoluble | |
| Copolymer a | PBG Substrate | Gy d | Graft Copolymer | ||||
|---|---|---|---|---|---|---|---|
| Mn (g/mol) b | % Alkyne c | Mn (g/mol) b | Mw/Mn b | fn e | % Shell f | ||
| G1PBG-eg-PGly | 322,000 | 7 | 98 | 906,000 | 1.09 | 89 | 69 |
| G2PBG-eg-PGly | 1.1 × 106 | 12 | 47 | 3.2 × 106 | 1.02 | 294 | 65 |
| G3PBG-eg-PGly | 3.0 × 106 | 11 | 30 | 6.3 × 106 | 1.01 | 467 | 52 |
| G1PBG-eg-PEO | 280,000 | 7 | 50 g | h | 1.30 | ||
| G2PBG-eg-PEO | 1.1 × 106 | 12 | 36 g | h | 1.23 | ||
| G3PBG-eg-PEO | 3.0 × 106 | 11 | 24 | 4.8 × 106 | 1.04 | 738 | 38 |
| Copolymer | PBG Core (DMF) a | Graft Copolymer (DMF) a | Graft Copolymer (PBS) b | |||
|---|---|---|---|---|---|---|
| dh1 (nm) c | dh2 (nm) d | dh1 (nm) c | dh2 (nm) d | dh1 (nm) c | dh2 (nm) d | |
| G1PBG-eg-PGly | 11.7 ± 1 | 10.0 ± 1 | 30.3 ± 1 | 26.3 ± 1 | 78.9 ± 1 | 54.6 ± 1 |
| G2PBG-eg-PGly | 18.9 ± 1 | 17.3 ± 1 | 39.7 ± 1 | 35.5 ± 1 | 72.5 ± 1 | 53.0 ± 1 |
| G3PBG-eg-PGly | 28.4 ± 1 | 26.8 ± 1 | 39.9 ± 1 | 38.9 ± 1 | 43.5 ± 1 | 39.8 ± 1 |
| G1PBG-eg-PEO | 11.7 ± 1 | 10.0 ± 1 | 46.8 ± 1 | 42.1 ± 1 | 61.7 ± 1 | 54.4 ± 1 |
| G2PBG-eg-PEO | 18.9 ± 1 | 17.3 ± 1 | 43.9 ± 1 | 39.4 ± 1 | 45.8 ± 1 | 41.7 ± 1 |
| G3PBG-eg-PEO | 28.4 ± 1 | 26.8 ± 1 | 38.3 ± 1 | 35.4 ± 1 | 56.9 ± 1 | 50.5 ± 1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gauthier, M.; Whitton, G. Arborescent Unimolecular Micelles: Poly(γ-Benzyl l-Glutamate) Core Grafted with a Hydrophilic Shell by Copper(I)-Catalyzed Azide–Alkyne Cycloaddition Coupling. Polymers 2017, 9, 540. https://doi.org/10.3390/polym9100540
Gauthier M, Whitton G. Arborescent Unimolecular Micelles: Poly(γ-Benzyl l-Glutamate) Core Grafted with a Hydrophilic Shell by Copper(I)-Catalyzed Azide–Alkyne Cycloaddition Coupling. Polymers. 2017; 9(10):540. https://doi.org/10.3390/polym9100540
Chicago/Turabian StyleGauthier, Mario, and Greg Whitton. 2017. "Arborescent Unimolecular Micelles: Poly(γ-Benzyl l-Glutamate) Core Grafted with a Hydrophilic Shell by Copper(I)-Catalyzed Azide–Alkyne Cycloaddition Coupling" Polymers 9, no. 10: 540. https://doi.org/10.3390/polym9100540