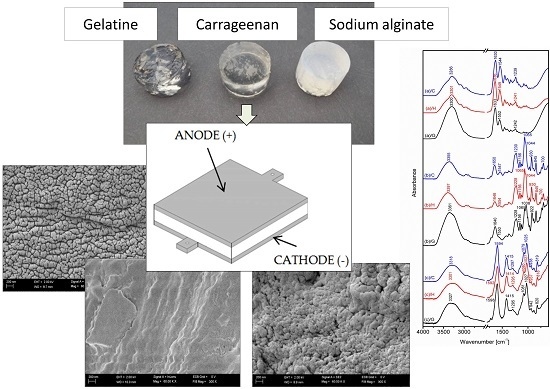
Physicochemical Properties of Biopolymer Hydrogels Treated by Direct Electric Current
Abstract
:1. Introduction
2. Experimental Section
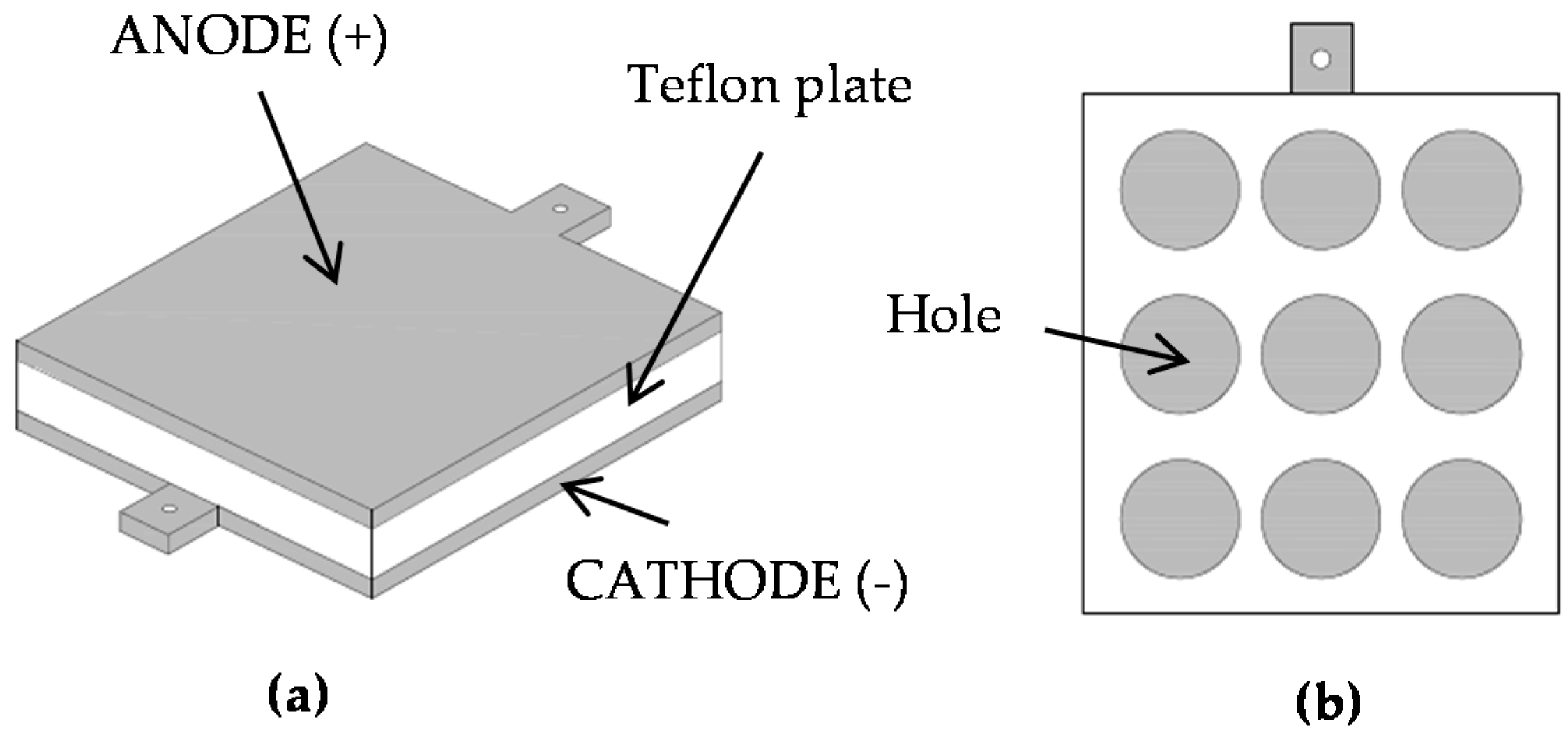
2.1. Apparatus
2.2. Materials
2.3. Preparation of Hydrogels
2.4. Hydrogels’ Characterization
2.4.1. pH Measurement
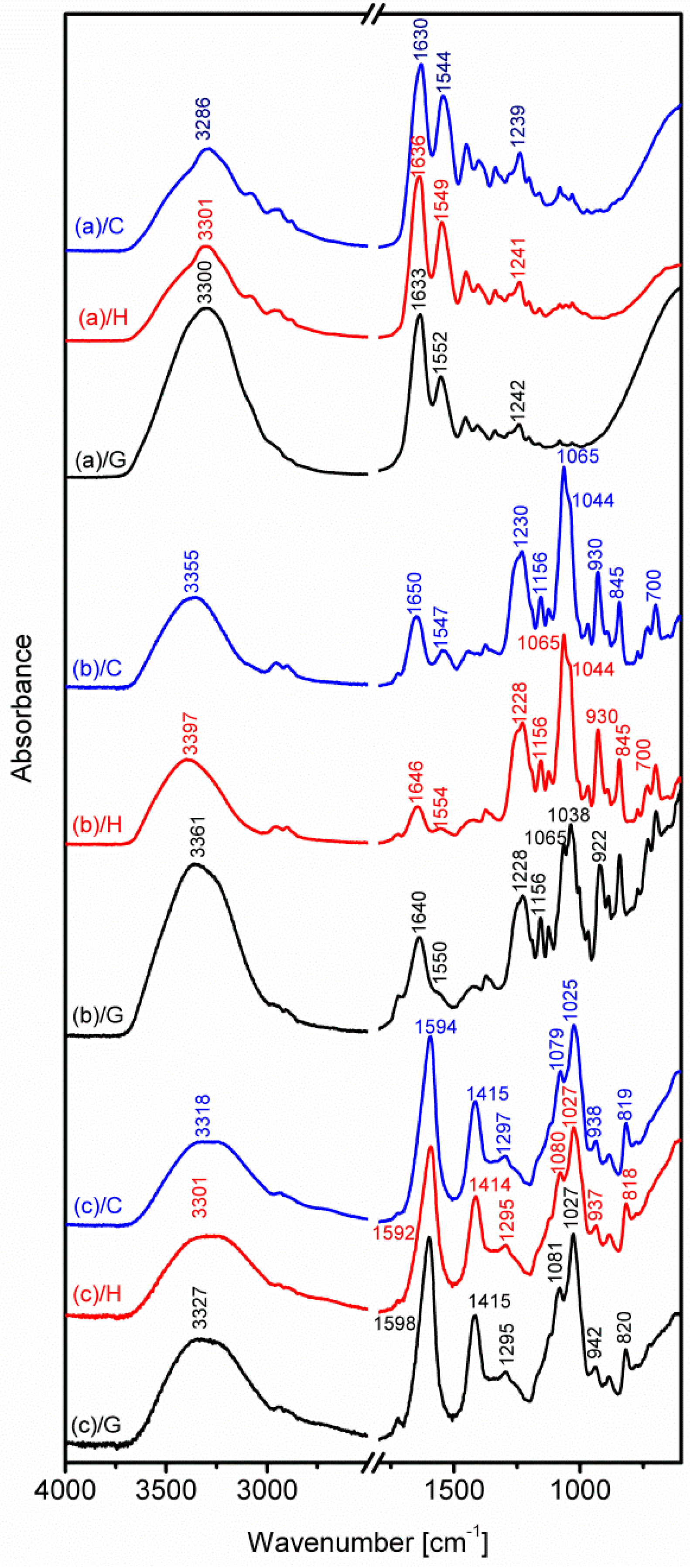
2.4.2. Fourier Transform Infrared Spectroscopy
2.4.3. Scanning Electron Microscopy
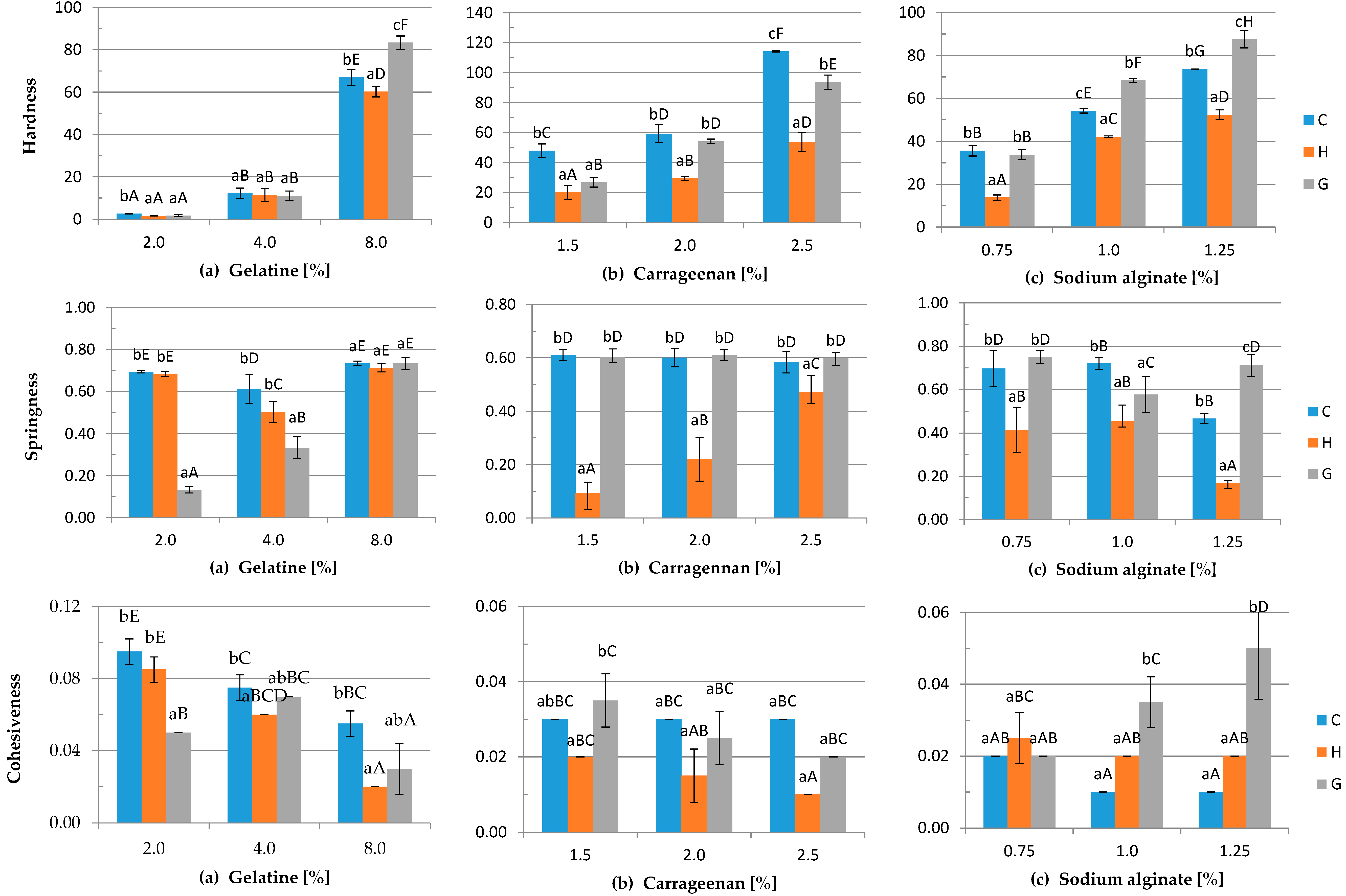
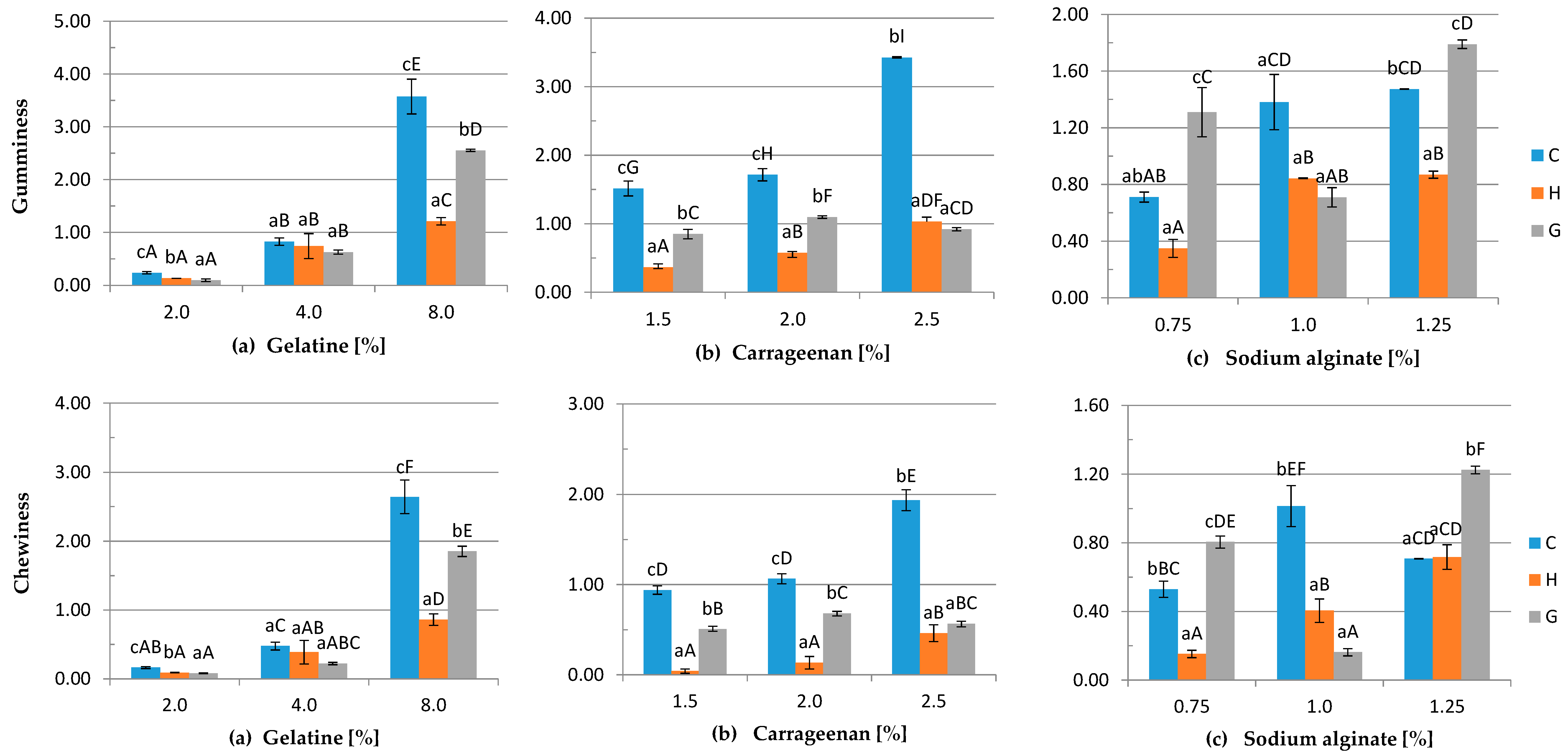
2.4.4. Texture Profiling Analysis
- hardness: maximum force required to compress the sample
- springiness: the distance the sample was compressed during the second compression to the peak force, divided by the initial sample height, reported as a percentage
- cohesiveness: the ratio of the area under the first and second compression
- gumminess: hardness × cohesiveness
- chewiness: gumminess × springiness
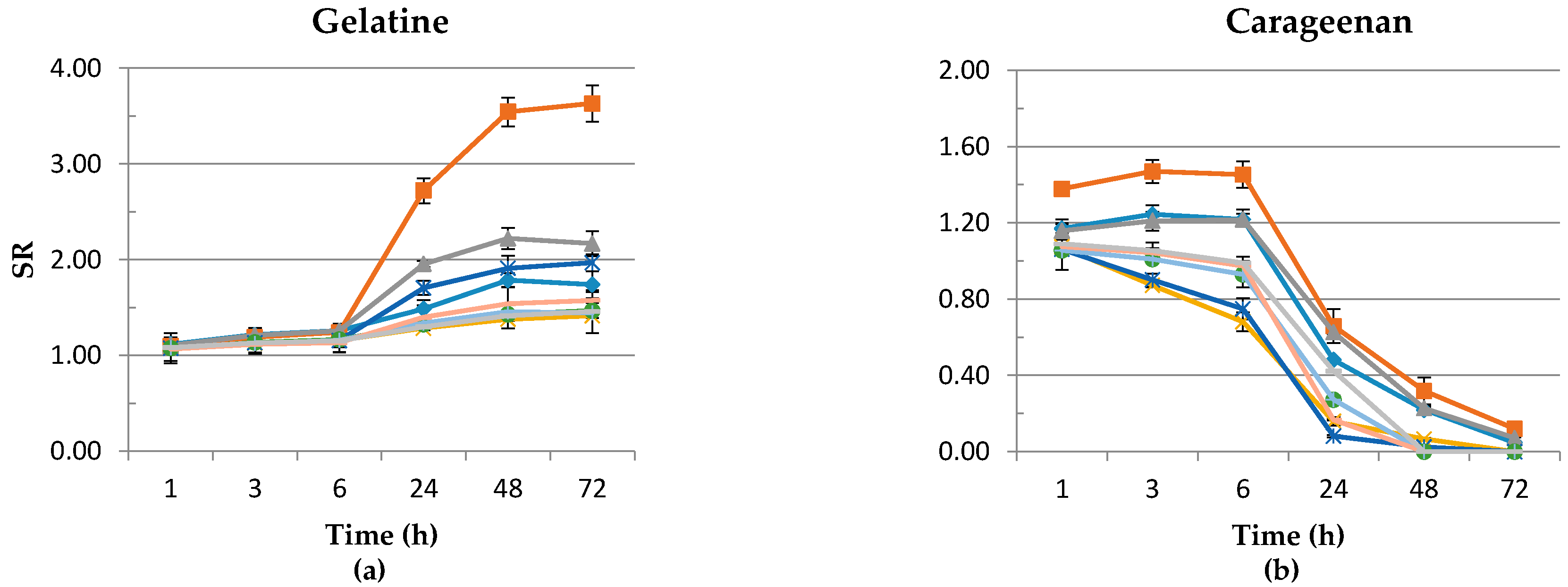
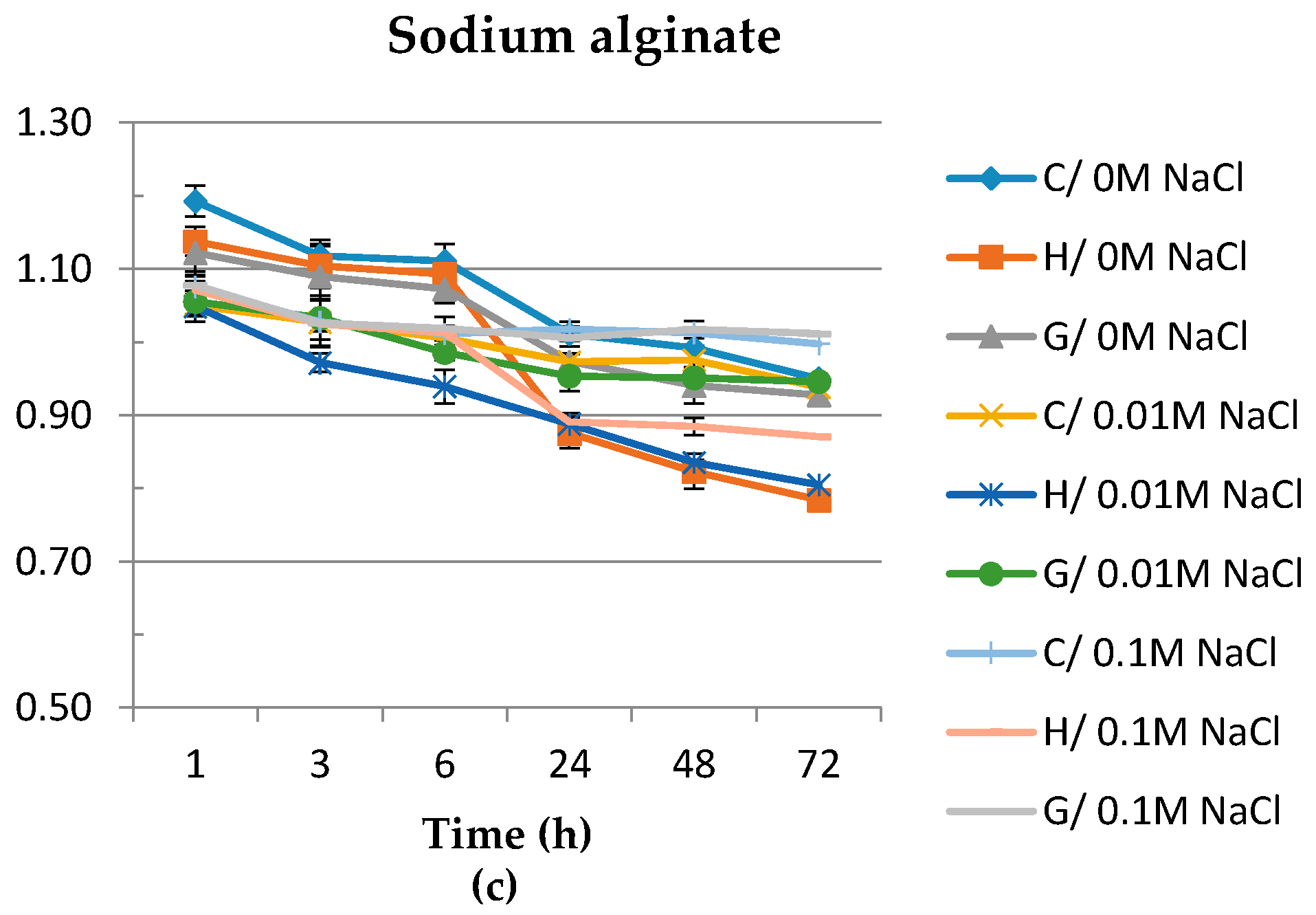
2.4.5. Swelling Ratio
2.4.6. Color Measurement
2.5. Statistical Analysis
3. Results and Discussion
3.1. pH Measurement
3.2. Fourier Transform Infrared Spectroscopy
3.3. Scanning Electron Microscopy
3.4. Mechanical Properties
3.5. Swelling Ratio
3.6. Color Measurement
4. Conclusion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bajpai, A.K.; Shukla, S.K.; Bhanu, S.; Kankane, S. Responsive polymers in controlled drug delivery. Prog. Polym. Sci. 2008, 33, 1088–1118. [Google Scholar] [CrossRef]
- Chen, S.; Shi, J.; Xu, X.; Ding, J.; Zhong, W.; Zhang, L.; Xing, M.; Zhang, L. Study of stiffness effects of poly(amidoamine)-poly(n-isopropyl acrylamide) hydrogen on wound healing. Coll. Surf. B Biointerfaces 2016, 140, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tian, Z.; Du, Y. Synthesis and pH sensitivity of carboxymethyl chitosan-based polyampholyte hydrogels for protein carrier matrices. Biomaterials 2004, 24, 3725–3732. [Google Scholar] [CrossRef] [PubMed]
- Vulpe, R.; Popa, M.; Picton, L.; Balan, V.; Dulong, V.; Butnaru, M.; Verestiuc, L. Crosslinked hydrogels based on biological marcomolecules with potential use in skin tissue engineering. J. Biol. Macromol. 2016, 84, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Gregorova, A.; Nabanita, S.; Kitano, T.; Saha, P. Hydrothermal effect and mechanical stress properties of carboxymethylcellulose based hydrogel food packaging. Carbohydr. Polym. 2015, 117, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, X.; Mu, C.; Zhang, H.; Qin, P.; Li, D. Freezing-thawing effects on properties of dialdehyde carboxymethyl cellulose crosslinked gelatin-MMT composite films. Food Hydrocoll. 2013, 33, 273–279. [Google Scholar] [CrossRef]
- Biswal, D.; Anupriya, B.; Uvanesh, K.; Anis, A.; Banerjee, I.; Pal, K. Effect of mechanical and electrical behaviour of gelatin hydrogels on drug release and cell proliferation. J. Mech. Behav. Biomed. Mater. 2016, 53, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Chi, W. Gelatin (Molecular Weight Distribution). In Polymer Materials Encyclopedia; Salamone, J.C., Ed.; CRC Press: Boca Raton, FL, USA, 1999; pp. 558–560. [Google Scholar]
- Pang, Z.; Deeth, H.; Sharma, R.; Bansal, N. Effect of addition of gelatin on the rheological and microstructural properties of acid milk protein gels. Food Hydrocoll. 2015, 43, 340–351. [Google Scholar] [CrossRef] [Green Version]
- Silva, R.S.G.; Bandeira, S.F.; Pinto, L.A.A. Characteristics and chemical composition of skins gelatin from cobia (Rachycentron conadum). LWT Food Sci. Technol. 2014, 57, 580–585. [Google Scholar] [CrossRef]
- Shyni, K.; Hema, G.S.; Ninan, G.; Mathew, S.; Joshy, C.G.; Lakshmanan, P.T. Isotalion and characterization of gelatin from the sins of skipjack tuna (Katsuwonus pelamis), dog shark (Scoliodon sorrakowah), and rohu (Labeo rohita). Food Hydrocoll. 2014, 39, 68–76. [Google Scholar] [CrossRef]
- Pang, Z.; Deeth, H.; Sopade, P.; Sharma, R.; Bansal, N. Rheology, texture and microstructure of gelatin gels with and without milk proteins. Food Hydrocoll. 2014, 35, 484–493. [Google Scholar] [CrossRef]
- Ooi, S.Y.; Ahmad, I.; Amin, M.C.I.M. Cellulose nanocrystals extracted from rice husks as a reinforcing material in gelatine hydrogels for use in controlled drug delivery systems. Ind. Crops Prod. 2016. In Press. [Google Scholar]
- Peng, L.H.; Chen, X.; Chen, L.; Li, N.; Liang, W.G.; Gao, J.Q. Topical astragaloside IV-releasing hydrogel improves healing of skin wounds in vivo. Biol. Pharm. Biull. 2012, 35, 881–888. [Google Scholar] [CrossRef]
- Kavoosi, G.; Dadfar, S.M.; Purfard, A.M. Mechanical, physical, antioxidant, and antimicrobial properties od gelatin films incorporated with thymol for potential use as nano wound dressing. J. Food Sci. 2013, 78, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ye, Y.; Li, D.; Li, X.; Mu, C. Biological properties of dialdehyde carbocymethyl celluloe crosslinked gelatin-PEG composite hydrogel fibres for wound dressings. Carbohydr. Polym. 2016, 137, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Benjakul, S.; Prodpran, T.; Agustini, T.W. Physico-mechanical and antimicrobial properties of gelatin film from the skin of unicorn leather jacket incorporated with essential oils. Food Hydrocoll. 2012, 28, 189–199. [Google Scholar] [CrossRef]
- Cardoso, G.P.; Dutra, M.P.; Fontes, P.R.; Ramos, A.L.S.; Gomide, L.A.M.; Ramos, E.M. Selection of chitosan gelatine-based edible coating for color preservation of beef in retail display. Meat Sci. 2016, 114, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Vet. Med. 2013, 58, 187–205. [Google Scholar]
- Prajapati, V.D.; Maheriya, P.M.; Jani, G.K.; Solanki, H.K. Carrageenan: A natural seaweed polysaccharide and its applications. Carbohydr. Polym. 2014, 105, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Stenner, R.; Matubayasi, N.; Shimizu, S. Gelation of carrageenan: Effects of sugars and polyols. Food Hydrocoll. 2016, 54, 284–292. [Google Scholar] [CrossRef]
- Tavassoli-Kafrani, E.; Shekarchzadeh, H.; Behabadi-Masoudpour, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Campelo, C.S.; Lima, L.D.; Rebelo, L.M.; Mantovani, D.; Beppu, M.M.; Vieira, R.S. In vitro evaluation of anti-calcification and anti-coagulation on sulfonated chitosan and carrageenan surfaces. Mater. Sci. Eng. C 2016, 59, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.L.D.; Moreira, J.; Neto, R.; Estrada, A.C.; Gil, A.M.; Trinidade, T. Impact of magnetic nanofillers in the swelling and release properties od k-carrageenan hydrogel nanocomposites. Carbohydr. Polym. 2012, 87, 328–335. [Google Scholar] [CrossRef]
- Du, L.; Brenner, T.; Xie, J.; Matsukawa, S. A study on phase separation behaviour in kappa/iota carrageenan mixtures by micro DSC, rheological measurements and simulating water and cations migration between phase. Food Hydrocoll. 2016, 81–88. [Google Scholar] [CrossRef]
- Draget, K.I.; Taylor, C. Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocoll. 2011, 25, 251–256. [Google Scholar] [CrossRef]
- Jabeen, S.; Chat, O.A.; Maswal, M.; Ashraf, U.; Rather, G.M.; Dar, A.A. Hydrogels of sodium alginate in cationic surfactants: Surfactant dependent modulation of encapsulation/release toward Ibuprofen. Carbohydr. Polym. 2015, 133, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Borba, P.A.A.; Pinotti, M.; Campos, C.E.M.; Pezzini, B.R.; Stulzer, H.K. Sodium alginate as a potential carrier in solid dispersion formulations to enhance dissolution rate and apparent water solubility of BCS II drugs. Carbohydr. Polym. 2016, 137, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Glikfeld, P.; Cullander, C.; Hinz, R.S.; Guy, R.H. A new system for in vitro studies of iontophoresis. Pharm. Res. 1988, 5, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Behl, C.R.; Kumar, S.; Malik, W.; DelTerzo, S.; Higushi, W.I.; Nash, R.A. Iontophoretic drug delivery: Effects of physico-chemical factors on the skin uptake of nanopeptide drugs. J. Pharm. Sci. 1989, 71, 355–360. [Google Scholar] [CrossRef]
- Ramanathan, S.; Block, L.H. The use of chitosan gels as matrices for electrically-modulated drug delivery. J. Controle. Release 2001, 70, 109–123. [Google Scholar] [CrossRef]
- Murdan, S. Electro-responsive drug delivery from hydrogels. J. Controle. Release 2003, 92, 1–17. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Rao, M.S.; Chawla, S.P.; Sharma, A. Effects of chitosan coating on shelf-life of ready-to-cook meat products during chilled storage. LWT Food Sci. Technol. 2013, 53, 321–326. [Google Scholar] [CrossRef]
- Sockett, P. Foodborne diseases: Prevalance of fodborne diseases in North America. Encycl. Food Saf. 2014, 1, 276–286. [Google Scholar]
- Król, Ż.; Jarmoluk, A. The effects of using a direct electric current on the chemical properties of gelatine gels and bacterial growth. J. Food Eng. 2015, 170, 1–7. [Google Scholar] [CrossRef]
- Rahman, S.M.E.; Park, J.H.; Wang, J.; Oh, D.H. Stability of low concentration electrolyzed water and its sanitization potential against foodborne pathogens. J. Food Eng. 2012, 113, 538–553. [Google Scholar] [CrossRef]
- Cao, W.; Zhu, Z.W.; Shi, Z.X.; Wang, Ch.Y.; Li, B.M. Efficiency of slightly acidic electrolyzed water for inactivation of Salmonella enteritisis and its contaminated shell eggs. Inter. J. Food Microbiol. 2009, 130, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Król, Ż.; Malik, M.; Marycz, K.; Jarmoluk, A. Characteristic of gelatine, carrageenan and sodium alginate treated with direct electric current. 2016. in preparation. [Google Scholar]
- Bourne, M.C. Food Texture and Viscosity: Concept and Measurement, 2nd ed.; Academic Press: San Diego, CA, USA, 2002; p. 15. [Google Scholar]
- Luo, Q.; Wang, H.; Zhang, X.; Qian, Y. Effect of direct electric current on the cell surface properties of phenol-degrading bacteria. Appl. Environ. Microbiol. 2005, 71, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.E.H. Drinking water disinfection by in-line electrolysis: Product and inorganic by-product formation. In Electrochemistry for the Environment; Comninellis, C., Chen, G., Eds.; Springer: New York, NY, USA, 2010; pp. 163–204. [Google Scholar]
- Hsu, S.Y. Effects of flow rate, temperature and salt concentration on chemical and physical properties of electrolyzed oxidizing water. J. Food Eng. 2005, 66, 171–176. [Google Scholar] [CrossRef]
- Kwon, I.C.; Bea, Y.H.; Okano, T.; Kim, S.W. Drug release from electric current sensitive polymers. J. Control. Release 1991, 17, 156–153. [Google Scholar] [CrossRef]
- Hoque, M.S.; Benjakul, S.; Prodpran, T. Effect of heat treatment of film-forming solution on the properties of film from cuttlefish (Sepia pharaonis) skin gelation. J. Food Eng. 2010, 96, 66–73. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Choubey, J. Design of gelatin nanoparticles as swelling controlled delivery system for chloroquine phosphate. J. Mater. Sci. Mater. Med. 2006, 17, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Bandekar, J. Amide modes and protein conformation. Biochim. Biophys. Acta 1992, 1120, 123–143. [Google Scholar] [CrossRef]
- Sen, M.; Erboz, E.N. Determination of critical gelation conditions of κ-carrageenan by viscosimetric and FT-IR analyses. Food Res. Int. 2010, 43, 1361–1364. [Google Scholar] [CrossRef]
- Prado, F.J.; Rodriguez, V.J.A.; Tojo, E.; Andrade, J.M. Quantitation of κ, l- and λ-carrageenans by mid-infrared spectroscopy and PLS regression. Anal. Chim. Acta 2003, 480, 23–37. [Google Scholar] [CrossRef]
- Leal, D.; Matsuhiro, B.; Rossi, M.; Caruso, F. FT-IR spectra of alginic acid block fractions in three species of brown seaweeds. Carbohydr. Res. 2008, 343, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Chandia, N.P.; Matsuhiro, B.; Vasquez, A.E. Alginic acids in Lessonia trabeculata: Characterization by formic acid hydrolysis and FT-IR spectroscopy. Carbohydr. Polym. 2001, 46, 81–87. [Google Scholar] [CrossRef]
- Bano, S.; Mohmood, A.; Kim, S.J.; Lee, K. Chlorine resistant binary complexed NaAlg/PVA composite membrane for nanofiltration. Sep. Purif. Technol. 2014, 137, 21–27. [Google Scholar] [CrossRef]
- Hsu, C.S.; Block, L.H. Anionic gels as vehicles for electrically-modulated drug delivery. I. solvent and drug transport phenomena. Pharm. Res. 1996, 13, 1865–1870. [Google Scholar] [CrossRef] [PubMed]
- Lau, M.H.; Tang, J.; Paulson, A.T. Texture profile and turbidity of gellan/gelatin mixed gels. Food Res. Inter. 2000, 33, 665–671. [Google Scholar] [CrossRef]
- Hambleton, A.; Perpinan-Saiz, N.; Fabra, M.J.; Voilley, A.; Debeaufort, F. The schroeder paradox or how the state of water affects the moisture transfer through edible films. Food Chem. 2012, 132, 1671–1678. [Google Scholar] [CrossRef]
- Haider, S.; Park, S.Y.; Saeed, K.; Farmer, B.L. Swelling and electroresponsive characteristic of gelatin immobilized onto multi-walled carbon nanotubes. Sens. Actuators B Chem. 2007, 124, 517–528. [Google Scholar] [CrossRef]
- Distantina, S.; Fahrurrozi, M. Hydrogels based on carrageenan extracted from Kappaphycus alvarezii. World Acad. Sci. Eng. Technol. 2013, 78, 987–990. [Google Scholar]
- Francis, S.; Kumar, M.; Varshney, L. Radiation synthesis of superabsorbent poly(acrylic acid)-carrageenan hydrogels. Radiat. Phys. Chem. 2004, 69, 481–486. [Google Scholar] [CrossRef]
- Jin, X.; Kang, H.; Huang, Y.; Liu, H.; Hu, Y. Synthesis and properties of electrically-sensitive poly(acrylic acid-co-acetoacetocxy ethyl methacrylate) Gels. J. Appl. Polym. Sci. 2008, 110, 3690–3696. [Google Scholar] [CrossRef]
- Hezaveh, H.; Muhamad, I.I. Modification and swelling kinetic study of kappa-carrageenan-based hydrogel for controlled release study. J. Taiwan Inst. Chem. E 2013, 44, 182–191. [Google Scholar] [CrossRef]
- Mirdarikvande, S.; Sadeghi, H.; Goderzi, A.; Alahyari, M.; Shasavari, H.; Khani, F. Effect of pH, and salinity onto swelling properties od hydrogels based on h-alginate-g-poly(AMPS). Biosci. Biotechnol. Res. Asia 2014, 11, 205–209. [Google Scholar] [CrossRef]
- Samanta, H.S.; Ray, S.K. Synthesis, characterization, swelling and drug release behaviour of semi-interpenetrating network hydrogels of sodium alginate and polyacrylamide. Carbohydr. Polym. 2014, 99, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T. Gels. Sci. Am. 1981, 244, 124–138. [Google Scholar] [CrossRef] [PubMed]


| Gelatine (%) | Carrageenan (%) | Sodium alginate (%) | NaCl (%) |
|---|---|---|---|
| 2.0 | 1.5 | 0.75 | 0.0 |
| 0.1 | |||
| 0.2 | |||
| 4.0 | 2.0 | 1.0 | 0.0 |
| 0.1 | |||
| 0.2 | |||
| 8.0 | 2.5 | 1.25 | 0.0 |
| 0.1 | |||
| 0.2 |
| Run code letter | Type of gels |
|---|---|
| C | control |
| H | gels on the basis of hydrosols treated with DC |
| G | gels treated with DC |
| Polymer | Concentration of polymer (%) | Type of gel | L * | a * | b * | ΔE*ab |
|---|---|---|---|---|---|---|
| Gelatine | 2 | C | 50.66 ± 0.34 b,c | 0.00 ± 0.00 a,b | 2.68 ± 0.21 a | 0.00 ± 0.00 a |
| H | 52.45 ± 0.87 c,d,e | 0.03 ± 0.01 b | 2.33 ± 0.17 a | 3.88 ± 0.32 b | ||
| G | 51.38 ± 0.52 c,d | −0.06 ± 0.02 a | 2.41 ± 0.04 a | 3.23 ± 0.52 b | ||
| 4 | C | 48.15 ± 0.21 a,b | −0.07 ± 0.02 a | 2.86 ± 0.16 a,b | 0.00 ± 0.00 a | |
| H | 54.89 ± 0.31 e | −0.29 ± 0.04 c | 2.77 ± 0.18 a,b | 6.75 ± 0.45 c | ||
| G | 52.81 ± 0.89 c,d,e | −0.02 ± 0.01 a,b | 3.25 ± 0.25 b | 2.55 ± 0.15 b | ||
| 8 | C | 46.71 ± 0.14 a | −0.43 ± 0.01 d | 3.82 ± 0.14 c | 0.00 ± 0.00 a | |
| H | 53.61 ± 0.57 d,e | −0.26 ± 0.05 c | 4.05 ± 0.13 c | 6.91 ± 1.12 c | ||
| G | 48.61 ± 0.97 a,b | −0.50 ± 0.09 d | 4.67 ± 0.25 d | 3.73 ± 0.71 b | ||
| Carrageenan | 1.5 | C | 47.76 ± 1.12 a,b | 0.10 ± 0.07 a,b,c | 3.20 ± 0.16 e | 0.00 ± 0.00 a |
| H | 55.07 ± 0.65 d | 0.08 ± 0.01 a,b | 1.95 ± 0.18 a | 7.42 ± 1.24 d | ||
| G | 52.09 ± 0.32 c | 0.04 ± 0.02 a | 2.44 ± 0.22 b | 4.59 ± 0.60 c | ||
| 2.0 | C | 48.31 ± 0.45 a,c | 0.12 ± 0.03 a,b,c | 3.71 ± 0.15 c | 0.00 ± 0.00 a | |
| H | 50.16 ± 0.87 b,c | 0.11 ± 0.03 a,b,c | 2.49 ± 0.21 b | 2.57 ± 0.35 b | ||
| G | 49.97 ± 0.45 b,c | 0.11 ± 0.02 a,b,c | 3.77 ± 0.17 b | 1.95 ± 0.18 b | ||
| 2.5 | C | 46.80 ± 0.63 a | 0.17 ± 0.05 b,c | 4.24 ± 0.13 c | 0.00 ± 0.00 a | |
| H | 51.07 ± 0.31 c | 0.19 ± 0.04 d | 2.61 ± 0.06 b | 4.88 ± 0.57 c | ||
| G | 48.02 ± 0.65 a,b | 0.12 ± 0.02 a,b,c | 3.83 ± 0.13 c,d | 1.83 ± 0.32 b | ||
| Sodium alginate | 0.75 | C | 47.08 ± 0.97 b,c | 0.20 ± 0.08 a,b | 2.90 ± 0.25 b,c,d | 0.00 ± 0.00 a |
| H | 50.29 ± 1.36 d | 0.18 ± 0.06 a,b | 3.50 ± 0.16 d,e | 3.29 ± 1.01 c | ||
| G | 48.43 ± 0.82 c,d | 0.20 ± 0.02 a,b | 2.46 ± 0.09 a,b,c | 1.42 ± 0.57 b | ||
| 1.0 | C | 44.73 ± 0.38 a,b | 0.32 ± 0.05 c,d | 2.33 ± 0.21 a,b | 0.00 ± 0.00 a | |
| H | 46.00 ± 0.46 a,b,c | 0.27 ± 0.03 b,c | 3.22 ± 0.19 c,d,e | 3.54 ± 0.16 c,d | ||
| G | 46.74 ± 0.73 a,b,c | 0.26 ± 0.06 b,c | 2.11 ± 0.15 a,b | 3.94 ± 0.19 d | ||
| 1.25 | C | 43.85 ± 0.32 a | 0.18 ± 0.01 a,b | 2.90 ± 0.31 b,c,d | 0.00 ± 0.00 a | |
| H | 46.10 ± 0.58 a,b,c | 0.15 ± 0.02 a | 3.81 ± 0.07 e | 2.90 ± 0.14 c | ||
| G | 45.38 ± 0.61 a,b,c | 0.40 ± 0.12 d | 1.93 ± 0.12 a | 3.66 ± 0.09 c,d |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Król, Ż.; Malik, M.; Marycz, K.; Jarmoluk, A. Physicochemical Properties of Biopolymer Hydrogels Treated by Direct Electric Current. Polymers 2016, 8, 248. https://doi.org/10.3390/polym8070248
Król Ż, Malik M, Marycz K, Jarmoluk A. Physicochemical Properties of Biopolymer Hydrogels Treated by Direct Electric Current. Polymers. 2016; 8(7):248. https://doi.org/10.3390/polym8070248
Chicago/Turabian StyleKról, Żaneta, Magdalena Malik, Krzysztof Marycz, and Andrzej Jarmoluk. 2016. "Physicochemical Properties of Biopolymer Hydrogels Treated by Direct Electric Current" Polymers 8, no. 7: 248. https://doi.org/10.3390/polym8070248