The Effect of Lithium Iodide to the Properties of Carboxymethyl κ-Carrageenan/Carboxymethyl Cellulose Polymer Electrolyte and Dye-Sensitized Solar Cell Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Electrolytes
2.3. Preparation and Characterization of Solid-State DSSC
3. Results
3.1. FTIR Study
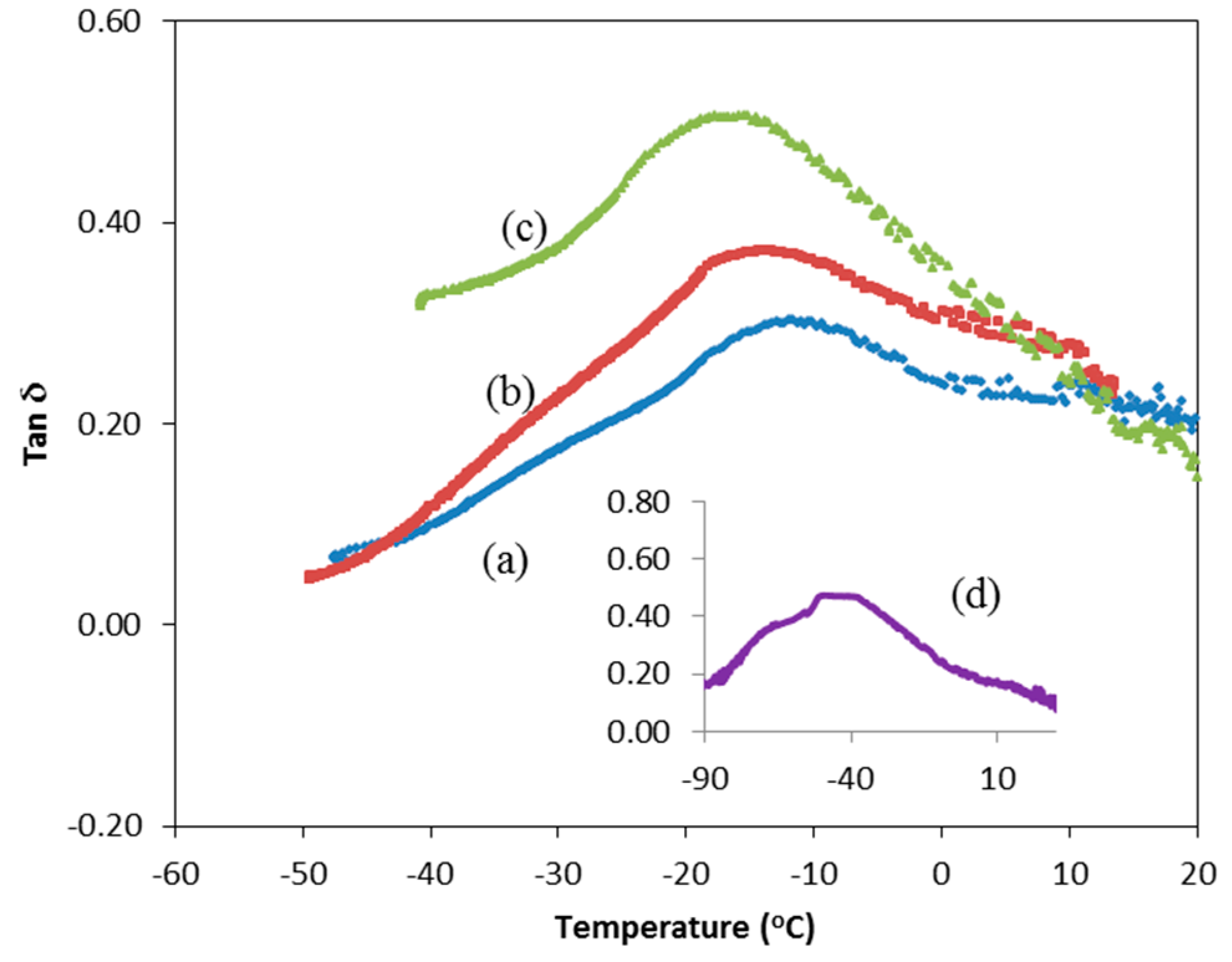
3.2. DMA Study
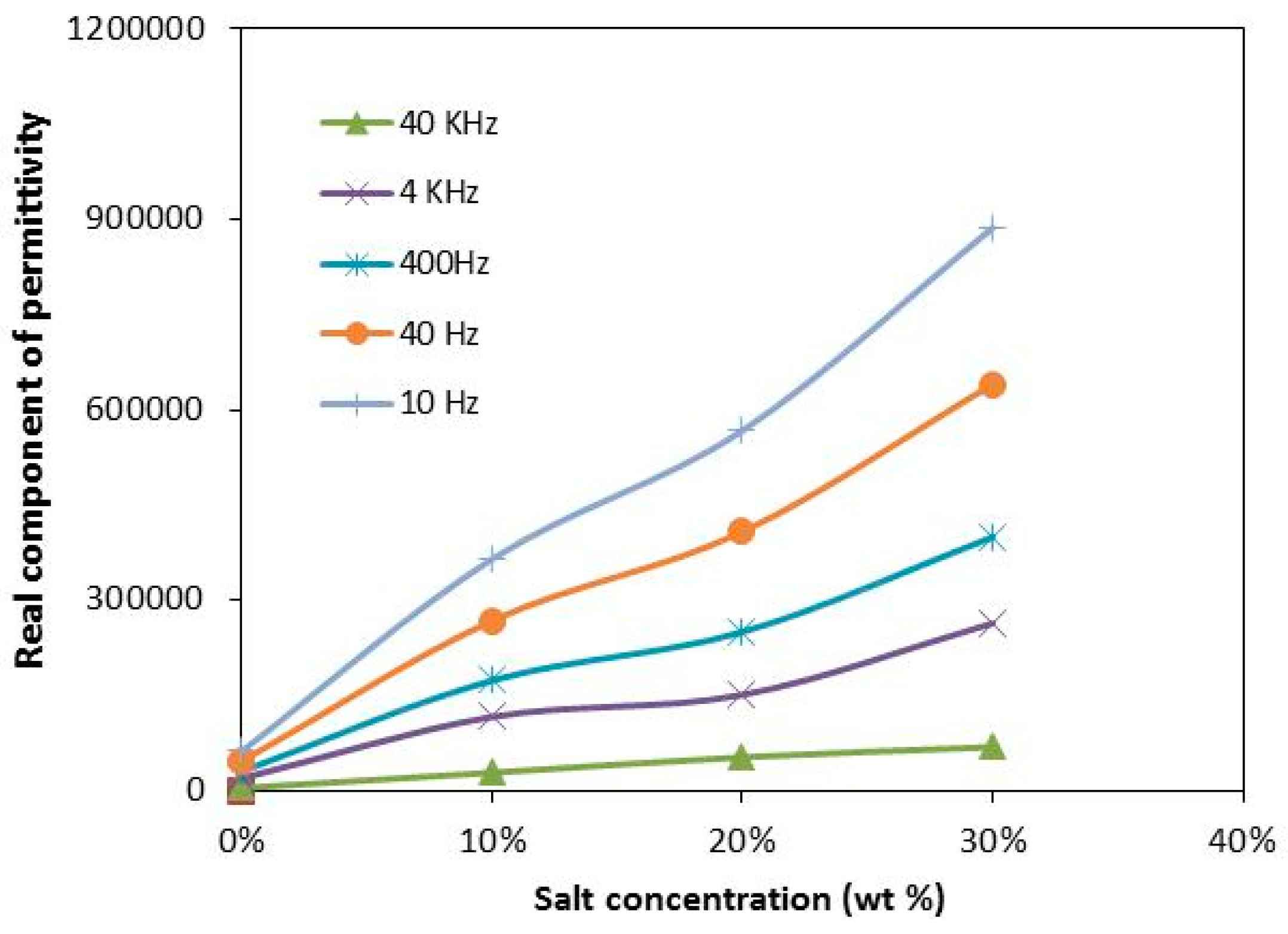
3.3. Room Temperature Conductivity Study
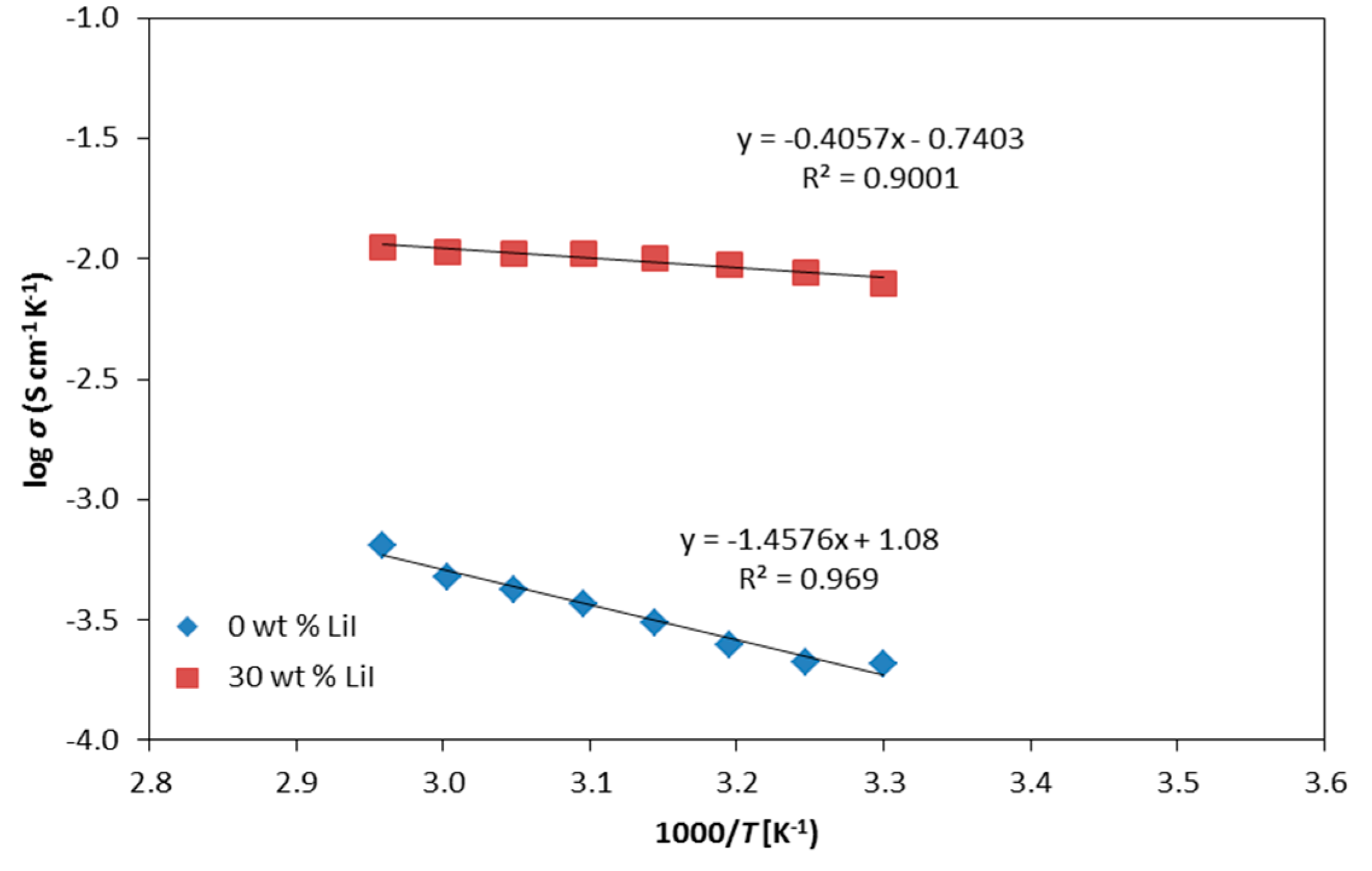
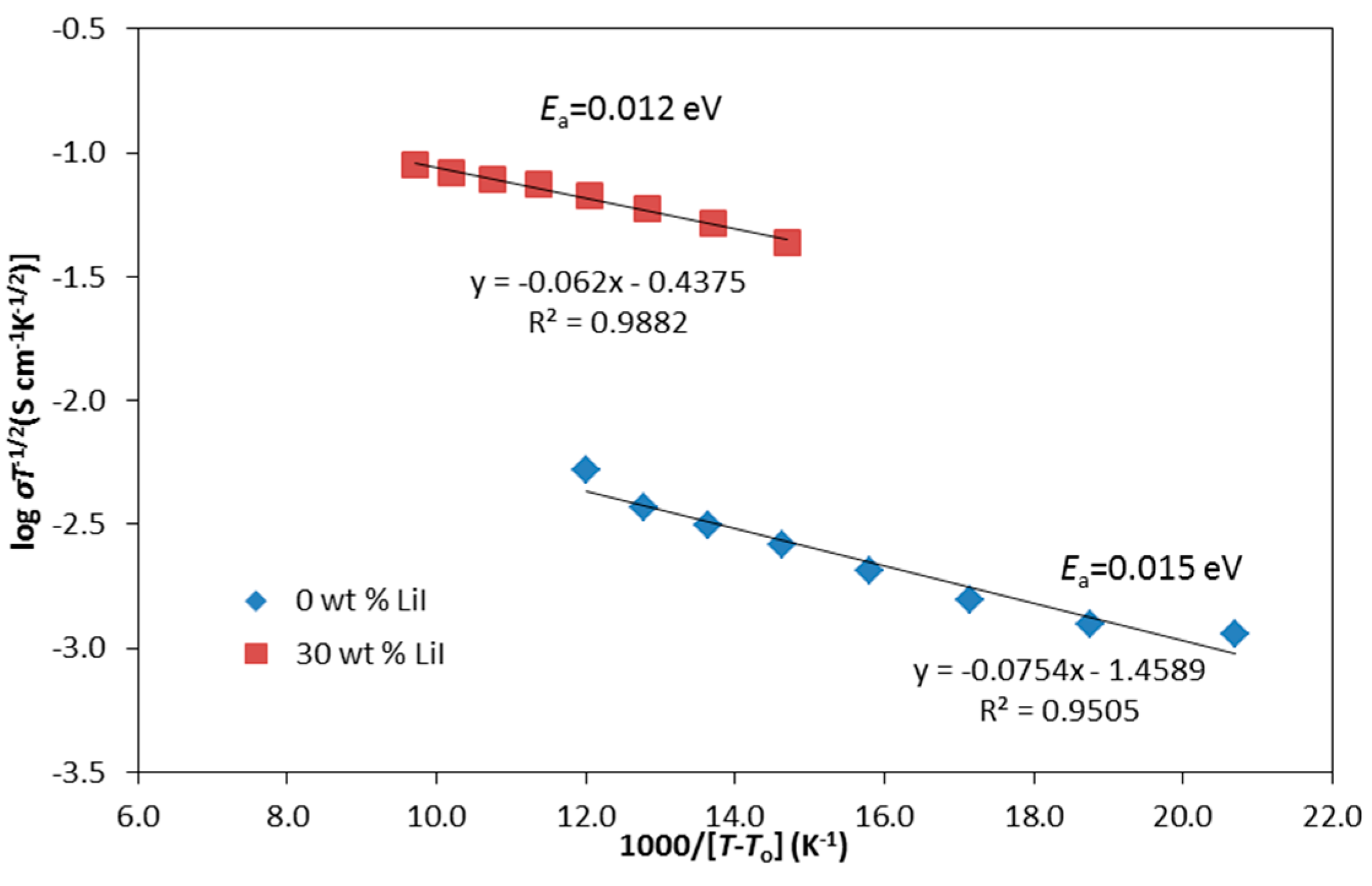
3.4. Conductivity at Various Temperatures
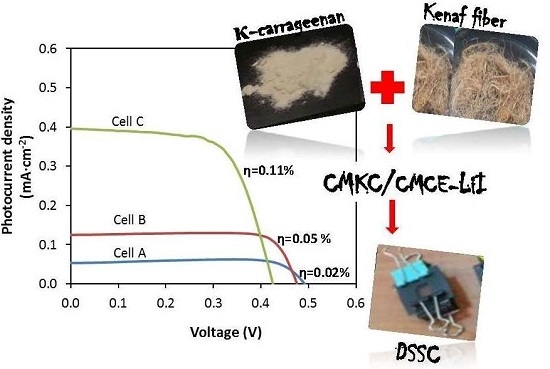
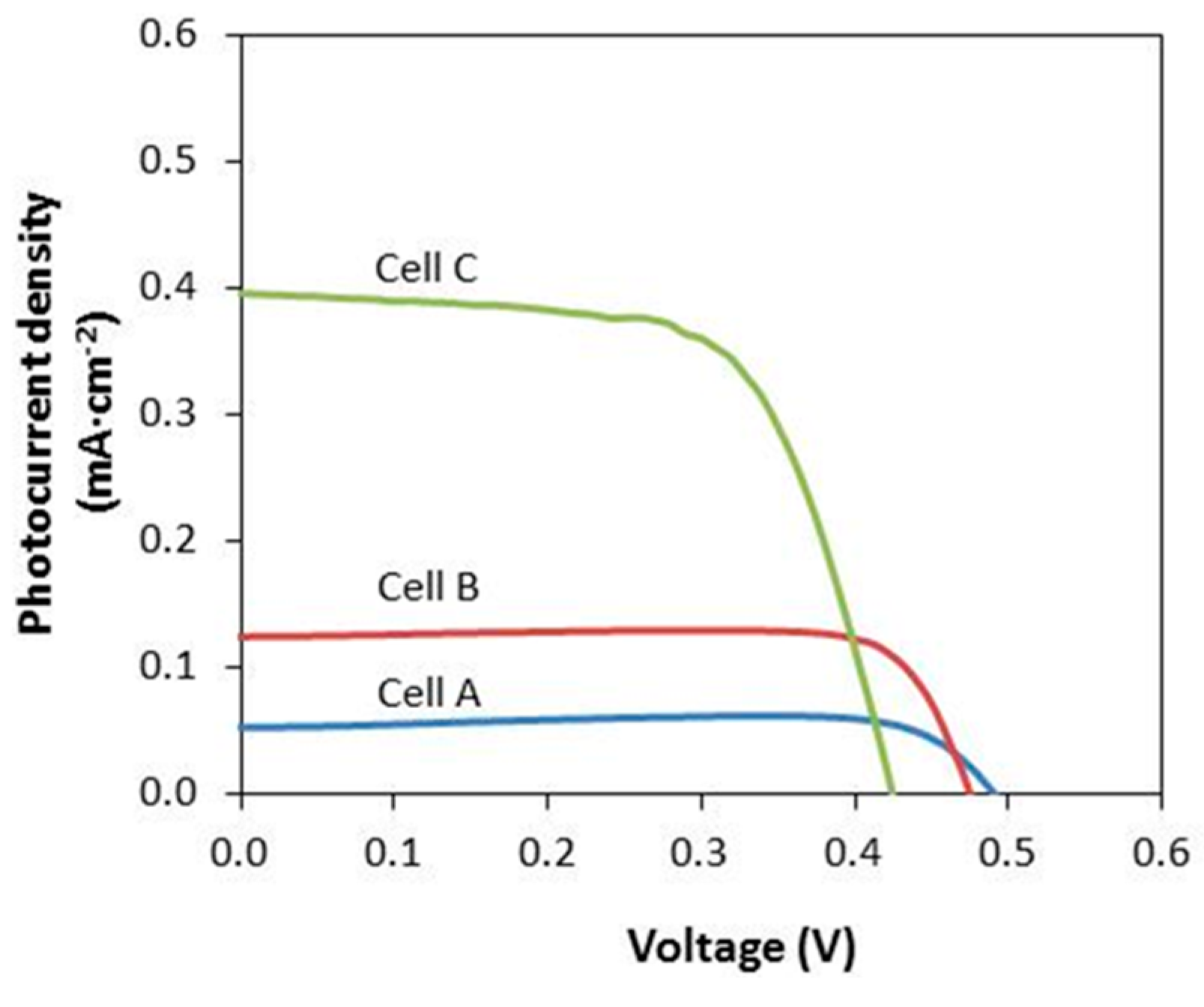
3.5. J–V Performance
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, J.; Peng, T.; Shi, W.; Li, R.; Xia, J. An efficient binary ionic liquid based quasi solid-state electrolyte for dye-sensitized solar cells. Electrochim. Acta 2013, 107, 231–237. [Google Scholar] [CrossRef]
- Grätzel, M. Conversion of sunlight to electric power by nanocrystalline dye-sensitized solar cells. J. Photochem. Photobiol. A 2004, 164, 3–14. [Google Scholar] [CrossRef]
- Nogueira, A.F.; Durrant, J.R.; de Paoli, M.A. Dye-sensitized nanocrystalline solar cells employing a polymer electrolyte. Adv. Mater. 2001, 13, 826–830. [Google Scholar] [CrossRef]
- Lee, J.Y.; Bhattacharya, B.; Kim, D.-W.; Park, J.-K. Poly(ethylene oxide)/poly(dimethylsiloxane) blend solid polymer electrolyte and its dye-sensitized solar cell applications. J. Phys. Chem. C 2008, 112, 12576–12582. [Google Scholar] [CrossRef]
- Roh, D.K.; Park, J.T.; Ahn, S.H.; Ahn, H.; Ryu, D.Y.; Kim, J.H. Amphiphilic poly(vinyl chloride)-g-poly(oxyethylene methacrylate) graft polymer electrolytes: Interactions, nanostructures and applications to dye-sensitized solar cells. Electrochim. Acta 2010, 55, 4976–4981. [Google Scholar] [CrossRef]
- Singh, M.; Singh, V.K.; Surana, K.; Bhattacharya, B.; Singh, P.K.; Rhee, H.-W. New polymer electrolyte for electrochemical application. J. Ind. Eng. Chem. 2013, 19, 819–822. [Google Scholar] [CrossRef]
- Su’ait, M.; Ahmad, A.; Badri, K.; Mohamed, N.S.; Rahman, M.; Ricardo, C.A.; Scardi, P. The potential of polyurethane bio-based solid polymer electrolyte for photoelectrochemical cell application. Int. J. Hydrog. Energy 2014, 39, 3005–3017. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, H.; Zhou, C.-H.; Xu, S.; Sebo, B.; Zhao, X.-Z. Novel agarose polymer electrolyte for quasi-solid state dye-sensitized solar cell. J. Power Sources 2011, 196, 2410–2415. [Google Scholar] [CrossRef]
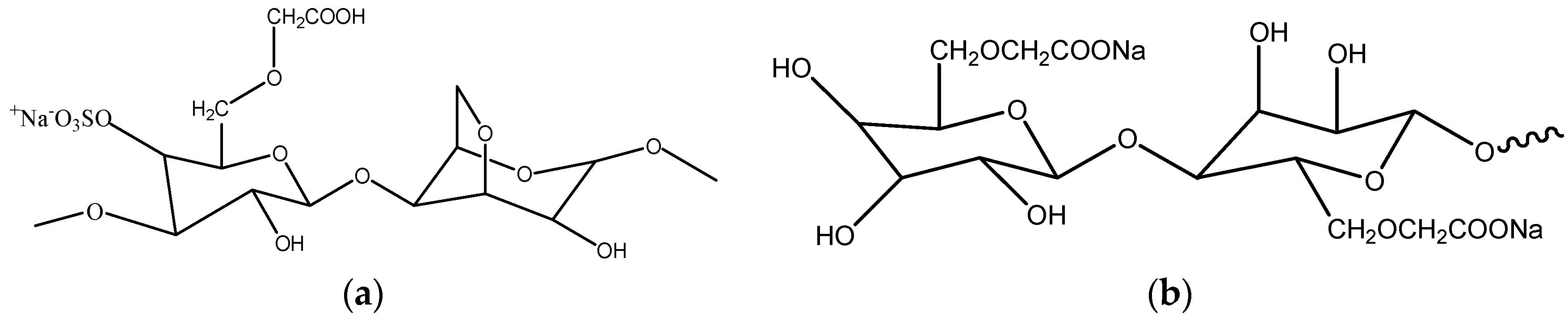
- Rudhziah, S.; Rani, M.S.A.; Ahmad, A.; Mohamed, N.S.; Kaddami, M. Potential of blend of κ-carrageenan and cellulose derivatives for green polymer electrolyte application. Ind. Crops Prod. 2015, 72, 133–141. [Google Scholar] [CrossRef]
- Rudhziah, S.; Ahmad, I.; Ahmad, A.; Mohamed, N.S. Biopolymer electrolytes based on blend of κ-carrageenan and cellulose derivatives for potential application in dye sensitized solar cell. Electrochimica Acta 2015, 175, 162–168. [Google Scholar] [CrossRef]
- Krossing, I.; Slattery, J.M.; Daguenet, C.; Dyson, P.J.; Oleinikova, A.; Weingärtner, H. Why are ionic liquids liquid? A simple explanation based on lattice and solvation energies. J. Am. Chem. Soc. 2006, 128, 13427–13434. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.-Z.; Mindemark, J.; Edström, K.; Brandell, D. Preparation of H-oleoyl-carboxymethyl-chitosan and the function as a coagulation agent for residual oil in aqueous system. Front. Mater. Sci. China 2008, 2, 105–112. [Google Scholar] [CrossRef]
- Fan, L.; Wang, L.; Gao, S.; Wu, P.; Li, M.; Xie, W.; Wang, W. Synthesis, characterization and properties of carboxymethyl -carrageenan. Carbohydr. Polym. 2011, 86, 1167–1174. [Google Scholar] [CrossRef]
- Le-Tien, C.; Millette, M.; Mateescu1, M.-A.; Lacroix, M. Modified alginate and chitosan for lactic acid bacteria immobilization. Biotechnol. Appl. Biochem. 2004, 39, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Tranquilan-Aranilla, C.; Nagasawa, N.; Bayquen, A.; Rosa, A.D. Synthesis and characterization of carboxymethyl derivatives of κ-carrageenan. Carbohydr. Polym. 2012, 87, 1810–1816. [Google Scholar] [CrossRef]
- Ali, A.; Subban, R.; Bahron, H.; Winie, T.; Latif, F.; Yahya, M. Grafted natural rubber-based polymer electrolytes: ATR-FTIR and conductivity studies. Ionics 2008, 14, 491–500. [Google Scholar] [CrossRef]
- Hema, M.; Selvasekerapandian, S.; Sakunthala, A.; Arunkumar, D.; Nithya, H. Structural, vibrational and electrical characterization of PVA–NH4Br polymer electrolyte system. Phys. B Condens. Matter 2008, 403, 2740–2747. [Google Scholar] [CrossRef]
- Rajendran, S.; Prabhu, M.R.; Rani, M.U. Characterization of PVC/PEMA based polymer blend electrolytes. Int. J. Electrochem. Sci. 2008, 3, 282–290. [Google Scholar]
- Kim, D.-W.; Min, B.R.; Won, J.; Kang, Y.S. Photovoltaic performance of dye-sensitized solar cell assembled with gel polymer electrolyte. J. Power Sources 2005, 149, 112–116. [Google Scholar] [CrossRef]
- Bella, F.; Sacco, A.; Pugliese, D.; Laurenti, M.; Bianco, S. Additives and salts for dye-sensitized solar cells electrolytes: What is the best choice? J. Power Sources 2014, 264, 333–343. [Google Scholar] [CrossRef]
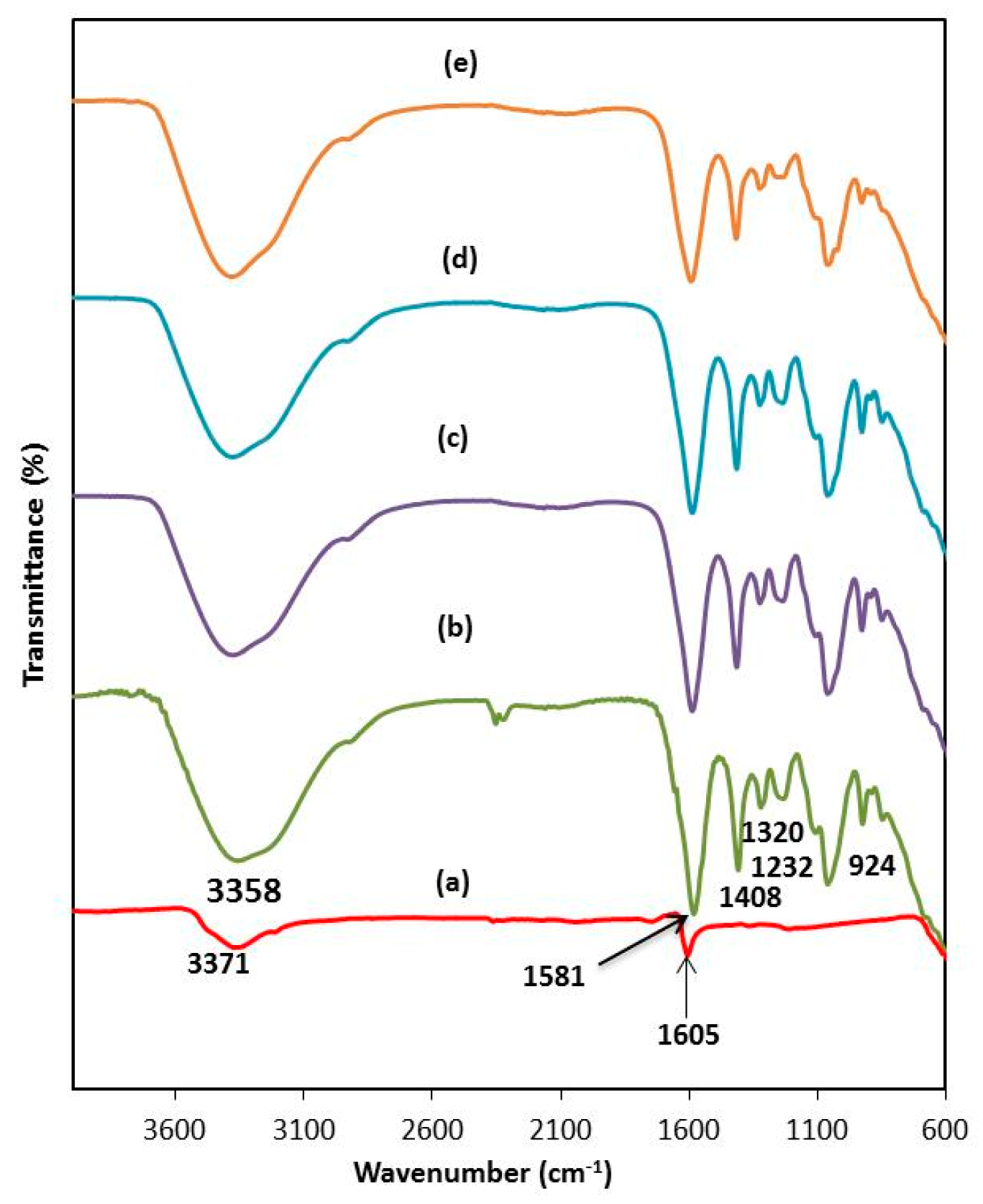
| Band assignment | Wavenumber (cm−1) | |||
|---|---|---|---|---|
| 0 wt % LiI | 10 wt % LiI | 20 wt % LiI | 30 wt % LiI | |
| OH stretching | 3,358 | 3,377 | 3,378 | 3,379 |
| COO− asymmetrical of carboxylate anion | 1,581 | 1,587 | 1,589 | 1,591 |
| COO– symmetric stretching | 1,408 | 1,414 | 1,415 | 1,416 |
| −CH2 scissoring | 1,320 | 1,324 | 1,324 | 1,324 |
| O=S=O symmetric vibration | 1,232 | 1,242 | 1,233 | 1,235 |
| C−O−C stretching | 924 | 927 | 927 | 927 |
| Concentration of LiI (wt %) | Glass transition temperature, Tg (°C) |
|---|---|
| 0 | −13.5 |
| 10 | −14.5 |
| 20 | −16.7 |
| 30 | −43.0 |
| CMKC/CMCE:Salt | Conductivity, (σ ± Δσ) (S·cm−1) |
|---|---|
| 100:0 | (3.25 ± 0.25) × 10−4 |
| 90:10 | (1.66 ± 0.16) × 10−3 |
| 80:20 | (2.73 ± 0.09 ) × 10−3 |
| 70:30 | (3.89 ± 0.27) × 10−3 |
| CMKC/CMCE:LiI (wt %) | I2 (M) | Voc (V) | Jsc (mA·cm−2) | ff | η% |
|---|---|---|---|---|---|
| 10 (Cell A) | 0.01 | 0.498 | 0.05 | 0.90 | 0.02 |
| 20 (Cell B) | 0.02 | 0.492 | 0.12 | 0.80 | 0.05 |
| 30 (Cell C) | 0.03 | 0.492 | 0.40 | 0.57 | 0.11 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che Balian, S.R.; Ahmad, A.; Mohamed, N.S. The Effect of Lithium Iodide to the Properties of Carboxymethyl κ-Carrageenan/Carboxymethyl Cellulose Polymer Electrolyte and Dye-Sensitized Solar Cell Performance. Polymers 2016, 8, 163. https://doi.org/10.3390/polym8050163
Che Balian SR, Ahmad A, Mohamed NS. The Effect of Lithium Iodide to the Properties of Carboxymethyl κ-Carrageenan/Carboxymethyl Cellulose Polymer Electrolyte and Dye-Sensitized Solar Cell Performance. Polymers. 2016; 8(5):163. https://doi.org/10.3390/polym8050163
Chicago/Turabian StyleChe Balian, Siti Rudhziah, Azizan Ahmad, and Nor Sabirin Mohamed. 2016. "The Effect of Lithium Iodide to the Properties of Carboxymethyl κ-Carrageenan/Carboxymethyl Cellulose Polymer Electrolyte and Dye-Sensitized Solar Cell Performance" Polymers 8, no. 5: 163. https://doi.org/10.3390/polym8050163