Synthesis and Electrochromism of Highly Organosoluble Polyamides and Polyimides with Bulky Trityl-Substituted Triphenylamine Units
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
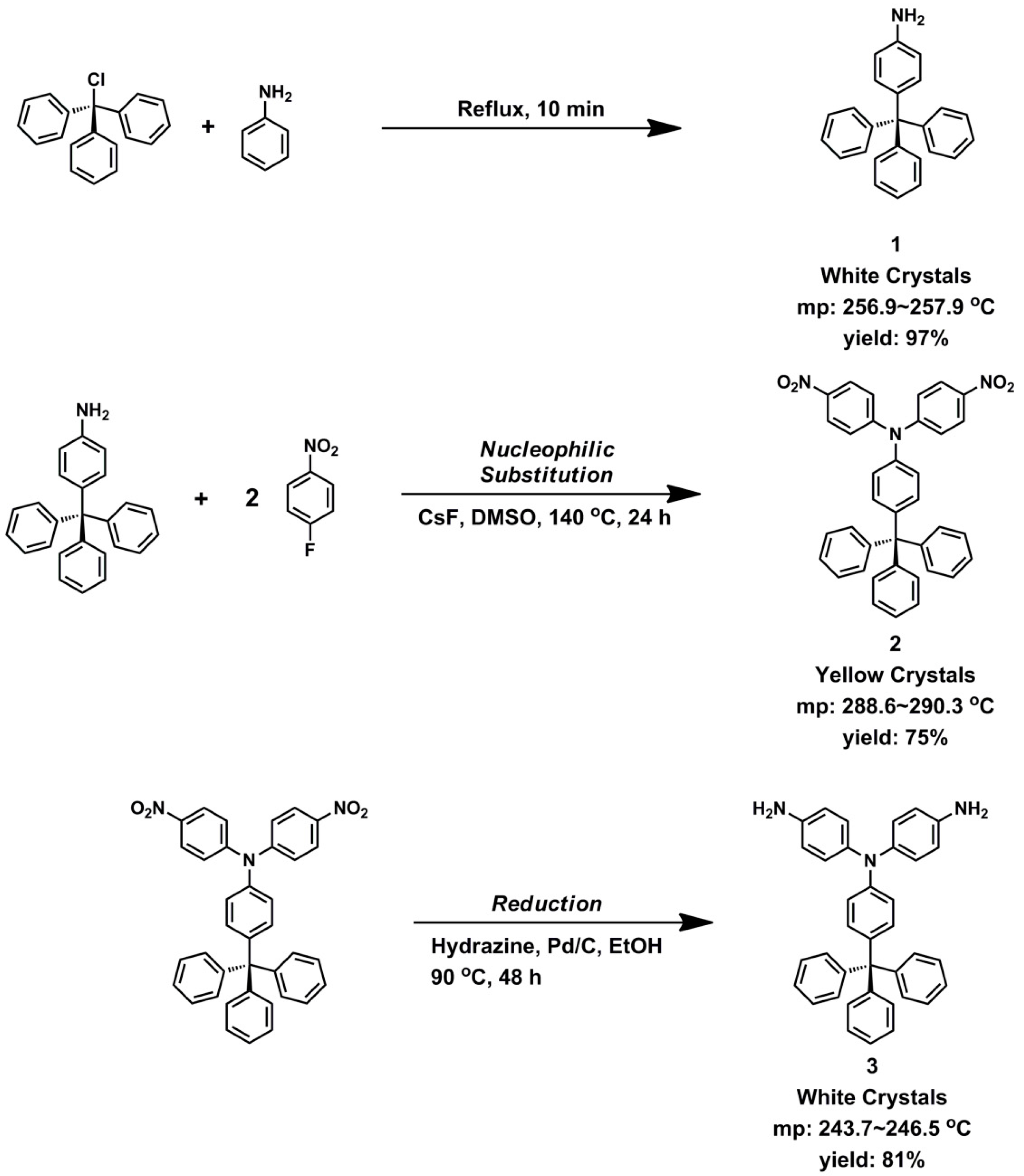
2.2. Monomer Synthesis
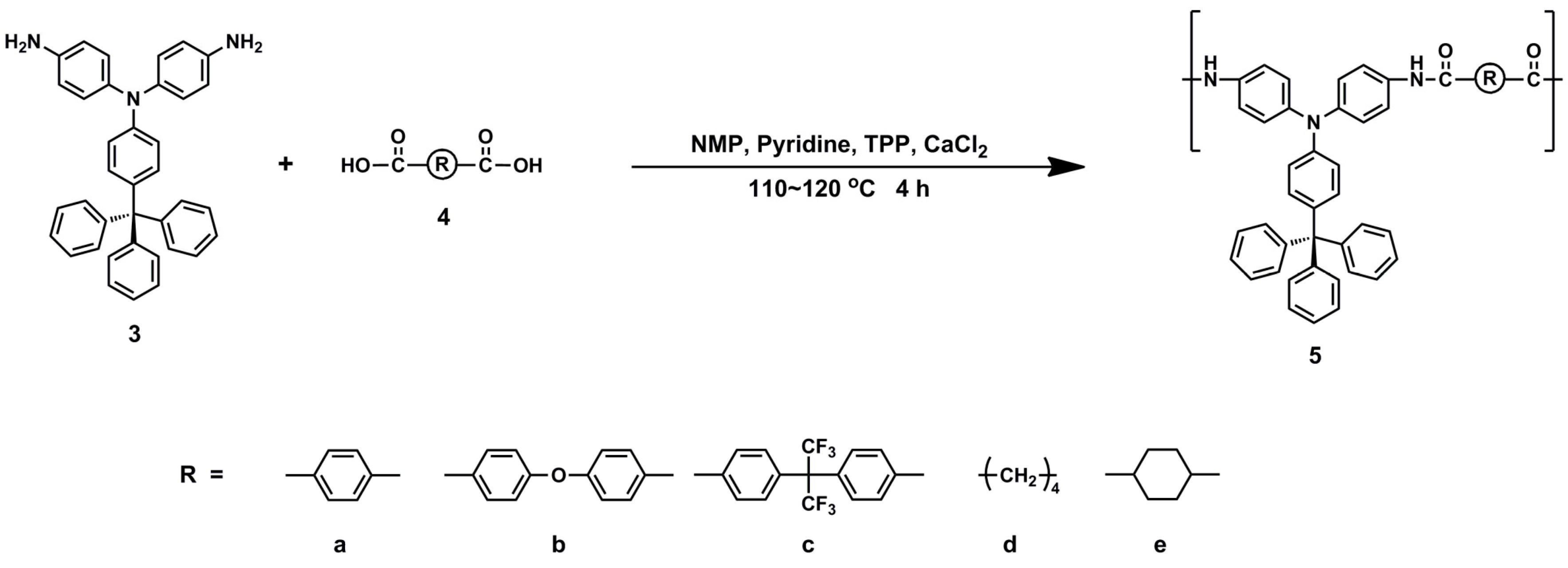
2.3. Synthesis of Polyamides
2.4. Synthesis of Polyimides
2.5. Fabrication of the Electrochromic Devices (ECDs)
2.5.1. Electrodes
2.5.2. Electrolytes
2.5.3. Assembly
3. Results and Discussion
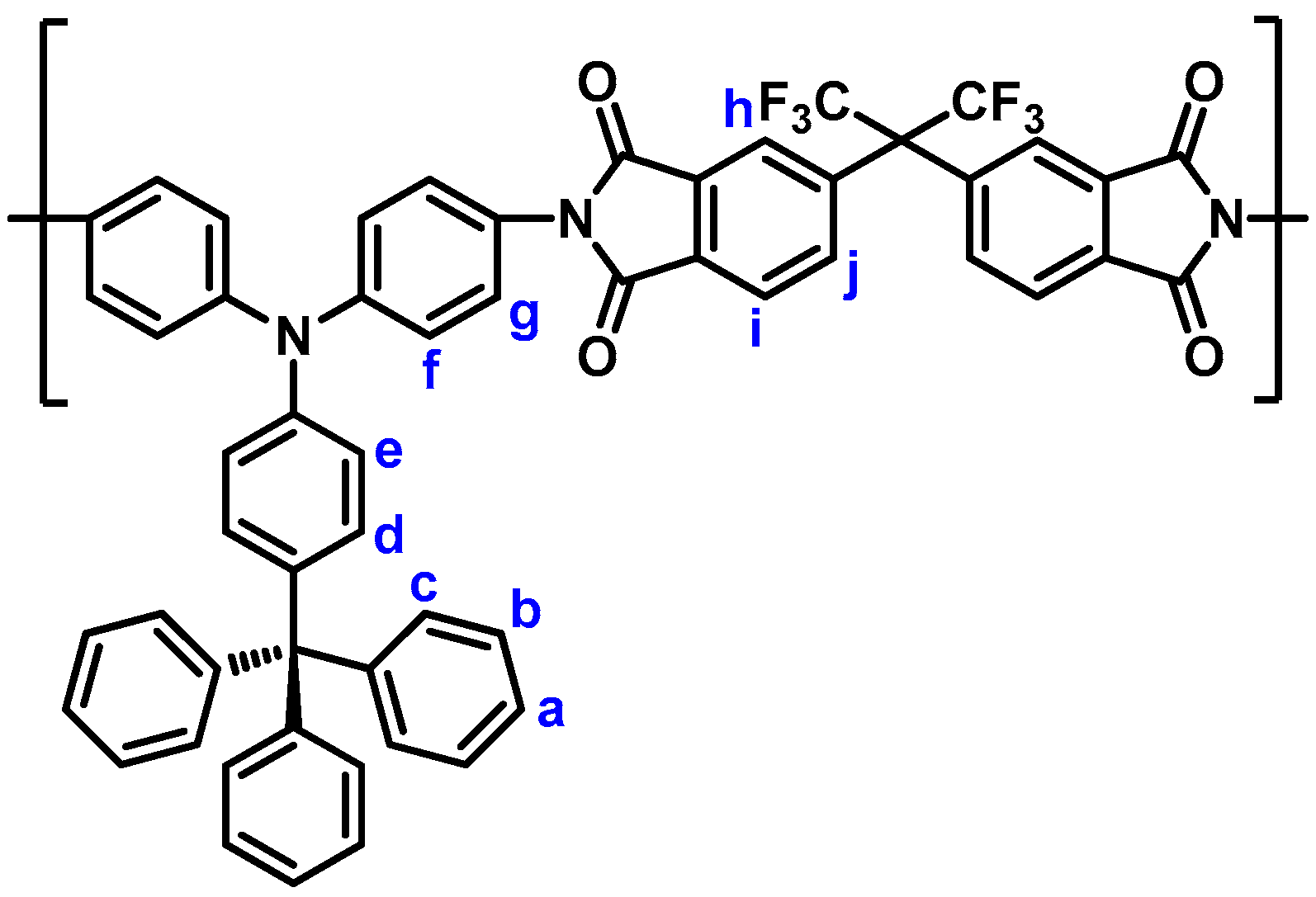
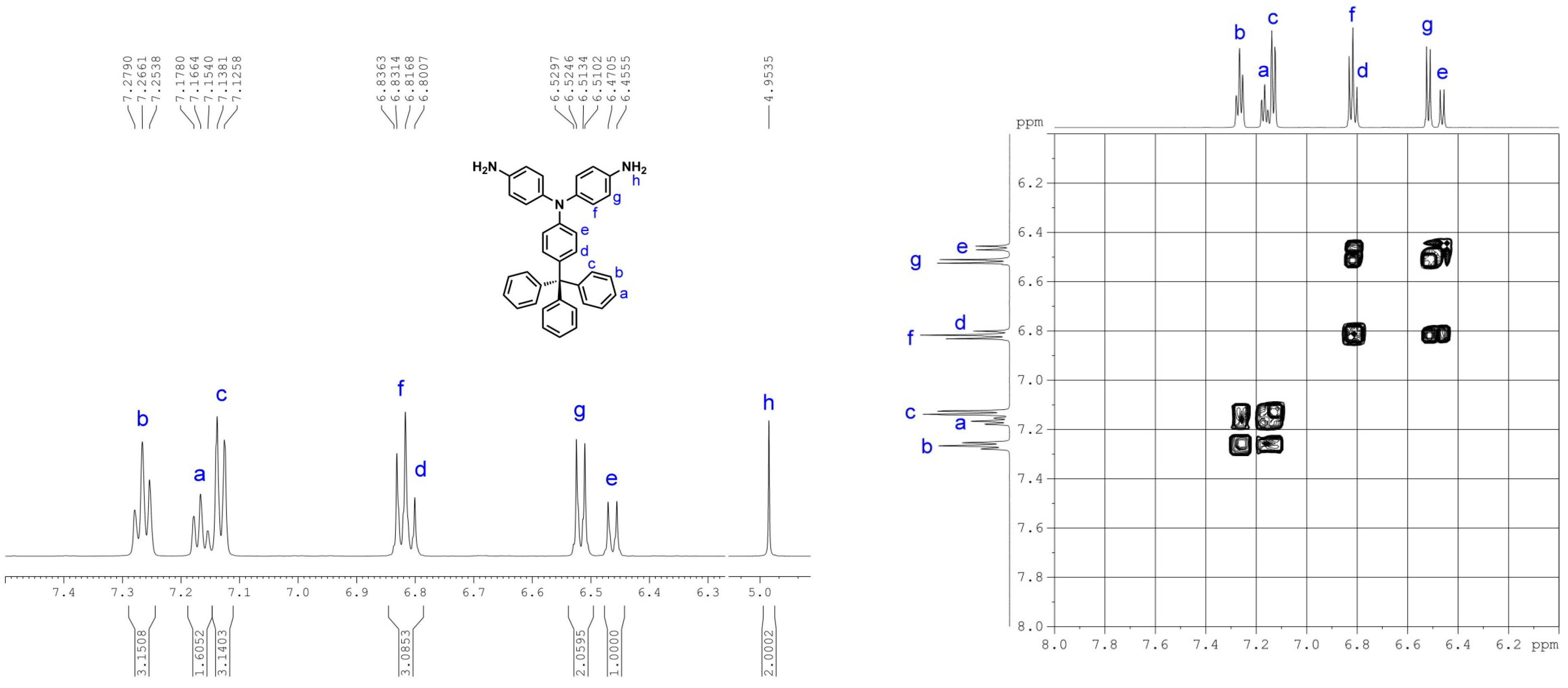
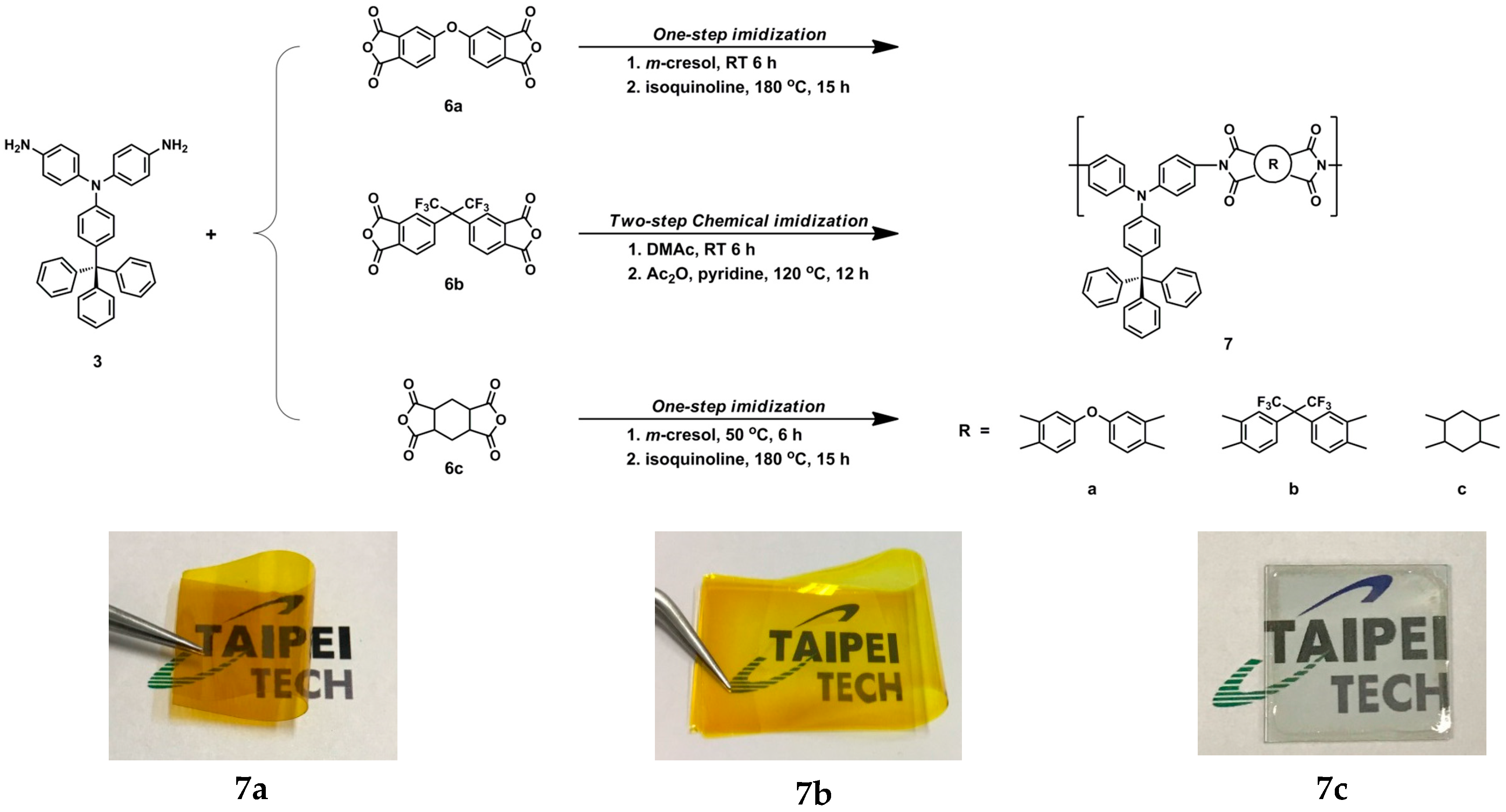
3.1. Monomer Synthesis
3.2. Synthesis of Polyamides
3.3. Synthesis of Polyimides
3.4. Properties of Polymers
3.4.1. Solubility
3.4.2. Thermal Stability
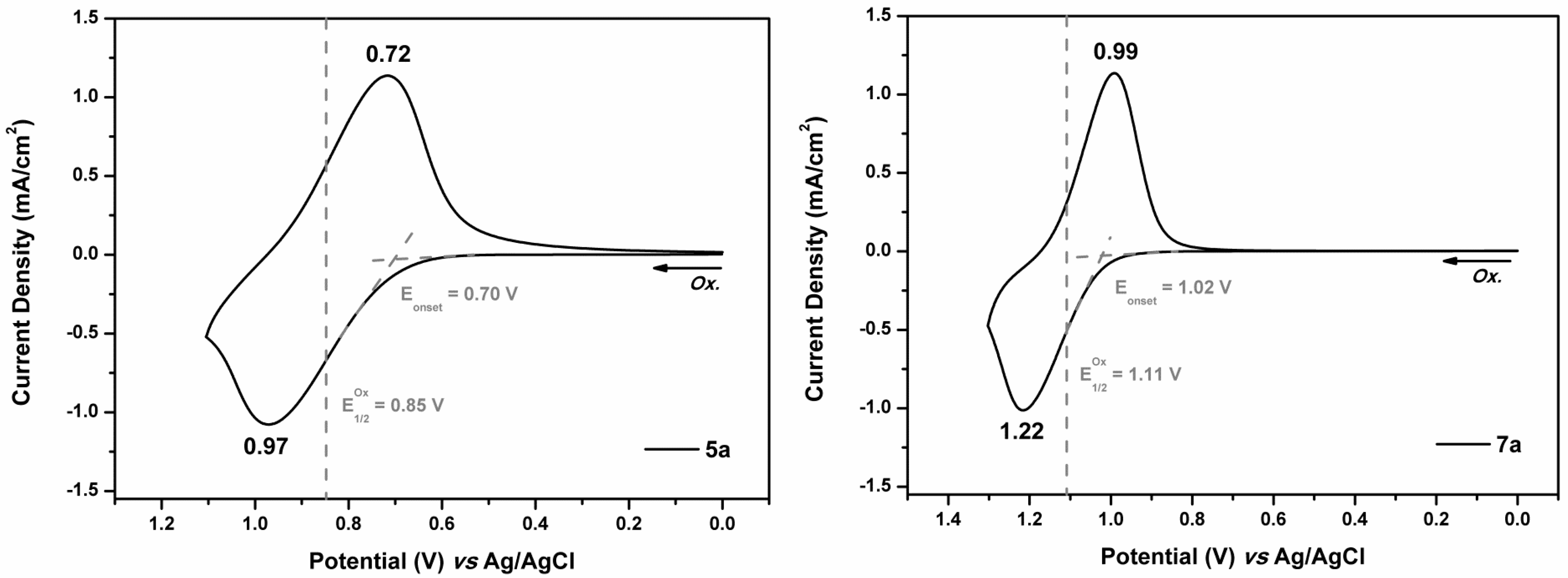
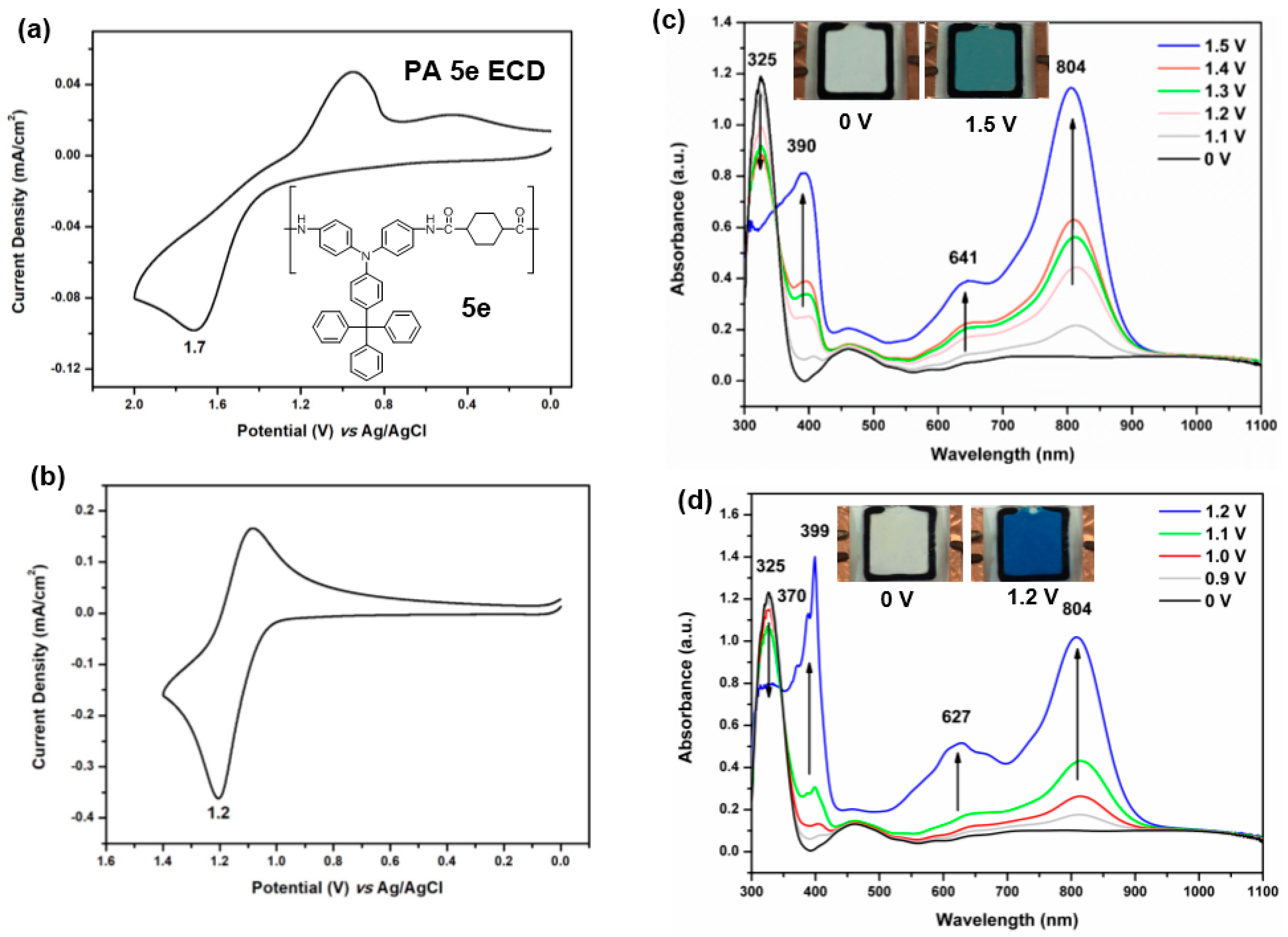
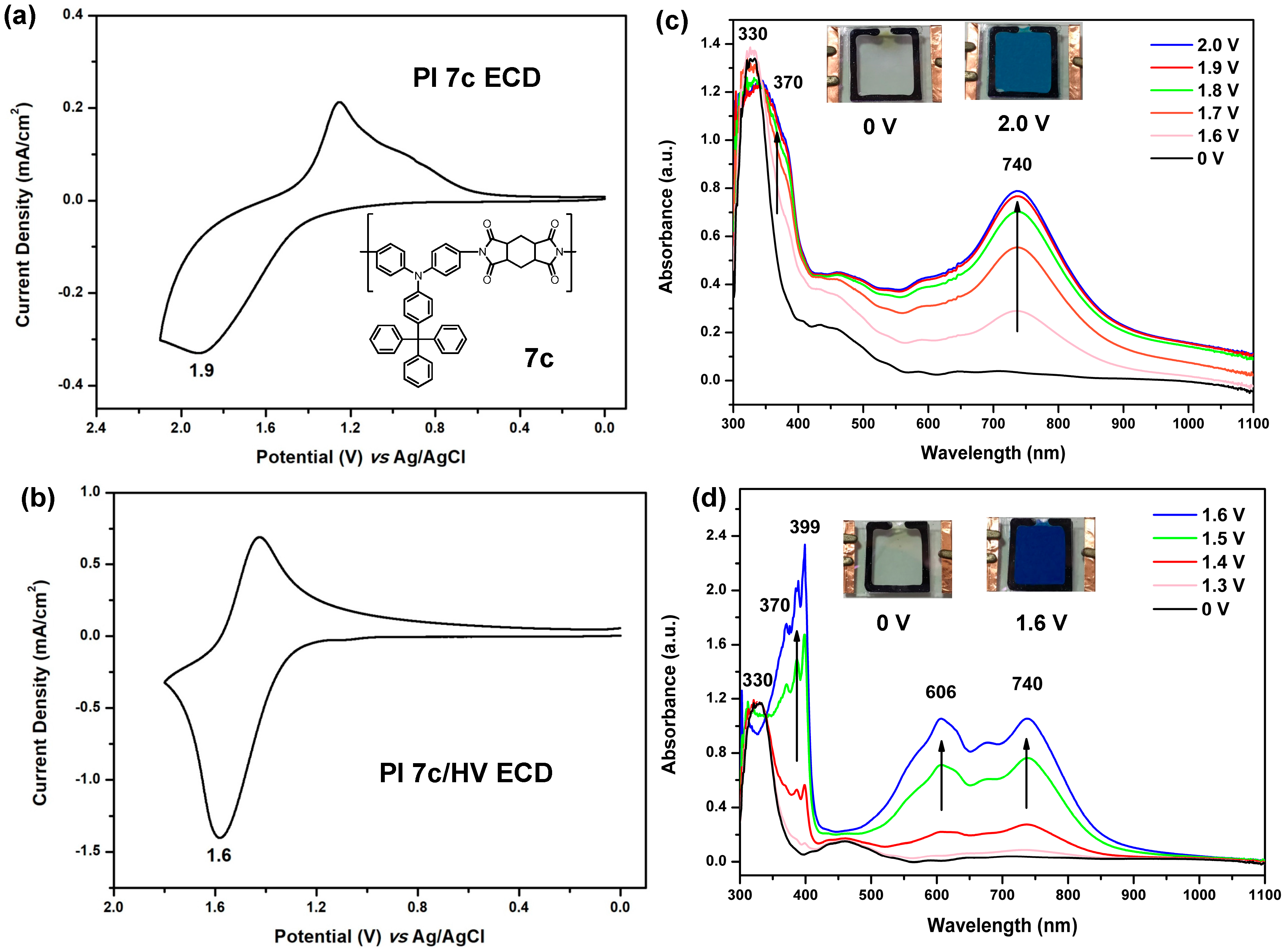
3.4.3. Electrochemical Property
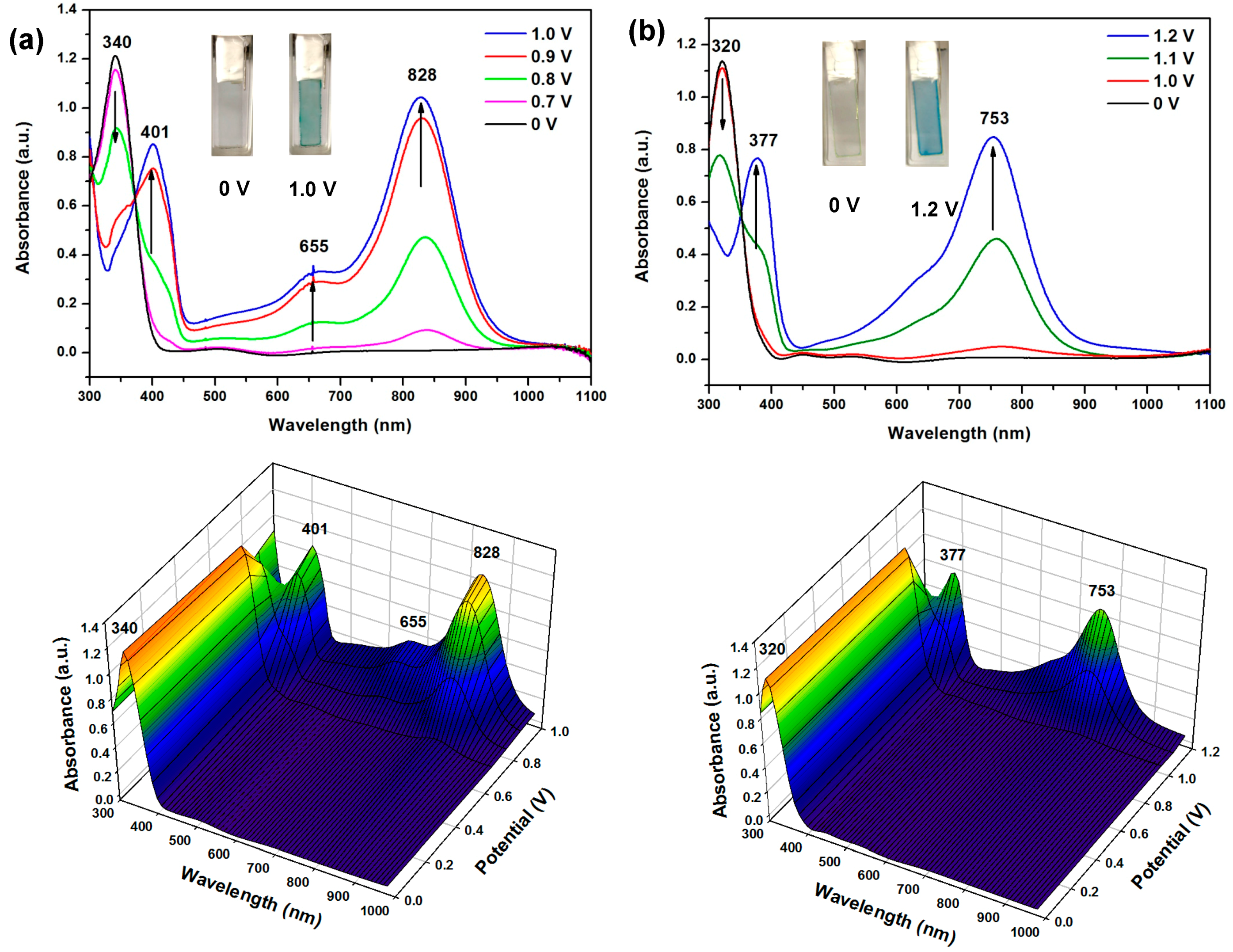
3.4.4. Electro-Optical Property
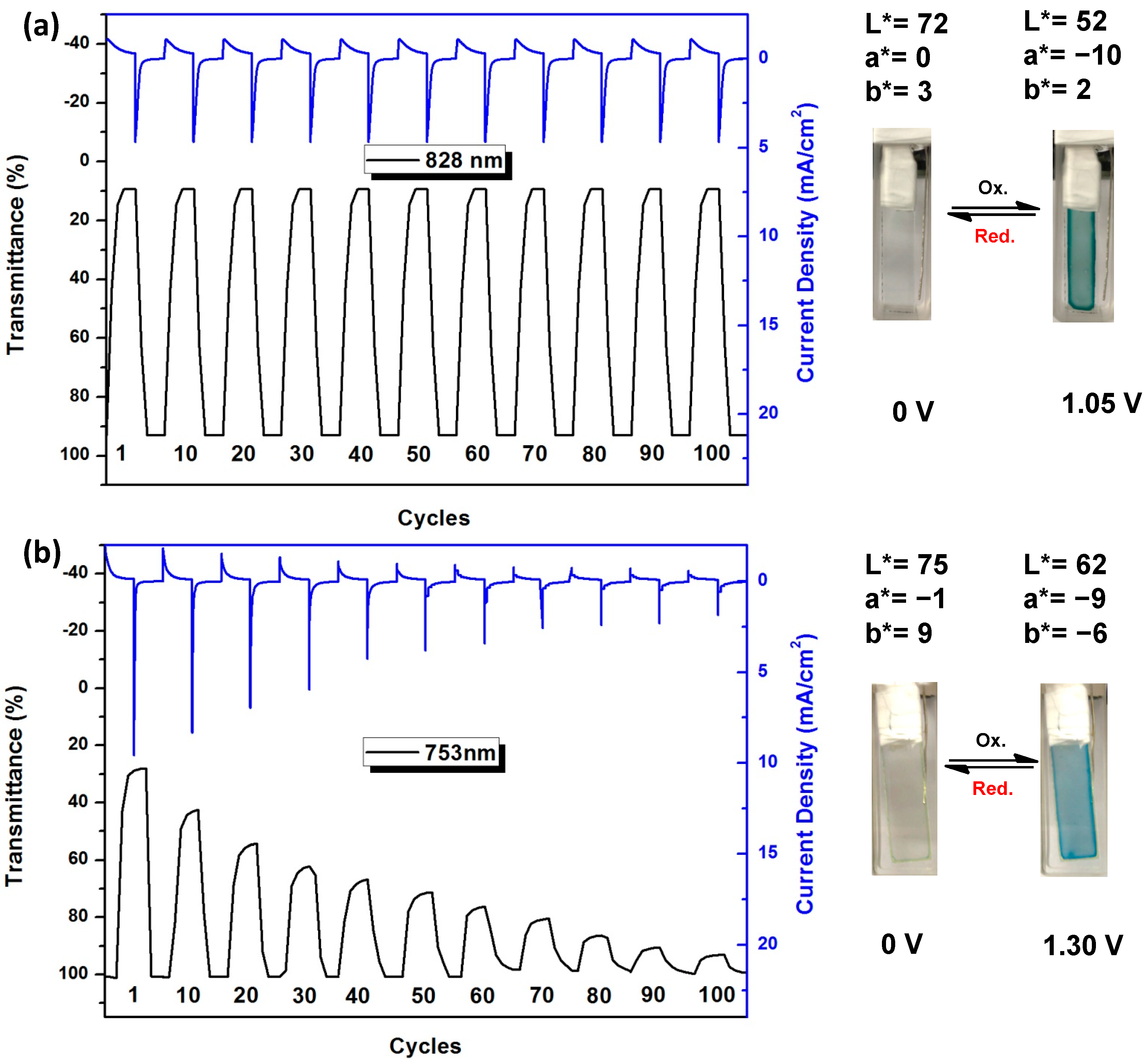
3.4.5. Electrochromic Switching and Stability
3.5. Electrochromic Devices
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, H.H. Aromatic High-Strength Fibers; John Wiley & Sons, Ltd.: New York, NY, USA, 1989. [Google Scholar]
- Yang, H.H. Kevlar Aramid Fiber; John Wiley & Sons, Ltd.: Chichester, UK, 1993. [Google Scholar]
- Garcia, J.M.; Garcia, F.C.; Serna, F.; de la Pena, J. High-performance aromatic polyamides. Prog. Polym. Sci. 2010, 35, 623–686. [Google Scholar] [CrossRef]
- Wilson, D.; Stenzenberger, H.D.; Hergenrother, P.M. (Eds.) Polyimides; Blackie & Son Ltd.: Glasgow/London, UK, 1990; ISBN 978-98-94-04-010-90-9663-83-8. [Google Scholar]
- Sroog, C.E. Polyimides. Prog. Polym. Sci. 1991, 16, 561–694. [Google Scholar] [CrossRef]
- Ghosh, M.K.; Mittal, K.L. (Eds.) Polyimides: Fundamentals and Applications; Marcel Dekker: New York, NY, USA, 1996. [Google Scholar]
- Liou, G.-S.; Yen, H.-J. Polyimides. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Moller, M., Eds.; Elsevier BV: Amsterdam, The Netherlands, 2012; Volume 5, pp. 497–535. [Google Scholar]
- Liaw, D.-J.; Wang, K.-L.; Huang, Y.-C.; Lee, K.-R.; Lai, J.-Y.; Ha, C.-S. Advanced polyimide materials: Synthesis, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Ghosh, A.; Sen, S.K.; Banerjee, S.; Voit, B. Solubility improvements in aromatic polyimides by macromoleculaer engineering. RSC Adv. 2012, 2, 5900–5926. [Google Scholar] [CrossRef]
- Yi, L.; Huang, W.; Yan, D. Polyimides with side groups: Synthesis and effects of side groups on their properties. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 533–559. [Google Scholar] [CrossRef]
- Ando, S.; Matsuura, T.; Sasaki, S. Coloration of aromatic polyimides and electronic properties of their source materials. Polym. J. 1997, 29, 69–76. [Google Scholar] [CrossRef]
- Hasegawa, M.; Horie, K. Photophysics, photochemistry, and optical properties of polyimides. Prog. Polym. Sci. 2001, 26, 259–335. [Google Scholar] [CrossRef]
- Chung, C.-L.; Yang, C.-P.; Hsiao, S.-H. Organosoluble and colorless fluorinated poly(ether imide)s from 1,2-bis(3,4-dicarboxyphenoxy)benzene dianhydride and trifluoromethyl-substituted aromatic bis(ether amine)s. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 3092–3102. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Yang, C.-P.; Hsiao, S.-H. Soluble and colorless poly(ether imide)s based on a benzonorbornane bis(ether anhydride) and trifluoromethyl-substituted aromatic bis(ether-amine)s. Macromol. Chem. Phys. 2006, 207, 1888–1898. [Google Scholar] [CrossRef]
- Hasegawa, M.; Hirano, D.; Fuji, M.; Haga, M.; Takezawa, E.; Yamaguchi, S.; Ishikawa, A.; Kagayama, T. Solution-processable colorless polyimides derived from hydrogenated pyromellitic dianhydride with controlled steric structure. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 575–592. [Google Scholar] [CrossRef]
- Hasegawa, M.; Fuji, M.; Ishii, J.; Yamaguchi, S.; Takezawa, E.; Kagayama, T.; Ishikawa, A. Colorless polyimides derived from 1S,2S,4R,5R-cyclohexane-tetracarboxylic dianhydride, self-orientation behavior during solution casting, and their optoelectronic applications. Polymer 2014, 55, 4693–4708. [Google Scholar] [CrossRef]
- Thelakkat, M. Star-shaped, dendrimeric and polymeric triarylamines as photoconductors and hole transport materials for electro-optical applications. Macromol. Mater. Eng. 2002, 287, 442–461. [Google Scholar] [CrossRef]
- Shirota, Y. Photo- and electroactive amorphous molecular materials—Molecular design, syntheses, reactions, properties, and applications. J. Mater. Chem. 2005, 15, 75–93. [Google Scholar] [CrossRef]
- Shirota, Y.; Kageyama, H. Charge carrier transporting molecular materials and their applications in devices. Chem. Rev. 2007, 107, 953–1010. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.; Tian, H. Triarylamine: A promising core unit for efficient photovoltaic materials. Chem. Commun. 2009, 5483–5495. [Google Scholar] [CrossRef] [PubMed]
- Iwan, A.; Sek, D. Polymers with triphenylamine units: Photonic and electroactive materials. Prog. Polym. Sci. 2011, 36, 1277–1325. [Google Scholar] [CrossRef]
- Liang, M.; Chen, J. Arylamine organic dyes for dye-sensitized solar cells. Chem. Soc. Rev. 2013, 42, 3453–3488. [Google Scholar] [CrossRef] [PubMed]
- Chou, M.-Y.; Leung, M.-K.; Su, Y.O.; Chiang, C.L.; Lin, C.-C.; Liu, J.-H.; Kuo, C.-K.; Mou, C.-Y. Electropolymerization of starburst triarylamines and their application to electrochromism and electroluminescence. Chem. Mater. 2004, 16, 654–661. [Google Scholar] [CrossRef]
- Otero, L.; Sereno, L.; Fungo, F.; Liao, Y.-L.; Lin, C.-Y.; Wong, K.-T. Synthesis and properties of a novel electrochromic polymer obtained from the electropolymerization of a 9,9′-spirobifluorene-bridged donor−acceptor (D−A) bichromophore. Chem. Mater. 2006, 18, 3495–3502. [Google Scholar] [CrossRef]
- Beapre, S.; Dumas, J.; Leclerc, M. Toward the development of new textile/plastic electrochromic cells using triphenylamine-based copolymers. Chem. Mater. 2006, 18, 4011–4018. [Google Scholar] [CrossRef]
- Huang, L.-T.; Yen, H.-J.; Liou, G.-S. Substituent effect on electrochemical and electrochromic behaviors on ambipolar aromatic polyimides based on aniline derivatives. Macromolecules 2011, 44, 9595–9610. [Google Scholar] [CrossRef]
- Kung, Y.-C.; Hsiao, S.-H. Solution-processable, high-Tg, ambipolar polyimide electrochromics bearing pyrenylamine units. J. Mater. Chem. 2011, 21, 1746–1754. [Google Scholar] [CrossRef]
- Yen, H.-J.; Chen, C.-J.; Liou, G.-S. Flexible multi-colored electrochromic and volatile polymer memory devices from starburst triarylamine-based electroactive polyimide. Adv. Funct. Mater. 2013, 23, 5307–5316. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Wang, H.-M.; Chang, P.-C.; Kung, Y.-R.; Lee, T.-M. Synthesis and electrochromic properties of aromatic polyetherimides based on a triphenylamine-dietheramine monomer. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 2925–2938. [Google Scholar] [CrossRef]
- Wang, H.-M.; Hsiao, S.-H. Ambipolar, multi-electrochromic polypyromellitimides and polynaphthalimides containing di(tert-butyl)-substituted bis(triarylamine) units. J. Mater. Chem. C 2014, 2, 1553–1564. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Peng, S.-C.; Kung, Y.-R.; Leu, C.-M.; Lee, T.-M. Synthesis and electro-optical properties of aromatic polyamides and polyimides bearing pendent 3,6-dimethoxycarbazole units. Eur. Polym. J. 2015, 73, 50–64. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Chen, Y.-Z. Electrochemical synthesis of stable ambipolar electrochromic polyimide film from a bis(triphenylamine) perylene diimide. J. Electroana. Chem. 2017, 799, 417–423. [Google Scholar] [CrossRef]
- Yen, H.-J.; Liou, G.-S. Solution-processable novel near-infrared electrochromic aromatic polyamides based on electroactive tetraphenyl-p-phenylenediamine moieties. Chem. Mater. 2009, 21, 4062–4070. [Google Scholar] [CrossRef]
- Kung, Y.-C.; Hsiao, S.-H. Fluorescent and electrochromic polyamides with pyrenylamine chromophore. J. Mater. Chem. 2010, 20, 5481–5492. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Wang, H.-M.; Liao, S.-H. Redox-stable and visible/near-infrared electrochromic aramids with main-chain triphenylamine and pendent 3,6-di-tert-butylcarbazole units. Polym. Chem. 2014, 5, 2473–2483. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Hsiao, Y.-H.; Kung, Y.-R.; Leu, C.-M.; Lee, T.-M. Triphenylamine-based redox-active aramids with 1-piperidinyl substituent as an auxiliary donor: Enhanced electrochemical stability and electrochromic performance. React. Funct. Polym. 2016, 108, 54–62. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Han, J.-S. Solution-processable transmissive-to-green switching electrochromic polyamides bearing 2,7-bis(diphenylamino)naphthalene units. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1409–1421. [Google Scholar] [CrossRef]
- Liu, H.-S.; Pan, B.-C.; Huang, D.-C.; Kung, Y.-R.; Leu, C.-M.; Liou, G.-S. Highly transparent to truly black electrochromic devices based on an ambipolar system of polyamides and viologen. NPG Asia Mater. 2017, 9, e388. [Google Scholar] [CrossRef]
- Yen, H.-J.; Liou, G.-S. Solution-processable triarylamine-based electroactive high performance polymers for anodically electrochromic applications. Polym. Chem. 2012, 3, 255–264. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, Z.; Yang, T.; Bei, R.; Zhang, Y.; Liu, S.; Chi, Z. Synthesis and properties of highly organosoluble and low dielectric constant polyimides containing non-polar bulky triphenyl methane moiety. React. Funct. Polym. 2016, 108, 71–77. [Google Scholar] [CrossRef]
- Mao, H.; Zhang, S. Synthesis, characterization and gas transport properties of novel poly(amine-imide)s containing tetraphenylmethane pendant groups. J. Mater. Chem. A 2014, 2, 9835–9843. [Google Scholar] [CrossRef]
- Bisoi, S.; Mandal, A.K.; Padmanabhan, V.; Banerjee, S. Aromatic polyamides containing trityl substituted triphenylamine: Gas transport properties and molecular dynamics simulations. J. Membr. Sci. 2017, 522, 77–90. [Google Scholar] [CrossRef]
- Wu, J.-H.; Liou, G.-S. High-performance electrofluorochromic devices based on electrochromism and photoluminescence-active novel poly(4-cyanotriphenylamine). Adv. Funct. Mater. 2014, 24, 6422–6429. [Google Scholar] [CrossRef]
- Mackenzie, C.A.; Chuchani, G. Tritylation of aromatic compounds. J. Org. Chem. 1955, 20, 336–345. [Google Scholar] [CrossRef]
- Yamazaki, N.; Matsumoto, M.; Higashi, F. Studies on reactions of the N-phosphonium salts of pyridines. XIV. Wholly aromatic polyamides by the direct polycondensation reaction by using phosphites in the presence of metal salts. J. Polym. Sci. Polym. Chem. Ed. 1975, 13, 1373–1380. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Kung, Y.-R. Synthesis and properties of poly(amine-amide)s and poly(amine-imide)s based on 4,4′-diamino-4′′-fluorotriphenylamine. J. Fluorine Chem. 2016, 186, 79–90. [Google Scholar] [CrossRef]
- Zhou, M.; Zhu, L.; Sun, Z.; Yang, Z.; Cao, D.; Li, Q. Tri-petal lilac-like perylene: Asymmetrical substituted platform for regioselective ether-exchange reaction. Synlett 2017, 28, 2121–2125. [Google Scholar]
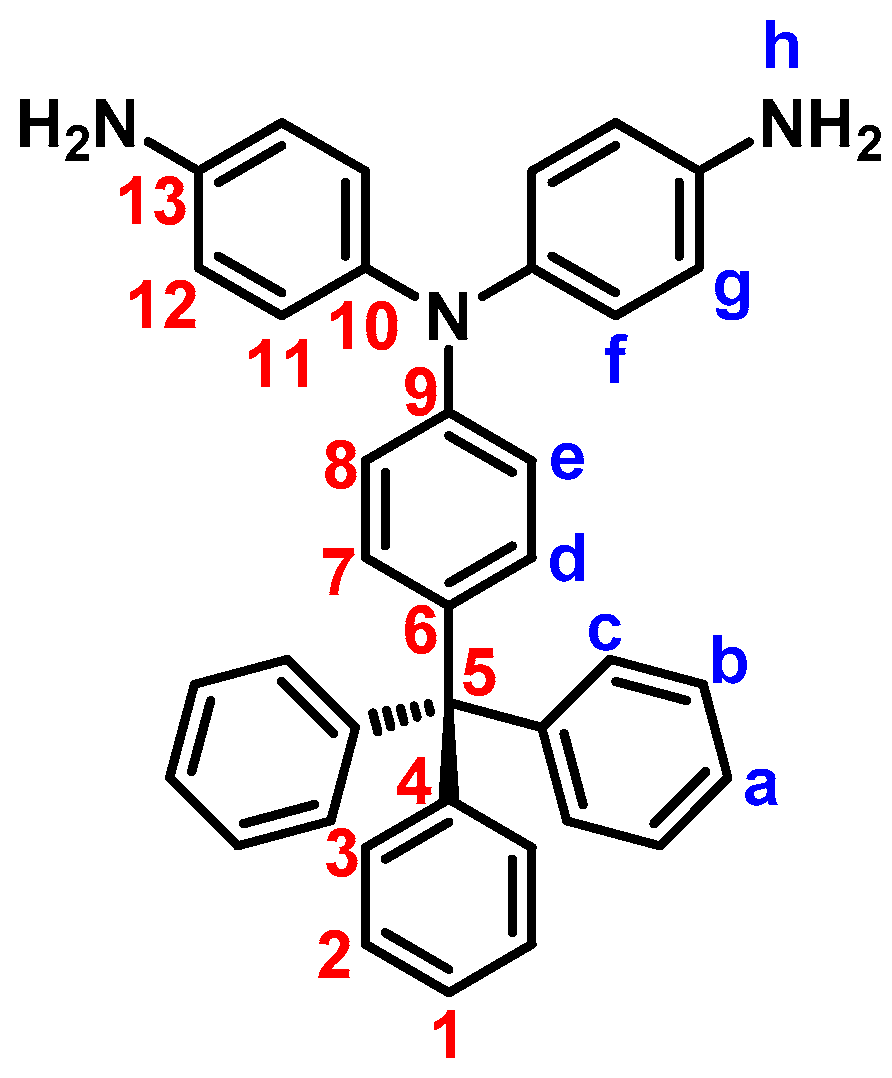
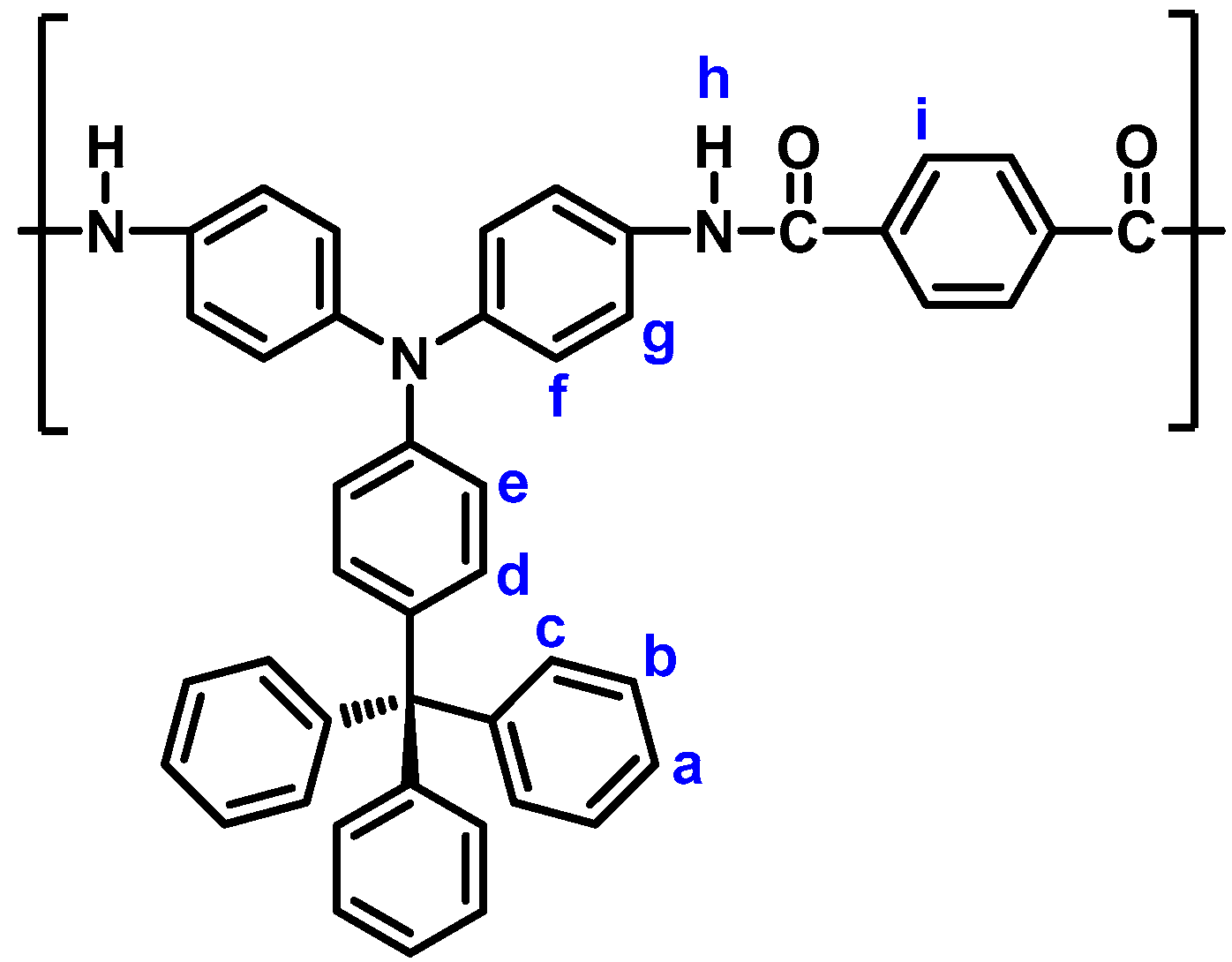


| Polymer Code | ηinh (dL g−1) a | Solubility in Various Solvents b,c | |||||
|---|---|---|---|---|---|---|---|
| NMP | DMAc | DMF | DMSO | m-Cresol | THF | ||
| 5a | 0.49 | ++ | ++ | ++ | ++ | ++ | +− |
| 5b | 0.62 | ++ | ++ | ++ | ++ | ++ | +− |
| 5c | 0.43 | ++ | ++ | ++ | ++ | ++ | ++ |
| 5d | 0.42 | ++ | ++ | ++ | ++ | ++ | ++ |
| 5e | 0.35 | ++ | ++ | ++ | ++ | ++ | ++ |
| 7a | 0.25 | ++ | ++ | + | + | ++ | ++ |
| 7b | 0.64 | ++ | ++ | ++ | + | ++ | ++ |
| 7c | 0.30 | ++ | ++ | ++ | ++ | ++ | ++ |
| Polymer | Tg (°C) | Td10 (°C) | Char Yield at 800 °C (wt %) | |
|---|---|---|---|---|
| in N2 | in Air | |||
| 5a | 312 | 410 | 420 | 55 |
| 5b | 288 | 430 | 430 | 55 |
| 5c | 305 | 435 | 460 | 55 |
| 5d | 206 | 400 | 400 | 27 |
| 5e | 295 | 395 | 390 | 32 |
| 7a | 292 | 570 | 560 | 60 |
| 7b | 314 | 570 | 550 | 67 |
| 7c | 336 | 525 | 520 | 39 |
| Polymer | UV-Vis Absorption (nm) a | Oxidation Potential (V) b | Eg (eV) c | HOMO (eV) d | LUMO (eV) d | ||
|---|---|---|---|---|---|---|---|
| λmax | λonset | Eonset | E1/2Ox | ||||
| 5a | 352 | 436 | 0.70 | 0.85 | 2.84 | −5.51 | −2.67 |
| 5b | 340 | 396 | 0.74 | 0.85 | 3.13 | −5.51 | −2.38 |
| 5c | 343 | 414 | 0.72 | 0.86 | 2.99 | −5.52 | −2.53 |
| 5d | 320 | 368 | 0.70 | 0.83 | 3.37 | −5.49 | −2.12 |
| 5e | 321 | 373 | 0.71 | 0.81 | 3.32 | −5.47 | −2.15 |
| 7a | 320 | 379 | 1.02 | 1.11 | 3.27 | −5.77 | −2.50 |
| 7b | 323 | 379 | 1.00 | 1.10 | 3.27 | −5.76 | −2.49 |
| 7c | 316 | 358 | 0.99 | 1.09 | 3.46 | −5.75 | −2.29 |
| Cycling Times a | ΔOD490 b | Qd (mC cm−2) c | CE (cm2 C−1) d | Decay in CE (%) |
|---|---|---|---|---|
| 1 | 0.99 | 5.33 | 186 | 0 |
| 10 | 0.99 | 5.33 | 186 | 0 |
| 20 | 0.98 | 5.31 | 185 | 0.5 |
| 30 | 0.98 | 5.31 | 185 | 0.5 |
| 40 | 0.98 | 5.32 | 184 | 1 |
| 50 | 0.97 | 5.30 | 183 | 1.6 |
| 60 | 0.96 | 5.29 | 181 | 2.7 |
| 70 | 0.96 | 5.30 | 181 | 2.7 |
| 80 | 0.96 | 5.29 | 180 | 3.2 |
| 90 | 0.95 | 5.30 | 179 | 3.7 |
| 100 | 0.94 | 5.28 | 178 | 4.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, S.-H.; Liao, W.-K.; Liou, G.-S. Synthesis and Electrochromism of Highly Organosoluble Polyamides and Polyimides with Bulky Trityl-Substituted Triphenylamine Units. Polymers 2017, 9, 511. https://doi.org/10.3390/polym9100511
Hsiao S-H, Liao W-K, Liou G-S. Synthesis and Electrochromism of Highly Organosoluble Polyamides and Polyimides with Bulky Trityl-Substituted Triphenylamine Units. Polymers. 2017; 9(10):511. https://doi.org/10.3390/polym9100511
Chicago/Turabian StyleHsiao, Sheng-Huei, Wei-Kai Liao, and Guey-Sheng Liou. 2017. "Synthesis and Electrochromism of Highly Organosoluble Polyamides and Polyimides with Bulky Trityl-Substituted Triphenylamine Units" Polymers 9, no. 10: 511. https://doi.org/10.3390/polym9100511






