Cluster Formation of Polyphilic Molecules Solvated in a DPPC Bilayer
Abstract
:1. Introduction
2. System Setup and Computational Details
3. Results and Discussion
3.1. Lateral Diffusion
3.2. B16/10 Radial Distribution Functions
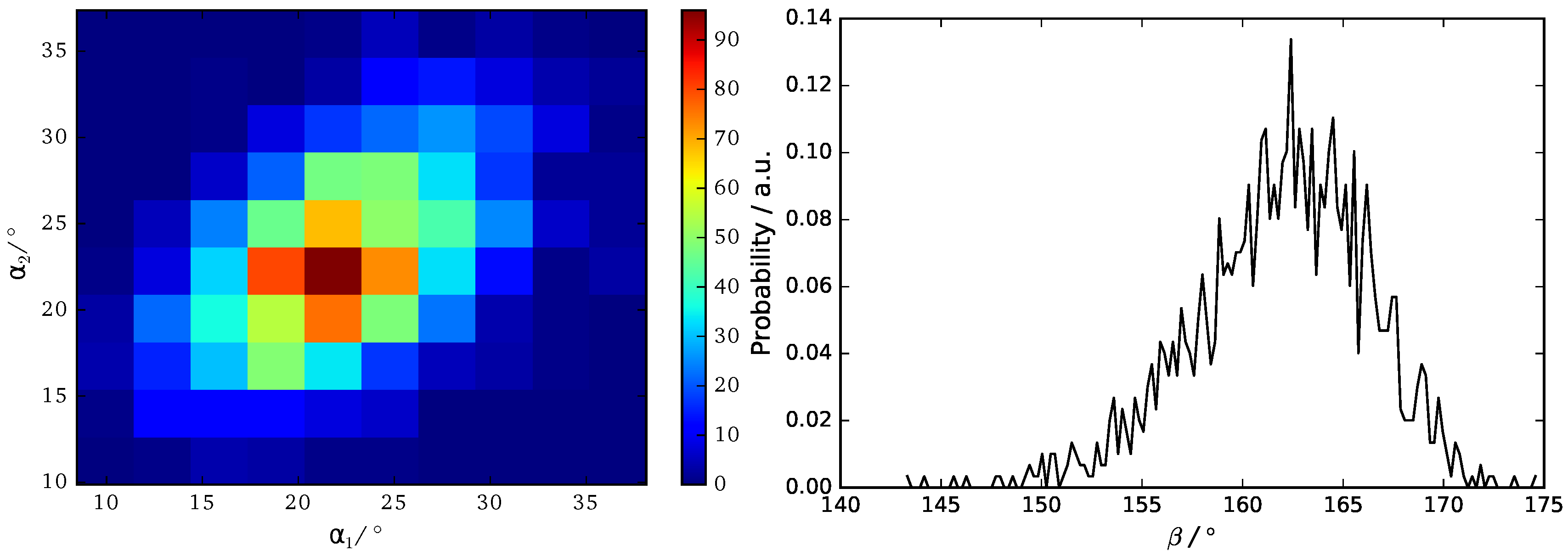
3.3. B16/10 Backbone Angles Distribution
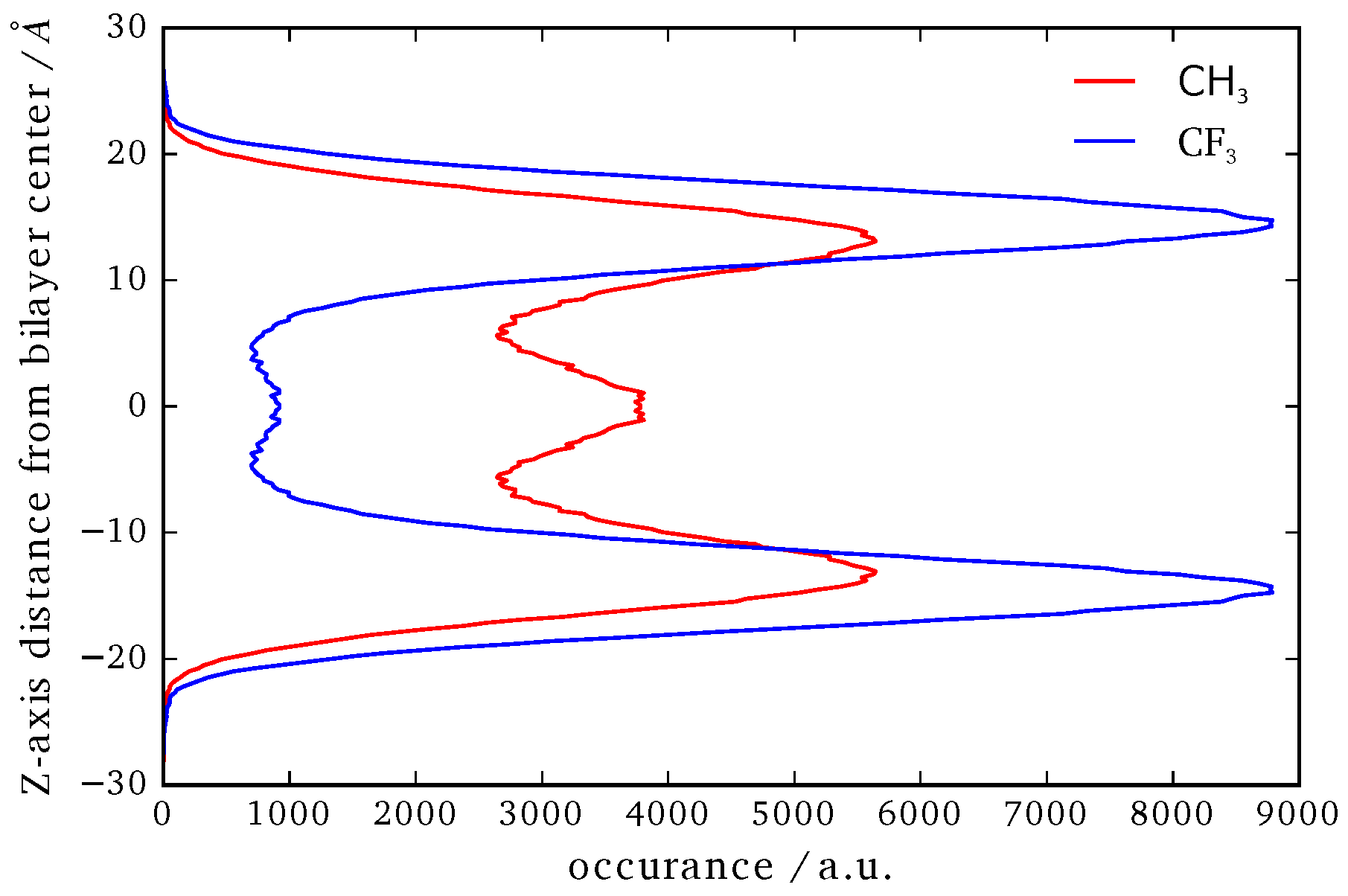
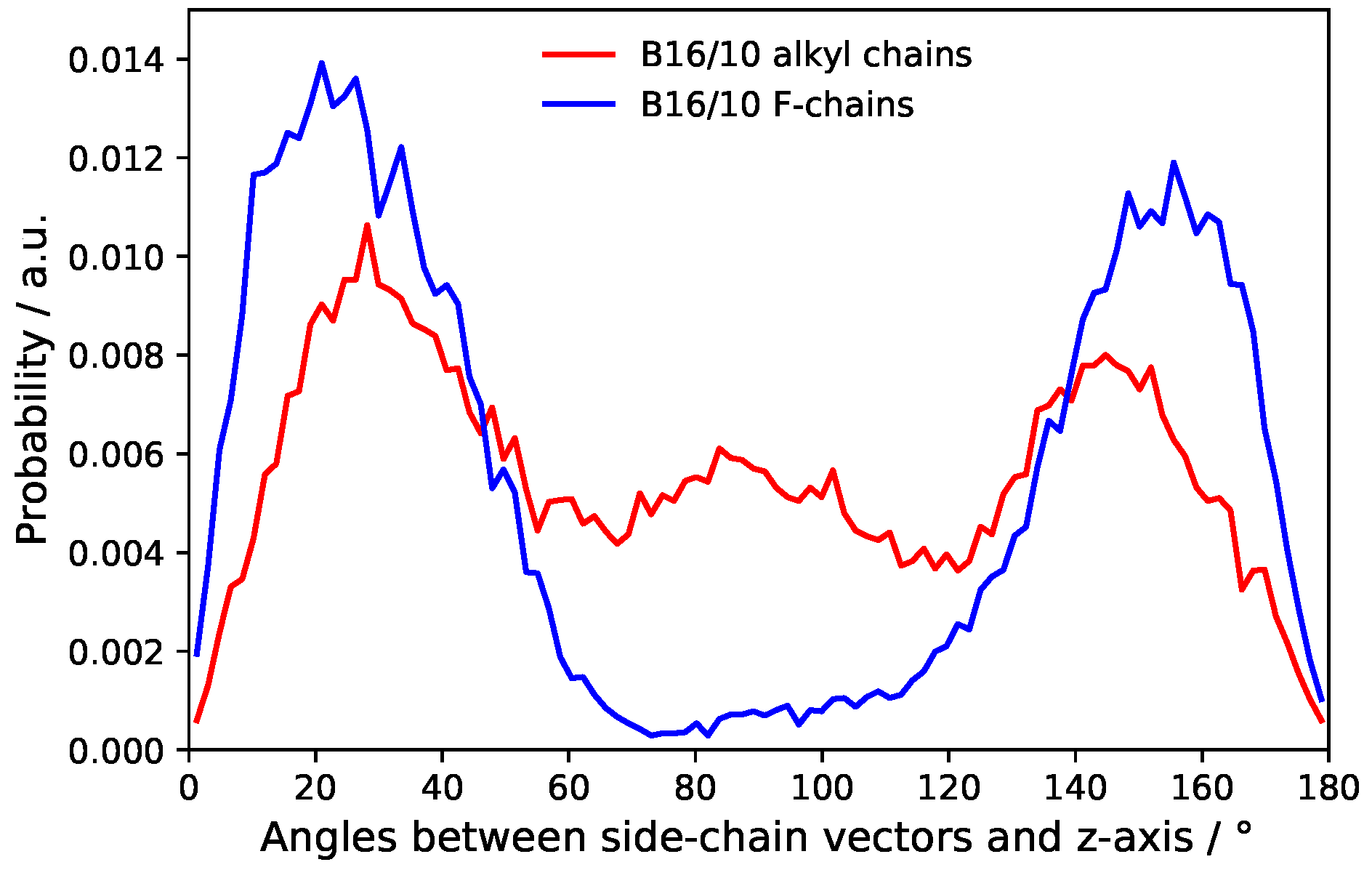
3.4. B16/10 Terminal Group Integration and Side Chain Orientations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ariga, K.; Hill, J.P.; Lee, M.V.; Vinu, A.; Charvet, R.; Acharya, S. Challenges and breakthroughs in recent research on self-assembly. Sci. Technol. Adv. Mater. 2008, 9, 014109. [Google Scholar] [CrossRef] [PubMed]
- White, S.H.; King, G.I.; Cain, J.E. Location of hexane in lipid bilayers determined by neutron diffraction. Nature 1981, 290, 161–163. [Google Scholar] [CrossRef]
- Brehm, M.; Saddiq, G.; Watermann, T.; Sebastiani, D. Influence of Small Fluorophilic and Lipophilic Organic Molecules on Dipalmitoylphosphatidylcholine Bilayers. J. Phys. Chem. B 2017, 121, 8311–8321. [Google Scholar] [CrossRef] [PubMed]
- Bates, M.; Walker, M. Dissipative particle dynamics simulation of T- and X-shaped polyphilic molecules exhibiting honeycomb columnar phases. Soft Matter 2009, 5, 346–353. [Google Scholar] [CrossRef]
- Crane, A.J.; Martínez-Veracoechea, F.J.; Escobedo, F.A.; Müller, E.A. Molecular dynamics simulation of the mesophase behaviour of a model bolaamphiphilic liquid crystal with a lateral flexible chain. Soft Matter 2008, 4, 1820–1829. [Google Scholar] [CrossRef]
- Hill, E.; Stratton, K.; Whitten, D.G.; Evans, D.G. Molecular Dynamics Simulation Study of the Interaction of Cationic Biocides with Lipid Bilayers: Aggregation Effects and Bilayer Damage. Langmuir 2012, 28, 14849–14854. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Olubummo, A.; Binder, W.H. Beyond the lipid-bilayer: Interaction of polymers and nanoparticles with membranes. Soft Matter 2012, 8, 4849–4864. [Google Scholar] [CrossRef]
- Hinks, J.; Wang, Y.; Poh, W.H.; Donose, B.C.; Thomas, A.W.; Wuertz, S.; Loo, S.C.J.; Bazan, G.C.; Kjelleberg, S.; Mu, Y.; et al. Modeling Cell Membrane Perturbation by Molecules Designed for Transmembrane Electron Transfer. Langmuir 2014, 30, 2429–2440. [Google Scholar] [CrossRef] [PubMed]
- James, P.V.; Sudeep, P.K.; Suresh, C.H.; Thomas, K.G. Photophysical and Theoretical Investigations of Oligo(p-phenyleneethynylene)s: Effect of Alkoxy Substitution and Alkyne–Aryl Bond Rotations. J. Phys. Chem. A 2006, 110, 4329–4337. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.H.; Whitten, D.G.; Evans, D.G. Computational Study of Bacterial Membrane Disruption by Cationic Biocides: Structural Basis for Water Pore Formation. J. Phys. Chem. B 2014, 118, 9722–9732. [Google Scholar] [CrossRef] [PubMed]
- Lechner, B.-D.; Ebert, H.; Prehm, M.; Werner, S.; Meister, A.; Hause, G.; Beerlink, A.; Saalwächter, K.; Bacia, K.; Tschierske, C.; et al. Temperature-Dependent In-Plane Structure Formation of an X-Shaped Bolapolyphile within Lipid Bilayers. Langmuir 2015, 31, 2839–2850. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Maitra, U. Chemistry and biology of bile acids. Curr. Sci. 2004, 87, 1666–1683. [Google Scholar]
- Krafft, M.P. Fluorocarbons and fluorinated amphiphiles in drug delivery and biomedical research. Adv. Drug Deliv. Rev. 2001, 47, 209–228. [Google Scholar] [CrossRef]
- Schwieger, C.; Achilles, A.; Scholz, S.; Rüger, J.; Bacia, K.; Saalwächter, K.; Kressler, J.; Blume, A. Binding of amphiphilic and triphilic block copolymers to lipid model membranes: The role of perfluorinated moieties. Soft Matter 2014, 10, 6147–6160. [Google Scholar] [CrossRef] [PubMed]
- Scholtysek, P.; Achilles, A.; Hoffmann, C.-V.; Lechner, B.-D.; Meister, A.; Tschierske, C.; Saalwächter, K.; Edwards, K.; Blume, A. A T-Shaped Amphiphilic Molecule Forms Closed Vesicles in Water and Bicelles in Mixtures with a Membrane Lipid. J. Phys. Chem. B 2012, 116, 4871–4878. [Google Scholar] [CrossRef] [PubMed]
- Achilles, A.; Bärenwald, R.; Lechner, B.; Werner, S.; Ebert, H.; Tschierske, C.; Blume, A.; Bacia, K.; Saalwächter, K. Self-Assembly of X-Shaped Bolapolyphiles in Lipid Membranes: Solid-State NMR Investigations. Langmuir 2016, 32, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Ebert, H.; Lechner, B.-D.; Lange, F.; Achilles, A.; Bärenwald, R.; Poppe, S.; Blume, A.; Saalwächter, K.; Tschierske, C.; et al. Dendritic Domains with Hexagonal Symmetry Formed by X-Shaped Bolapolyphiles in Lipid Membranes. Chem. Eur. J. 2015, 21, 8840–8850. [Google Scholar] [CrossRef] [PubMed]
- Bärenwald, R.; Achilles, A.; Lange, F.; Mendes, T.F.; Saalwächter, K. Applications of Solid-State NMR Spectroscopy for the Study of Lipid Membranes with Polyphilic Guest (Macro)Molecules. Polymers 2016, 8, 439. [Google Scholar] [CrossRef]
- Permadi, H.; Lundgren, B.; Andersson, K.; DePierre, J.W. Effects of perfluoro fatty acids on xenobiotic-metabolizing enzymes, enzymes which detoxify reactive forms of oxygen and lipid peroxidation in mouse liver. Biochem. Pharmacol. 1992, 44, 1183–1191. [Google Scholar] [CrossRef]
- Heuvel, J.P.V.; Kuslikis, B.I.; Van Rafelghem, M.J.; Peterson, R.E. Tissue distribution, metabolism, and elimination of perfluorooctanoic acid in male and female rats. J. Biochem. Toxicol. 1991, 6, 83–92. [Google Scholar] [CrossRef]
- Park, K.-H.; Berrier, C.; Lebaupain, F.; Pucci, B.; Popot, J.-L.; Ghazi, A.; Zito, F. Fluorinated and hemifluorinated surfactants as alternatives to detergents for membrane protein cell-free synthesis. Biochem. J. 2007, 403, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Brehm, M.; Weber, H.; Thomas, M.; Hollóczki, O.; Kirchner, B. Domain Analysis in Nanostructured Liquids: A Post-Molecular Dynamics Study at the Example of Ionic Liquids. ChemPhysChem 2015, 16, 3271–3277. [Google Scholar] [CrossRef] [PubMed]
- Nagle, J.F.; Tristram-Nagle, S. Structure of lipid bilayers. Biochim. Biophys. Acta 2000, 1469, 159–195. [Google Scholar] [CrossRef]
- Liu, B.; Hoopes, M.I.; Karttunen, M. Molecular Dynamics Simulations of DPPC/CTAB Monolayers at the Air/Water Interface. J. Phys. Chem. B 2014, 118, 11723–11737. [Google Scholar] [CrossRef] [PubMed]
- Repáková, J.; Holopainen, J.M.; Morrow, M.R.; McDonald, M.C.; Capková, P.; Vattulainen, I. Influence of DPH on the Structure and Dynamics of a DPPC Bilayer. Biophys. J. 2005, 88, 3398–3410. [Google Scholar] [CrossRef] [PubMed]
- Hughes, Z.E.; Mark, A.E.; Mancera, R.L. Molecular Dynamics Simulations of the Interactions of DMSO with DPPC and DOPC Phospholipid Membranes. J. Phys. Chem. B 2012, 116, 11911–11923. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Kieffer, R.; Glettner, B.; Nürnberger, C.; Liu, F.; Pelz, K.; Prehm, M.; Baumeister, U.; Hahn, H.; Lang, H.; et al. Complex Multicolor Tilings and Critical Phenomena in Tetraphilic Liquid Crystals. Science 2011, 331, 1302–1306. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, M.; Kikukawa, T.; Takahashi, H.; Takagi, T.; Yokoyama, Y.; Amii, H.; Baba, T.; Kanamori, T.; Sonoyama, M. Physicochemical Studies of Bacteriorhodopsin Reconstituted in Partially Fluorinated Phosphatidylcholine Bilayers. J. Phys. Chem. B 2013, 117, 5422–5429. [Google Scholar] [CrossRef] [PubMed]
- Bennett, W.F.D.; Tieleman, D.P. Computer simulations of lipid membrane domains. Biochim. Biophys. Acta 2013, 1828, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Lehmler, H.-J.; Bummer, P.M. Mixing of perfluorinated carboxylic acids with dipalmitoylphosphatidylcholine. Biochim. Biophys. Acta 2004, 1664, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Korlach, J.; Schwille, P.; Webb, W.W.; Feigenson, G.W. Characterization of lipid bilayer phases by confocal microscopy and fluorescence correlation spectroscopy. Proc. Natl. Acad. Sci. USA 1999, 96, 8461–8466. [Google Scholar] [CrossRef] [PubMed]
- Klauda, J.B.; Venable, R.M.; Freites, J.A.; O’Connor, J.W.; Tobias, D.J.; Mondragon-Ramirez, C.; Vorobyov, I.; MacKerell, A.D.; Pastor, R.W. Update of the CHARMM All-Atom Additive Force Field for Lipids: Validation on Six Lipid Types. J. Phys. Chem. B 2010, 114, 7830–7843. [Google Scholar] [CrossRef] [PubMed]
- Krafft, M.P. Controlling phospholipid self-assembly and film properties using highly fluorinated components—Fluorinated monolayers, vesicles, emulsions and microbubbles. Biochimie 2012, 10, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Feller, S.E.; Venable, R.M.; Pastor, R.W. Computer Simulation of a DPPC Phospholipid Bilayer: Structural Changes as a Function of Molecular Surface Area. Langmuir 1997, 13, 6555–6561. [Google Scholar] [CrossRef]
- Von Rudorff, G.F.; Watermann, T.; Guo, X.Y.; Sebastiani, D. Conformational Space of a Polyphilic Molecule with a Fluorophilic Side Chain Integrated in a DPPC Bilayer. J. Comput. Chem. 2017, 38, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kalé, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef] [PubMed]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM General Force Field: A Force Field for Drug-Like Molecules Compatible with the CHARMM All-Atom Additive Biological Force Fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef] [PubMed]
- Von Rudorff, G.F.; Watermann, T.; Sebastiani, D. Perfluoroalkane Force Field for Lipid Membrane Environments. J. Phys. Chem. B. 2014, 118, 12531–12540. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Michaud-Agrawal, N.; Denning, E.J.; Woolf, T.B.; Beckstein, O. MDAnalysis: A toolkit for the analysis of molecular dynamics simulations. J. Comput. Chem. 2011, 32, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Brehm, M.; Kirchner, B. TRAVIS—A Free Analyzer and Visualizer for Monte Carlo and Molecular Dynamics Trajectories. J. Chem. Inf. Model. 2011, 51, 2007–2023. [Google Scholar] [CrossRef] [PubMed]
- Junglas, M.; Danner, B.; Bayerl, T.M. Molecular Order Parameter Profiles and Diffusion Coefficients of Cationic Lipid Bilayers on a Solid Support. Langmuir 2003, 19, 1914–1917. [Google Scholar] [CrossRef]
- McQuarrie, D.A. Statistical Mechanics; University Science Books: Davis, CA, USA, 1976; p. 641. [Google Scholar]
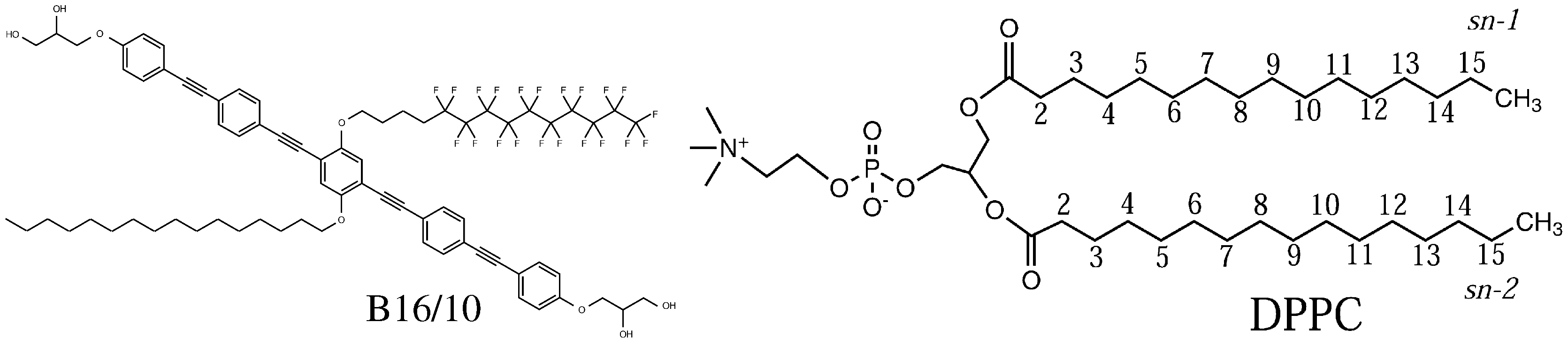
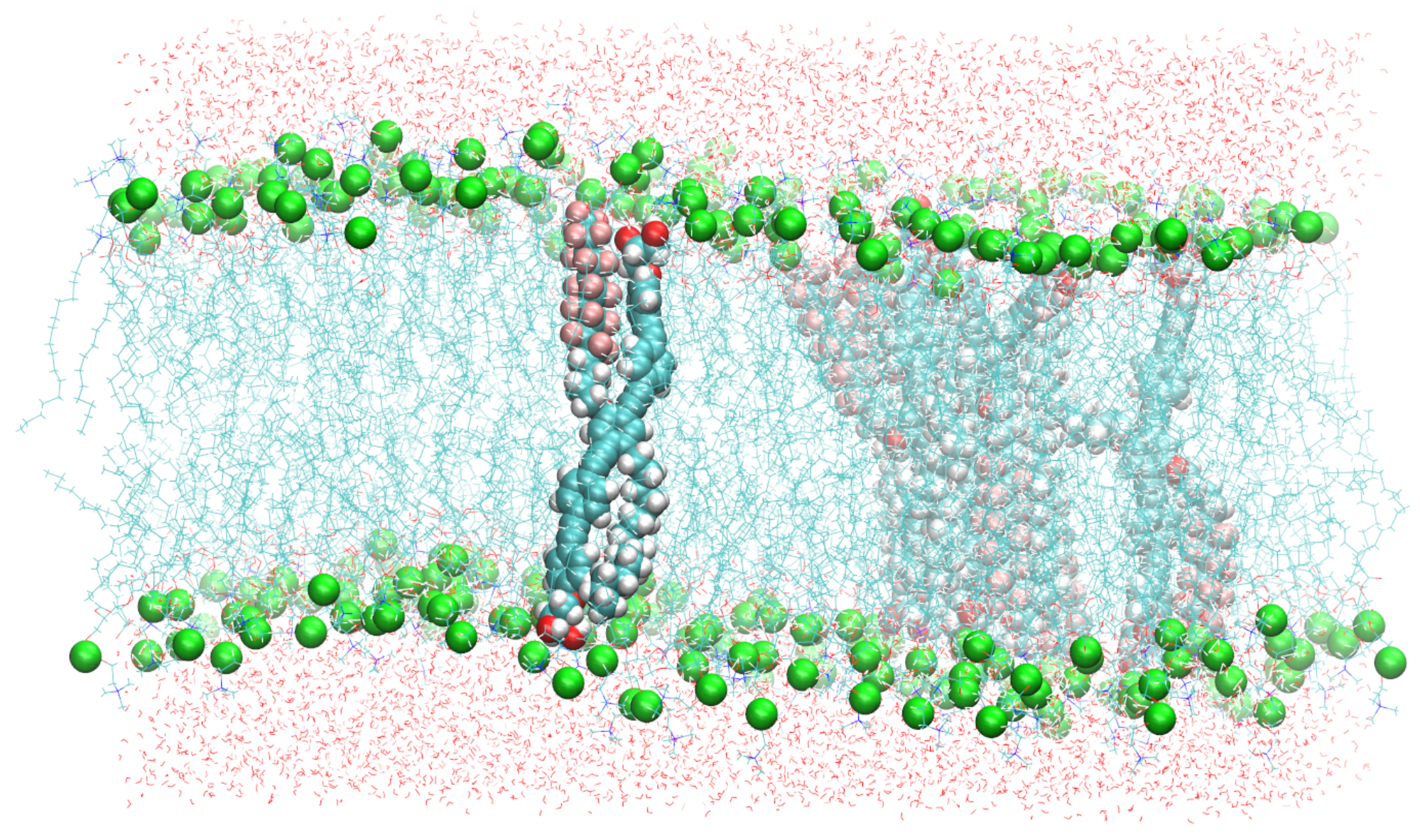

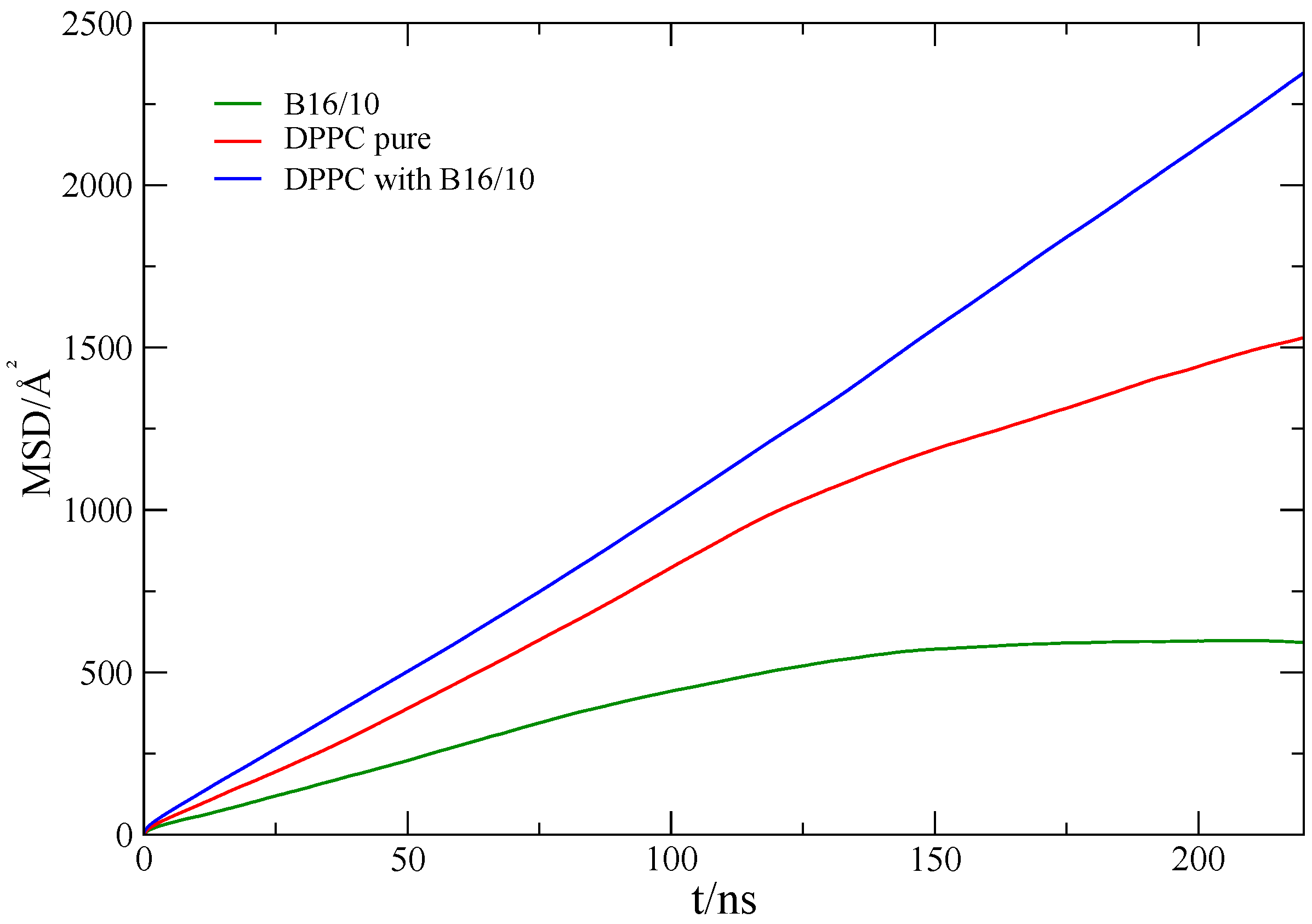

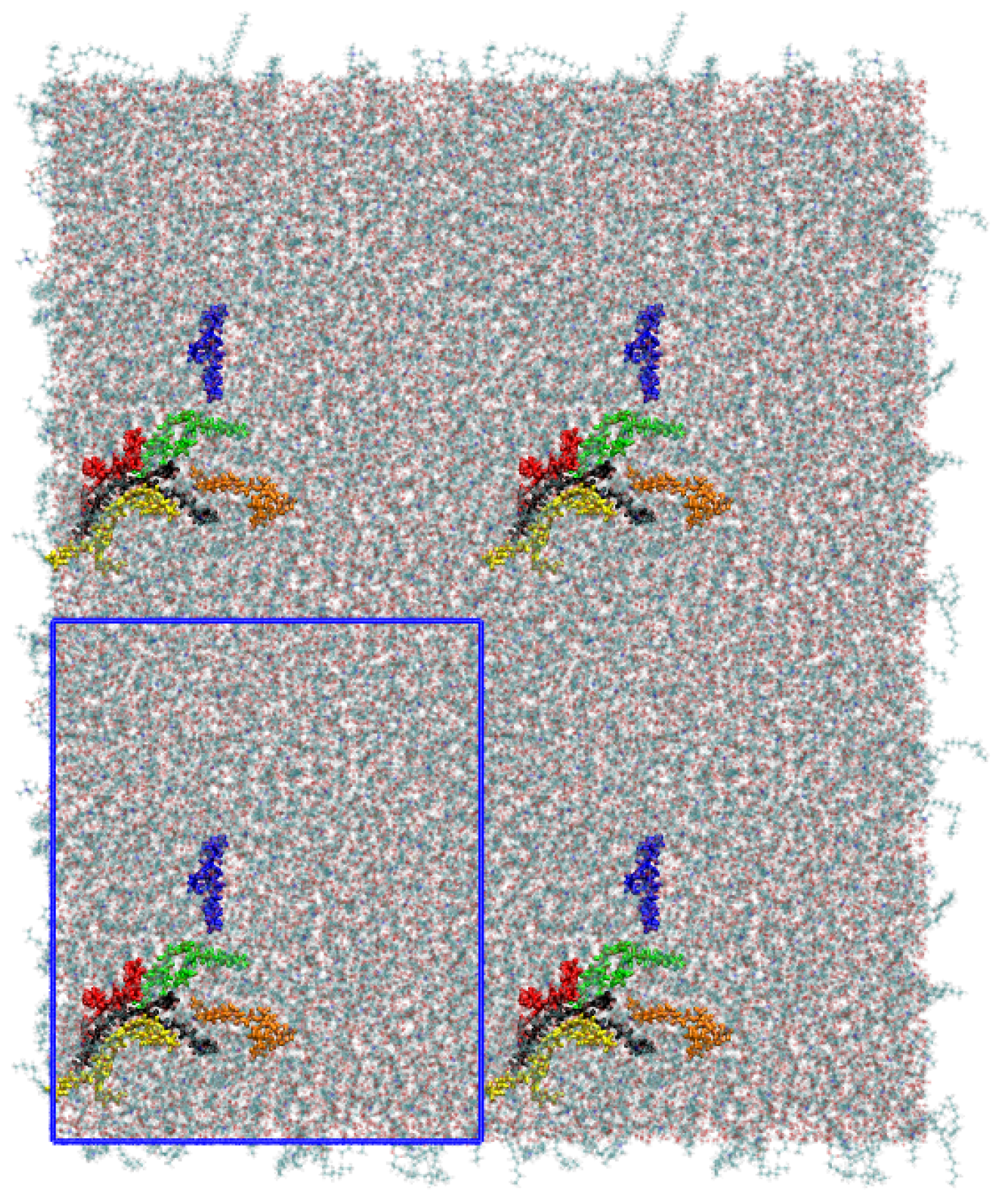

| D/() | Lipids (Pure DPPC) | Lipids (mix) | B16/10 (mix) |
|---|---|---|---|
| MD | 13.9 ± 0.3 | 24 ± 0.6 | 10.7 ± 0.3 |
| exp | 14.2 ± 1.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.-Y.; Peschel, C.; Watermann, T.; Rudorff, G.F.v.; Sebastiani, D. Cluster Formation of Polyphilic Molecules Solvated in a DPPC Bilayer. Polymers 2017, 9, 488. https://doi.org/10.3390/polym9100488
Guo X-Y, Peschel C, Watermann T, Rudorff GFv, Sebastiani D. Cluster Formation of Polyphilic Molecules Solvated in a DPPC Bilayer. Polymers. 2017; 9(10):488. https://doi.org/10.3390/polym9100488
Chicago/Turabian StyleGuo, Xiang-Yang, Christopher Peschel, Tobias Watermann, Guido Falk von Rudorff, and Daniel Sebastiani. 2017. "Cluster Formation of Polyphilic Molecules Solvated in a DPPC Bilayer" Polymers 9, no. 10: 488. https://doi.org/10.3390/polym9100488





