3D Culture of Chondrocytes in Gelatin Hydrogels with Different Stiffness
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of GelMA Macromers
2.2. 1H Nuclear Magnetic Resonance (NMR)
2.3. RGD Density Measurement
2.4. Preparation of GelMA Hydrogels
2.5. Mechanical Testing
2.6. Swelling Ratio Measurement
2.7. Measurement of Enzymatic Degradation of Hydrogels
2.8. Culture of Chondrocytes in Hydrogels
2.9. Cell Viability Assay
2.10. Cytoskeleton Actin Filament Staining
2.11. Histological Stainings
2.12. Quantification of DNA and Sulfated Glycosaminoglycan (sGAG)
2.13. Real-Time PCR Analysis
2.14. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of GelMA Macromers
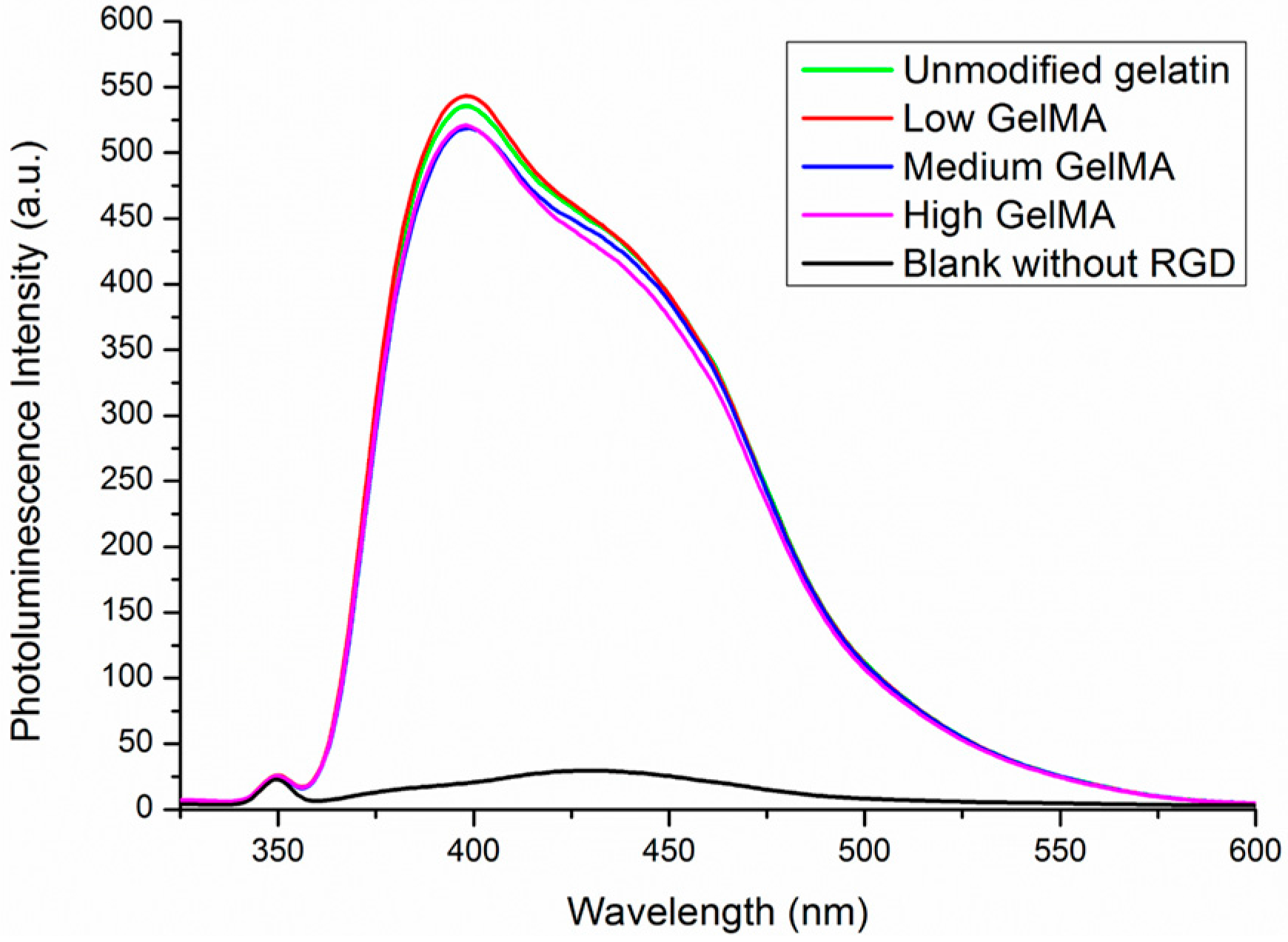
3.2. RGD Density in Gelatin and GelMA Macromers
3.3. Young’s Modulus and Swelling Ratio of GelMA Hydrogels
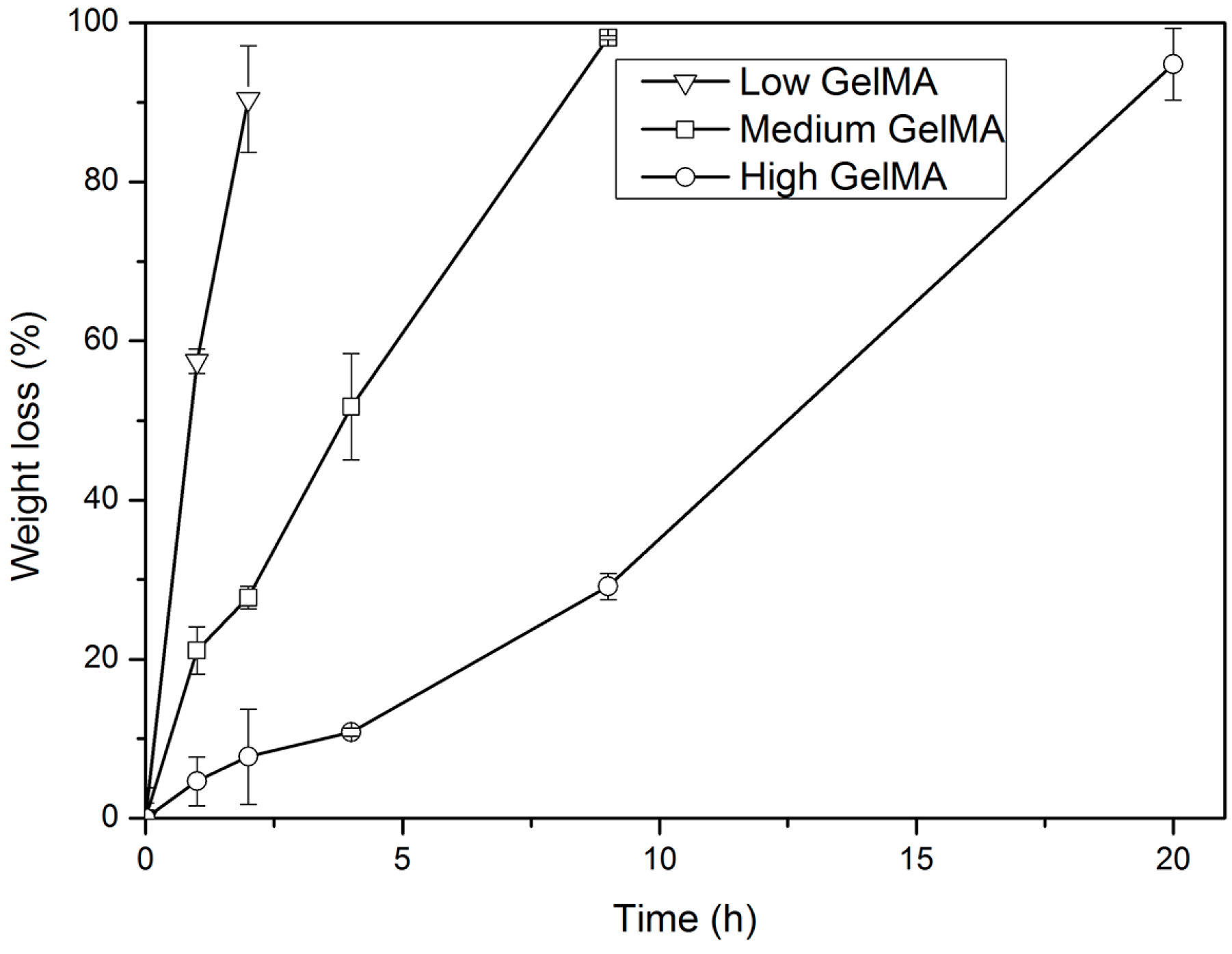
3.4. Enzymatic Degradation of GelMA Hydrogels
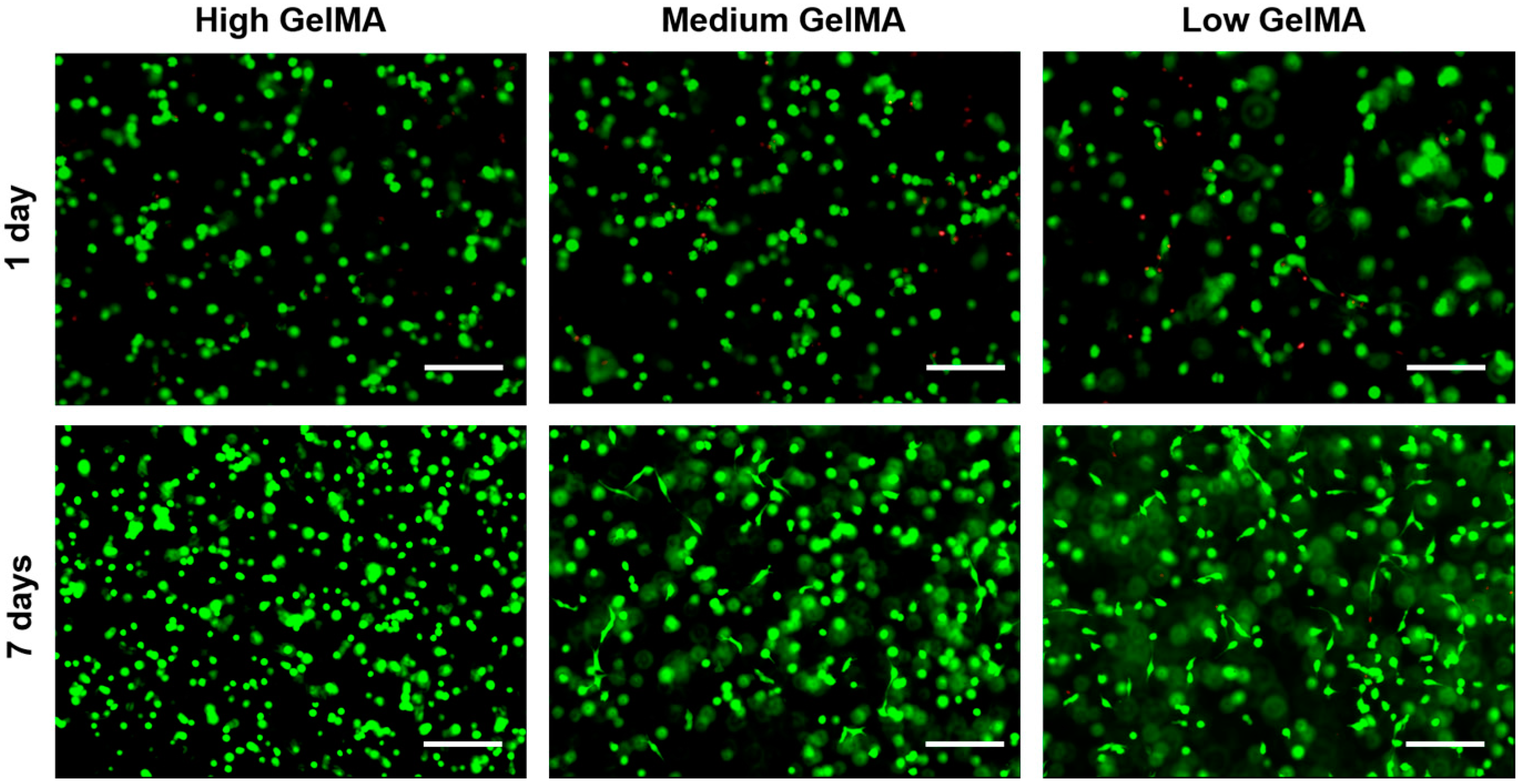
3.5. Cell Viability in GelMA Hydrogels
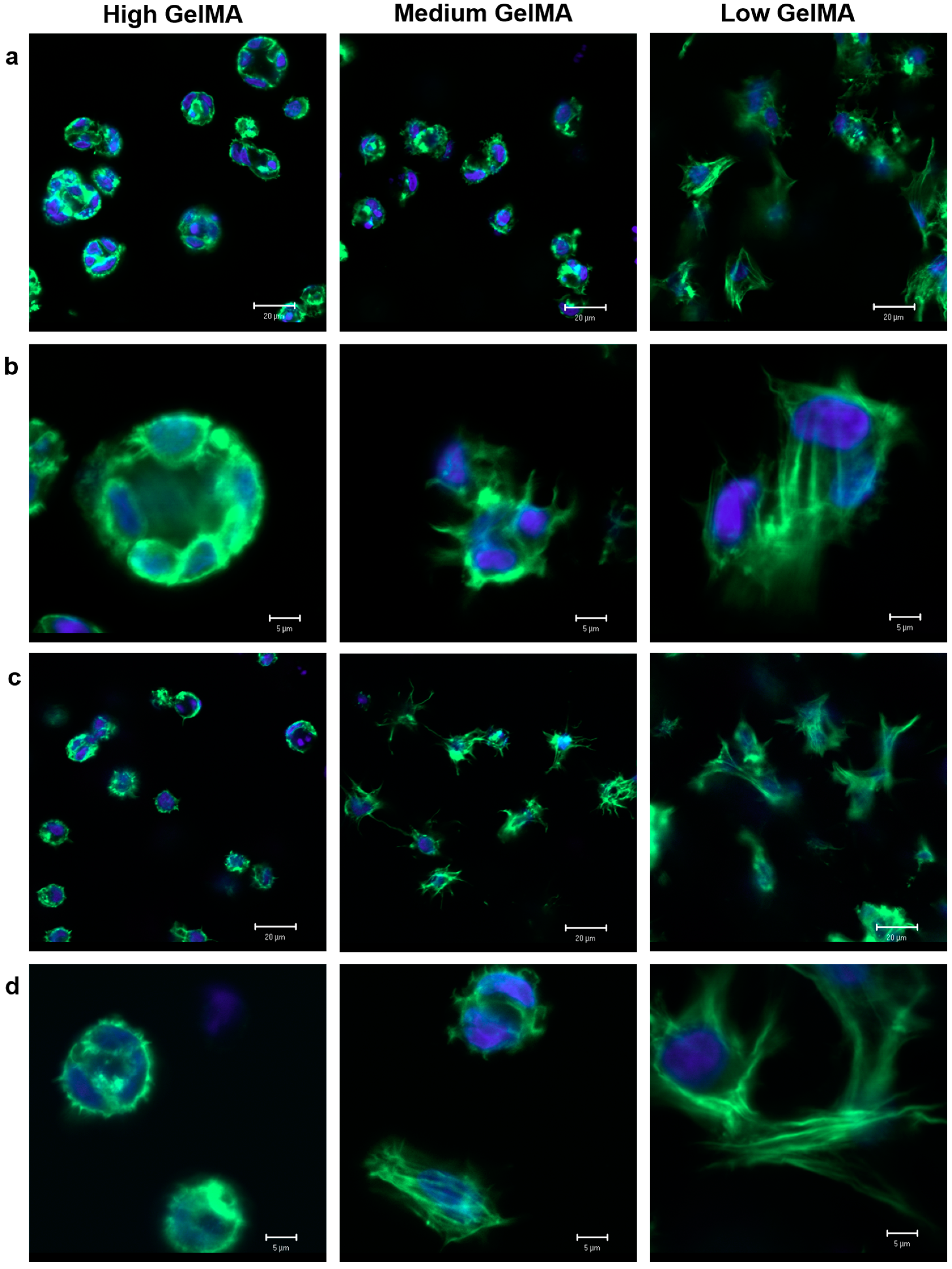
3.6. Cytoskeleton Organization in GelMA Hydrogels
3.7. Histological Stainings
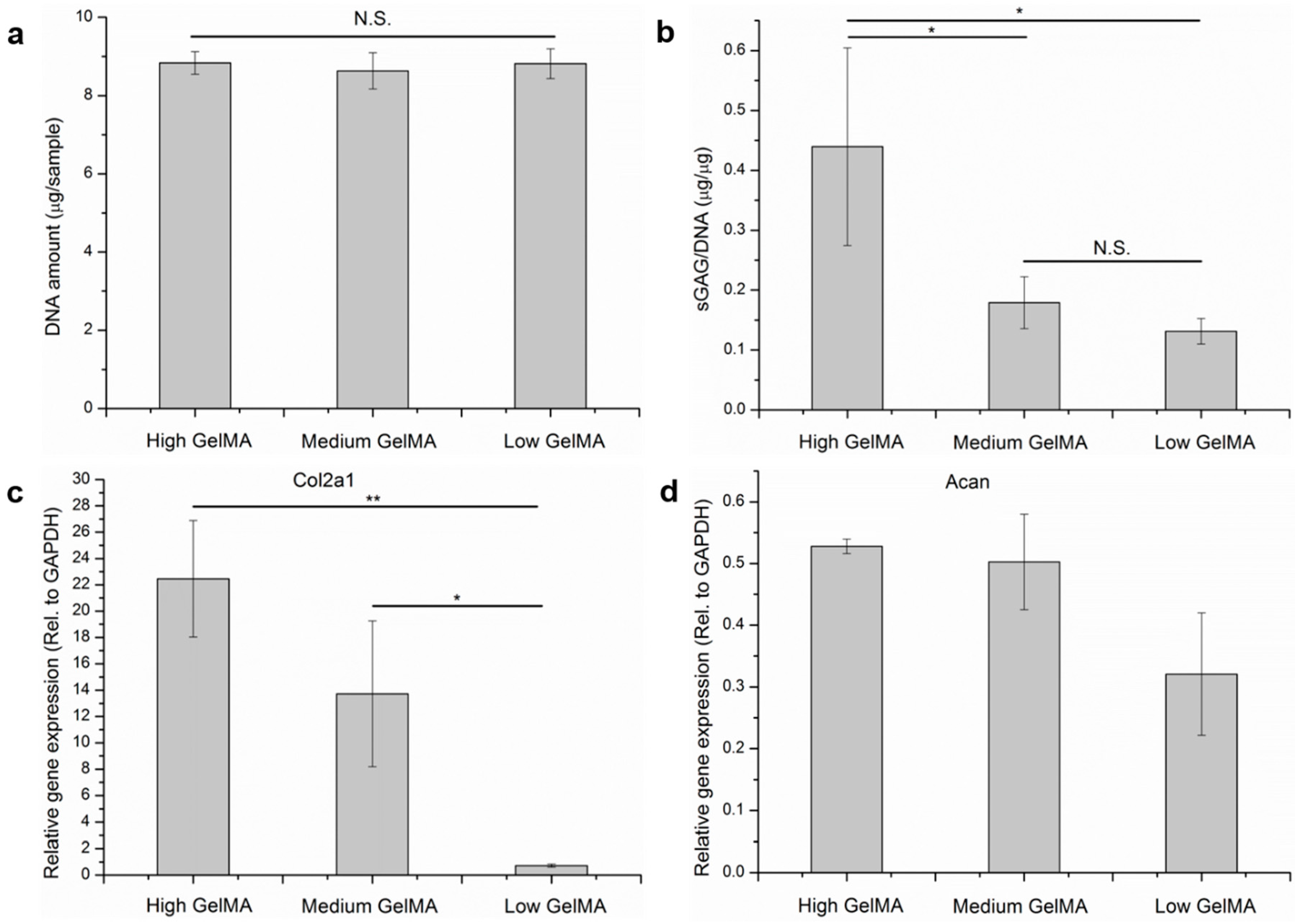
3.8. Quantification of DNA, Cartilaginous Matrices, and Gene Expression
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jeng, L.; Hsu, H.-P.; Spector, M. Tissue-engineered cartilaginous constructs for the treatment of caprine cartilage defects, including distribution of laminin and type IV collagen. Tissue Eng. Part A 2013, 19, 2267–2274. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, W.; Kawazoe, N.; Chen, G. Influence of cell protrusion and spreading on adipogenic differentiation of mesenchymal stem cells on micropatterned surfaces. Soft Matter 2013, 9, 4160–4166. [Google Scholar] [CrossRef]
- Kumar, S.S.; Hsiao, J.-H.; Ling, Q.-D.; Dulinska-Molak, I.; Chen, G.; Chang, Y.; Chang, Y.; Chen, Y.H.; Chen, D.-C.; Hsu, S.-T. The combined influence of substrate elasticity and surface-grafted molecules on the ex vivo expansion of hematopoietic stem and progenitor cells. Biomaterials 2013, 34, 7632–7644. [Google Scholar] [CrossRef] [PubMed]
- Hoshiba, T.; Kawazoe, N.; Tateishi, T.; Chen, G. Development of extracellular matrices mimicking stepwise adipogenesis of mesenchymal stem cells. Adv. Mater. 2010, 22, 3042–3047. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Kawazoe, N.; Kitajima, T.; Myoken, Y.; Tomita, M.; Umezawa, A.; Chen, G.; Ito, Y. Spatial immobilization of bone morphogenetic protein-4 in a collagen-PLGA hybrid scaffold for enhanced osteoinductivity. Biomaterials 2012, 33, 6140–6146. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.; Jana, S.; Tsao, C.-T.; Zhang, M. Tenogenic differentiation of human bone marrow stem cells via a combinatory effect of aligned chitosan-poly-caprolactone nanofibers and TGF-β3. J. Mater. Chem. B 2013, 1, 6516–6524. [Google Scholar] [CrossRef]
- Guo, L.; Kawazoe, N.; Hoshiba, T.; Tateishi, T.; Chen, G.; Zhang, X. Osteogenic differentiation of human mesenchymal stem cells on chargeable polymer-modified surfaces. J. Biomed. Mater. Res. A 2008, 87, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Nakamoto, T.; Dulińska-Molak, I.; Kawazoe, N.; Chen, G. Regulating the stemness of mesenchymal stem cells by tuning micropattern features. J. Mater. Chem. B 2016, 4, 37–45. [Google Scholar] [CrossRef]
- Higuchi, A.; Ling, Q.-D.; Kumar, S.S.; Chang, Y.; Alarfaj, A.A.; Munusamy, M.A.; Murugan, K.; Hsu, S.-T.; Umezawa, A. Physical cues of cell culture materials lead the direction of differentiation lineages of pluripotent stem cells. J. Mater. Chem. B 2015, 3, 8032–8058. [Google Scholar] [CrossRef]
- Sunyer, R.; Jin, A.J.; Nossal, R.; Sackett, D.L. Fabrication of hydrogels with steep stiffness gradients for studying cell mechanical response. PLoS ONE 2013, 7, e46107. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Wolf, K. Plasticity of cell migration: A multiscale tuning model. J. Cell Biol. 2010, 188, 11–19. [Google Scholar] [PubMed]
- Higuchi, A.; Ling, Q.-D.; Chang, Y.; Hsu, S.-T.; Umezawa, A. Physical cues of biomaterials guide stem cell differentiation fate. Chem. Rev. 2013, 113, 3297–3328. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Levental, I.; Georges, P.C.; Janmey, P.A. Soft biological materials and their impact on cell function. Soft Matter 2007, 3, 299–306. [Google Scholar] [CrossRef]
- Da Cunha, C.B.; Klumpers, D.D.; Li, W.A.; Koshy, S.T.; Weaver, J.C.; Chaudhuri, O.; Granja, P.L.; Mooney, D.J. Influence of the stiffness of three-dimensional alginate/collagen-I interpenetrating networks on fibroblast biology. Biomaterials 2014, 35, 8927–8936. [Google Scholar] [CrossRef] [PubMed]
- Yeung, T.; Georges, P.C.; Flanagan, L.A.; Marg, B.; Ortiz, M.; Funaki, M.; Zahir, N.; Ming, W.; Weaver, V.; Janmey, P.A. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskelet. 2005, 60, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Janmey, P.; Wang, Y.-L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Schuh, E.; Kramer, J.; Rohwedel, J.; Notbohm, H.; Müller, R.; Gutsmann, T.; Rotter, N. Effect of matrix elasticity on the maintenance of the chondrogenic phenotype. Tissue Eng. Part A 2010, 16, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Bryant, S.J.; Anseth, K.S. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly (ethylene glycol) hydrogels. J. Biomed. Mater. Res. 2002, 59, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Callahan, L.A.S.; Ganios, A.M.; Childers, E.P.; Weiner, S.D.; Becker, M.L. Primary human chondrocyte extracellular matrix formation and phenotype maintenance using RGD-derivatized PEGDM hydrogels possessing a continuous young’s modulus gradient. Acta Biomater. 2013, 9, 6095–6104. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Chung, C.; Jia, X.; Randolph, M.A.; Langer, R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules 2005, 6, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Schuh, E.; Hofmann, S.; Stok, K.; Notbohm, H.; Müller, R.; Rotter, N. Chondrocyte redifferentiation in 3D: The effect of adhesion site density and substrate elasticity. J. Biomed. Mater. Res. A 2012, 100, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Jeon, O.; Bouhadir, K.H.; Mansour, J.M.; Alsberg, E. Photocrosslinked alginate hydrogels with tunable biodegradation rates and mechanical properties. Biomaterials 2009, 30, 2724–2734. [Google Scholar] [CrossRef] [PubMed]
- Stainsby, G.; Ward, G.; Courts, A. The Science and Technology of Gelatin; Ward, A.G., Courts, A., Eds.; Academic Press Inc.: New York, NY, USA, 1977; p. 179. [Google Scholar]
- Sell, S.A.; Wolfe, P.S.; Garg, K.; McCool, J.M.; Rodriguez, I.A.; Bowlin, G.L. The use of natural polymers in tissue engineering: A focus on electrospun extracellular matrix analogues. Polymers 2010, 2, 522–553. [Google Scholar] [CrossRef]
- Awad, H.A.; Wickham, M.Q.; Leddy, H.A.; Gimble, J.M.; Guilak, F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials 2004, 25, 3211–3222. [Google Scholar] [CrossRef] [PubMed]
- Lien, S.-M.; Ko, L.-Y.; Huang, T.-J. Effect of pore size on ECM secretion and cell growth in gelatin scaffold for articular cartilage tissue engineering. Acta Biomater. 2009, 5, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Schuurman, W.; Levett, P.A.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.; Hutmacher, D.W.; Melchels, F.P.; Klein, T.J.; Malda, J. Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol. Biosci. 2013, 13, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Shoichet, M.S. A photolabile hydrogel for guided three-dimensional cell growth and migration. Nat. Mater. 2004, 3, 249–253. [Google Scholar] [CrossRef] [PubMed]
- West, J.L. Protein-patterned hydrogels: Customized cell microenvironments. Nat. Mater. 2011, 10, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Benton, J.A.; DeForest, C.A.; Vivekanandan, V.; Anseth, K.S. Photocrosslinking of gelatin macromers to synthesize porous hydrogels that promote valvular interstitial cell function. Tissue Eng. Part A 2009, 15, 3221–3230. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Ito, Y. Visible light-curable polymers for biomedical applications. Sci. China Chem. 2014, 57, 510–521. [Google Scholar] [CrossRef]
- Wei, D.; Xiao, W.; Sun, J.; Zhong, M.; Guo, L.; Fan, H.; Zhang, X. A biocompatible hydrogel with improved stiffness and hydrophilicity for modular tissue engineering assembly. J. Mater. Chem. B 2015, 3, 2753–2763. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bulcke, A.I.; Bogdanov, B.; de Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Hoch, E.; Hirth, T.; Tovar, G.E.; Borchers, K. Chemical tailoring of gelatin to adjust its chemical and physical properties for functional bioprinting. J. Mater. Chem. B 2013, 1, 5675–5685. [Google Scholar] [CrossRef]
- Das, P.; Jana, N.R. Highly colloidally stable hyperbranched polyglycerol grafted red fluorescent silicon nanoparticle as bioimaging probe. ACS Appl. Mater. Interfaces 2014, 6, 4301–4309. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.E.; MacQuarrie, R. A sensitive fluorometric method for the determination of arginine using 9,10-phenanthrenequinone. Anal. Biochem. 1978, 90, 246–255. [Google Scholar] [CrossRef]
- Xiao, W.; He, J.; Nichol, J.W.; Wang, L.; Hutson, C.B.; Wang, B.; Du, Y.; Fan, H.; Khademhosseini, A. Synthesis and characterization of photocrosslinkable gelatin and silk fibroin interpenetrating polymer network hydrogels. Acta Biomater. 2011, 7, 2384–2393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lu, H.; Kawazoe, N.; Chen, G. Pore size effect of collagen scaffolds on cartilage regeneration. Acta Biomater. 2014, 10, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Ko, Y.-G.; Kawazoe, N.; Chen, G. Cartilage tissue engineering using funnel-like collagen sponges prepared with embossing ice particulate templates. Biomaterials 2010, 31, 5825–5835. [Google Scholar] [CrossRef] [PubMed]
- Edep, M.E.; Shirani, J.; Wolf, P.; Brown, D.L. Matrix metalloproteinase expression in nonrheumatic aortic stenosis. Cardiovasc. Pathol. 2000, 9, 281–286. [Google Scholar] [CrossRef]
- Kim, I.L.; Khetan, S.; Baker, B.M.; Chen, C.S.; Burdick, J.A. Fibrous hyaluronic acid hydrogels that direct MSC chondrogenesis through mechanical and adhesive cues. Biomaterials 2013, 34, 5571–5580. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, P.S.; Loboa, E.G. Cytoskeletal and focal adhesion influences on mesenchymal stem cell shape, mechanical properties, and differentiation down osteogenic, adipogenic, and chondrogenic pathways. Tissue Eng. Part B Rev. 2012, 18, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Moreira Teixeira, L.; Leijten, J.; Sobral, J.; Jin, R.; Apeldoorn, V.A.; Feijen, J.; Blitterswijk, V.C.; Dijkstra, P.; Karperien, H. High throughput generated micro-aggregates of chondrocytes stimulate cartilage formation in vitro and in vivo. Eur. Cell Mater. 2012, 23, 387–399. [Google Scholar] [PubMed]
- Fang, J.; Yong, Q.; Zhang, K.; Sun, W.; Yan, S.; Cui, L.; Yin, J. Novel injectable porous poly (γ-benzyl-l-glutamate) microspheres for cartilage tissue engineering: Preparation and evaluation. J. Mater. Chem. B 2015, 3, 1020–1031. [Google Scholar] [CrossRef]
- Liu, Y.; Chan-Park, M.B. A biomimetic hydrogel based on methacrylated dextran-graft-lysine and gelatin for 3D smooth muscle cell culture. Biomaterials 2010, 31, 1158–1170. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Li, L.; Sun, M.; Zhang, Y.; Chen, L.; Rong, Y.; Li, Y. Mechanism of regulation of stem cell differentiation by matrix stiffness. Stem Cell Res. Ther. 2015, 6, 103. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.L.; Cooke, M.E.; Alliston, T. ECM stiffness primes the TGFβ pathway to promote chondrocyte differentiation. Mol. Biol. Cell 2012, 23, 3731–3742. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Burdick, J.A. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng. Part A 2008, 15, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Acharya, C.; Adesida, A.; Zajac, P.; Mumme, M.; Riesle, J.; Martin, I.; Barbero, A. Enhanced chondrocyte proliferation and mesenchymal stromal cells chondrogenesis in coculture pellets mediate improved cartilage formation. J. Cell. Physiol. 2012, 227, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Marklein, R.A.; Burdick, J.A. Controlling stem cell fate with material design. Adv. Mater. 2010, 22, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, Q.; Nakamoto, T.; Kawazoe, N.; Chen, G. Gelatin scaffolds with controlled pore structure and mechanical property for cartilage tissue engineering. Tissue Eng. Part C Methods 2016, 22, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, L.; Han, Q.; Heng, B.C.; Yang, Z.; Ge, Z. Relationship between cell function and initial cell seeding density of primary porcine chondrocytes in vitro. Biomed. Eng. Appl. Basis Commun. 2013, 25, 1340001. [Google Scholar] [CrossRef]
- Khetan, S.; Guvendiren, M.; Legant, W.R.; Cohen, D.M.; Chen, C.S.; Burdick, J.A. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater. 2013, 12, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Lee, H.-P.; Lippens, E.; Duda, G.N.; et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016, 15, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, S.; Huang, J.; Guo, W.; Chen, J.; Zhang, L.; Zhao, B.; Peng, J.; Wang, A.; Wang, Y. The ECM-cell interaction of cartilage extracellular matrix on chondrocytes. Biomed. Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z. Chondrons and the pericellular matrix of chondrocytes. Tissue Eng. Part B Rev. 2014, 21, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, L.G.; Setton, L.A.; Guilak, F. The biomechanical role of the chondrocyte pericellular matrix in articular cartilage. Acta Biomater. 2005, 1, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Buxboim, A.; Ivanovska, I.L.; Discher, D.E. Matrix elasticity, cytoskeletal forces and physics of the nucleus: How deeply do cells ‘feel’outside and in? J. Cell Sci. 2010, 123, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.-G.; Revzin, A.; Pishko, M.V. Poly(ethylene glycol) hydrogel microstructures encapsulating living cells. Langmuir 2002, 18, 2459–2462. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.B.; Lin, C.-C.; Kuntzler, D.V.; Anseth, K.S. The performance of human mesenchymal stem cells encapsulated in cell-degradable polymer-peptide hydrogels. Biomaterials 2011, 32, 3564–3574. [Google Scholar] [CrossRef] [PubMed]



| Sample | Feed Ratio of Gel (g)/MA (mL) | DoF (%) |
|---|---|---|
| Low GelMA | 5/0.2 | 25.8 ± 0.7 |
| Medium GelMA | 5/1.0 | 52.5 ± 1.2 |
| High GelMA | 5/5.0 | 91.7 ± 1.4 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Chen, S.; Li, J.; Wang, X.; Zhang, J.; Kawazoe, N.; Chen, G. 3D Culture of Chondrocytes in Gelatin Hydrogels with Different Stiffness. Polymers 2016, 8, 269. https://doi.org/10.3390/polym8080269
Li X, Chen S, Li J, Wang X, Zhang J, Kawazoe N, Chen G. 3D Culture of Chondrocytes in Gelatin Hydrogels with Different Stiffness. Polymers. 2016; 8(8):269. https://doi.org/10.3390/polym8080269
Chicago/Turabian StyleLi, Xiaomeng, Shangwu Chen, Jingchao Li, Xinlong Wang, Jing Zhang, Naoki Kawazoe, and Guoping Chen. 2016. "3D Culture of Chondrocytes in Gelatin Hydrogels with Different Stiffness" Polymers 8, no. 8: 269. https://doi.org/10.3390/polym8080269







