Polymer Inclusion Membranes (PIM) for the Recovery of Potassium in the Presence of Competitive Cations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Membrane Preparation
2.3. Membrane Characterization
2.3.1. Transport Experiments
2.3.2. Analyses
3. Results and Discussion
3.1. Effect of CTA and 2-NPOE on K+ Transport
3.1.1. Effect of CTA
3.1.2. Effect of CTA and 2-NPOE
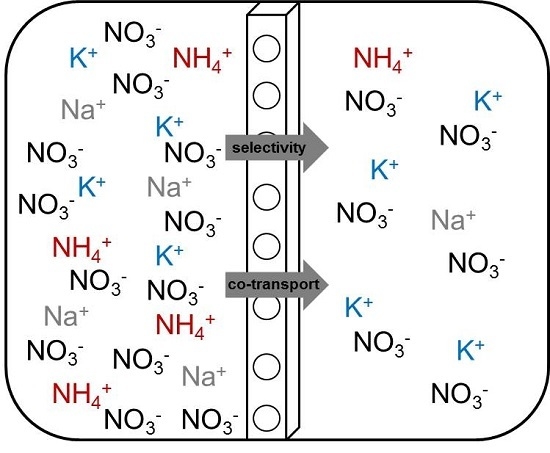
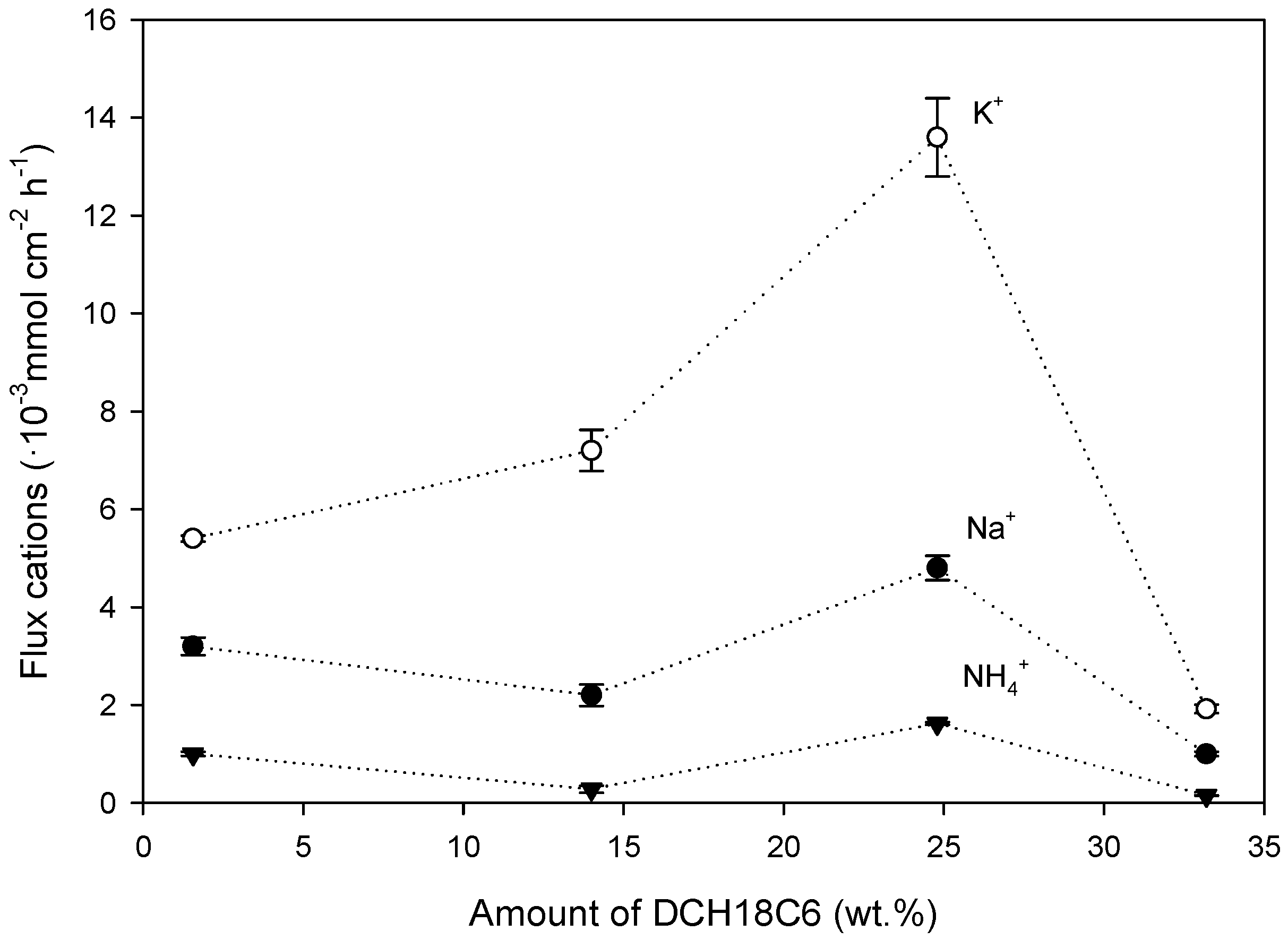
3.2. Effect of the PIM Composition on K+, Na+ and NH4+ Flux and Selectivity
Effect of DCH18C6
- Effect on the Cation Flux
- Effect on selectivity
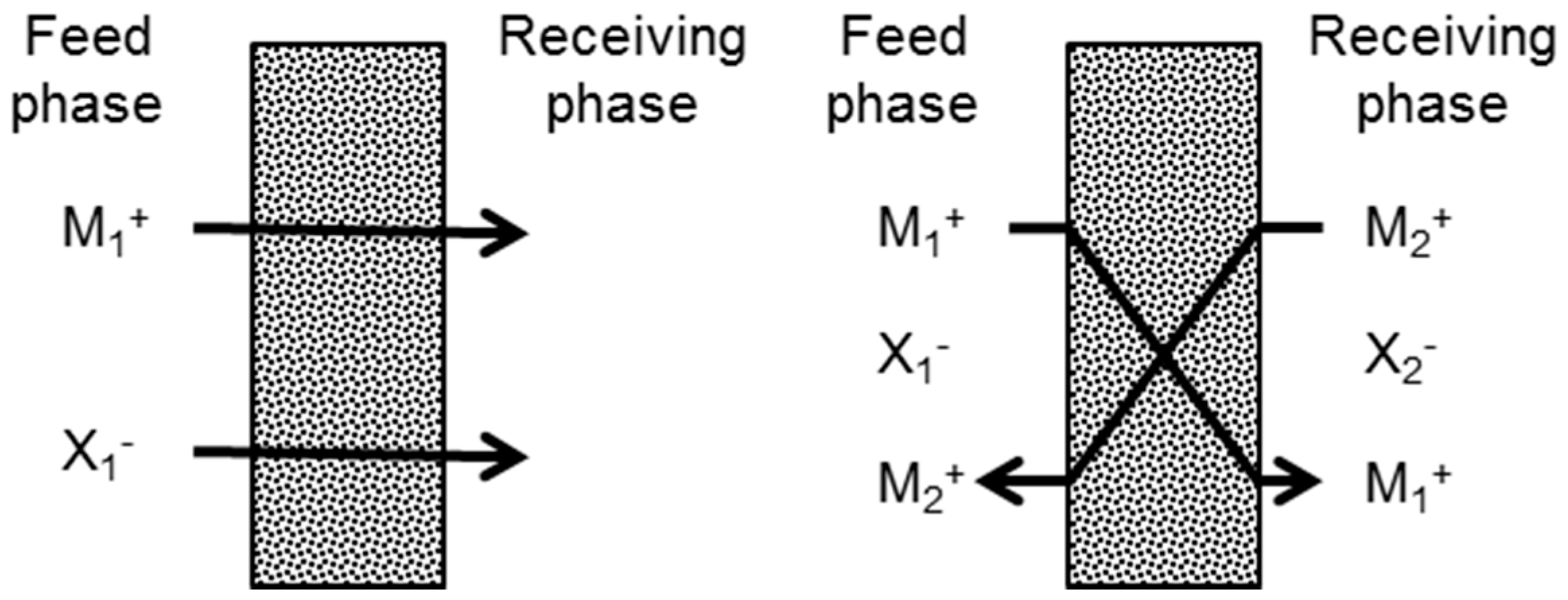
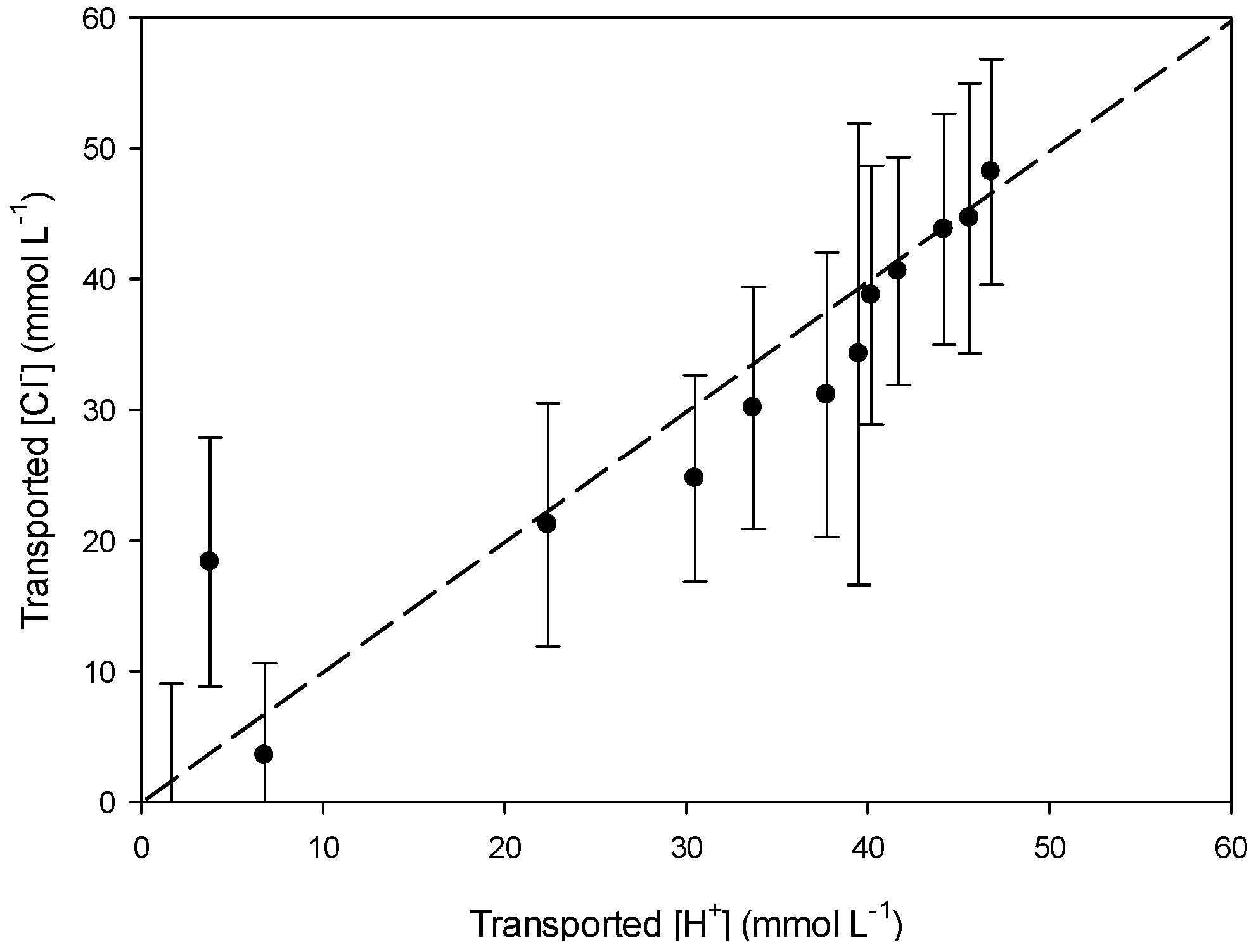
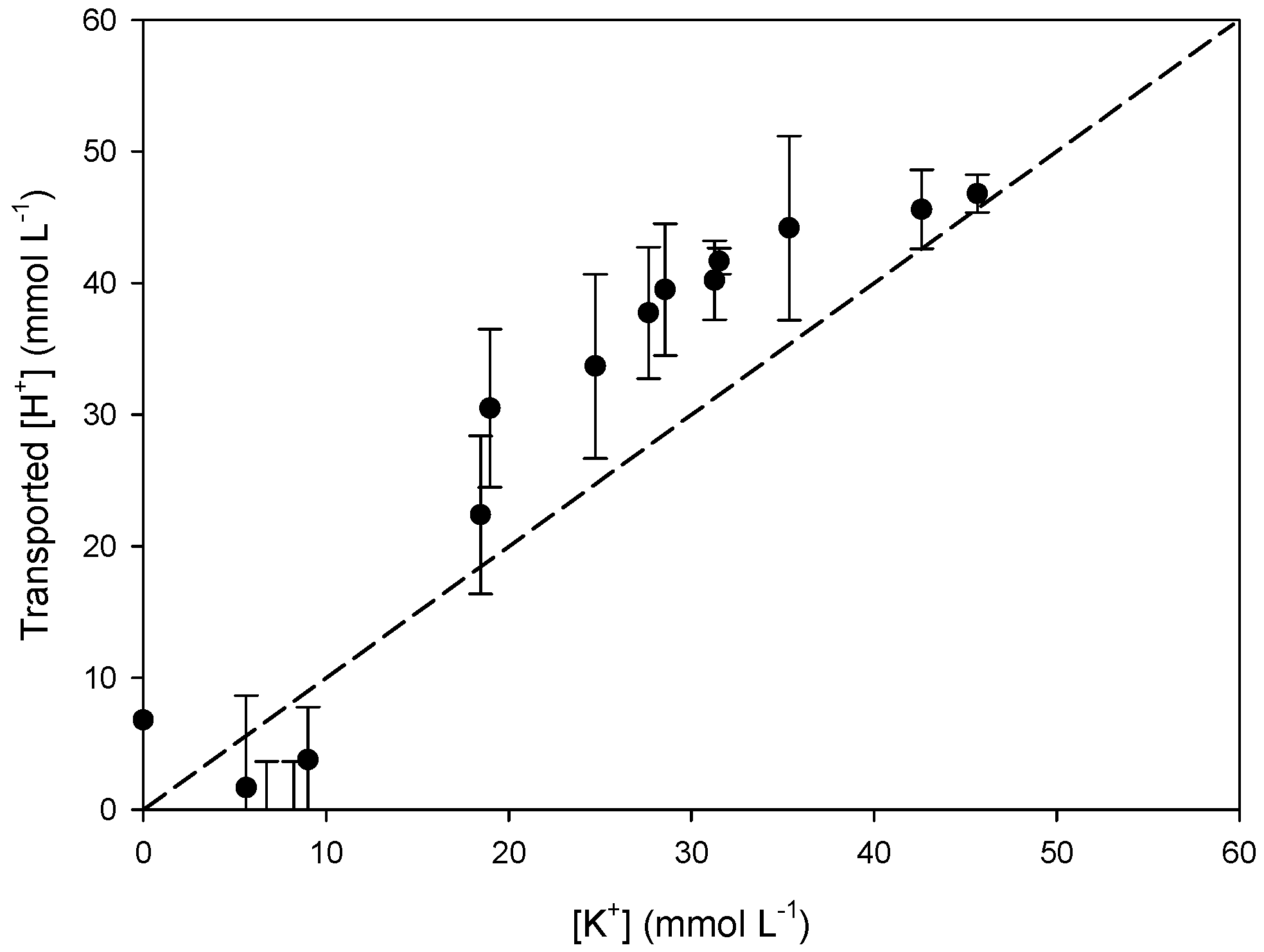
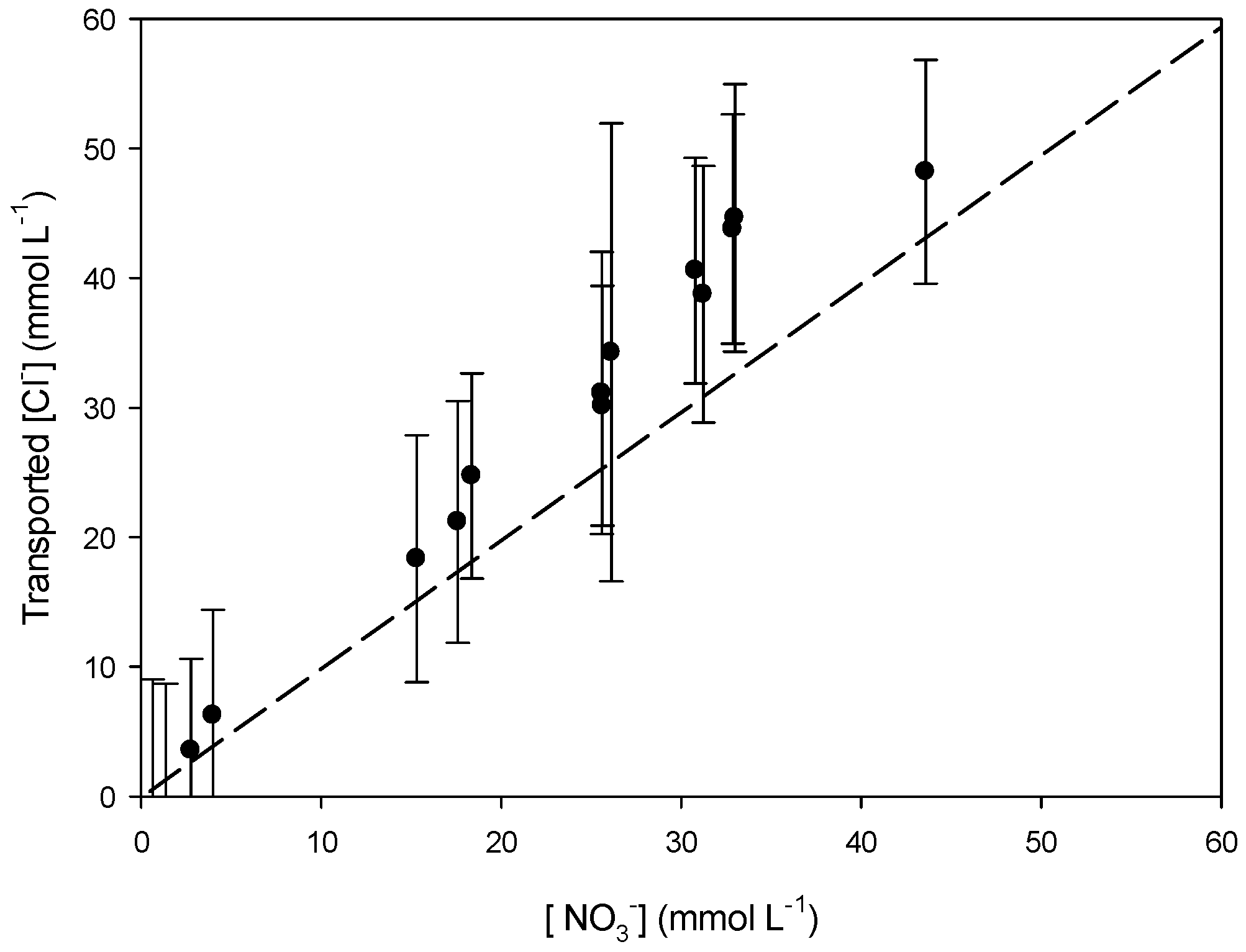
3.3. Transport Mechanism
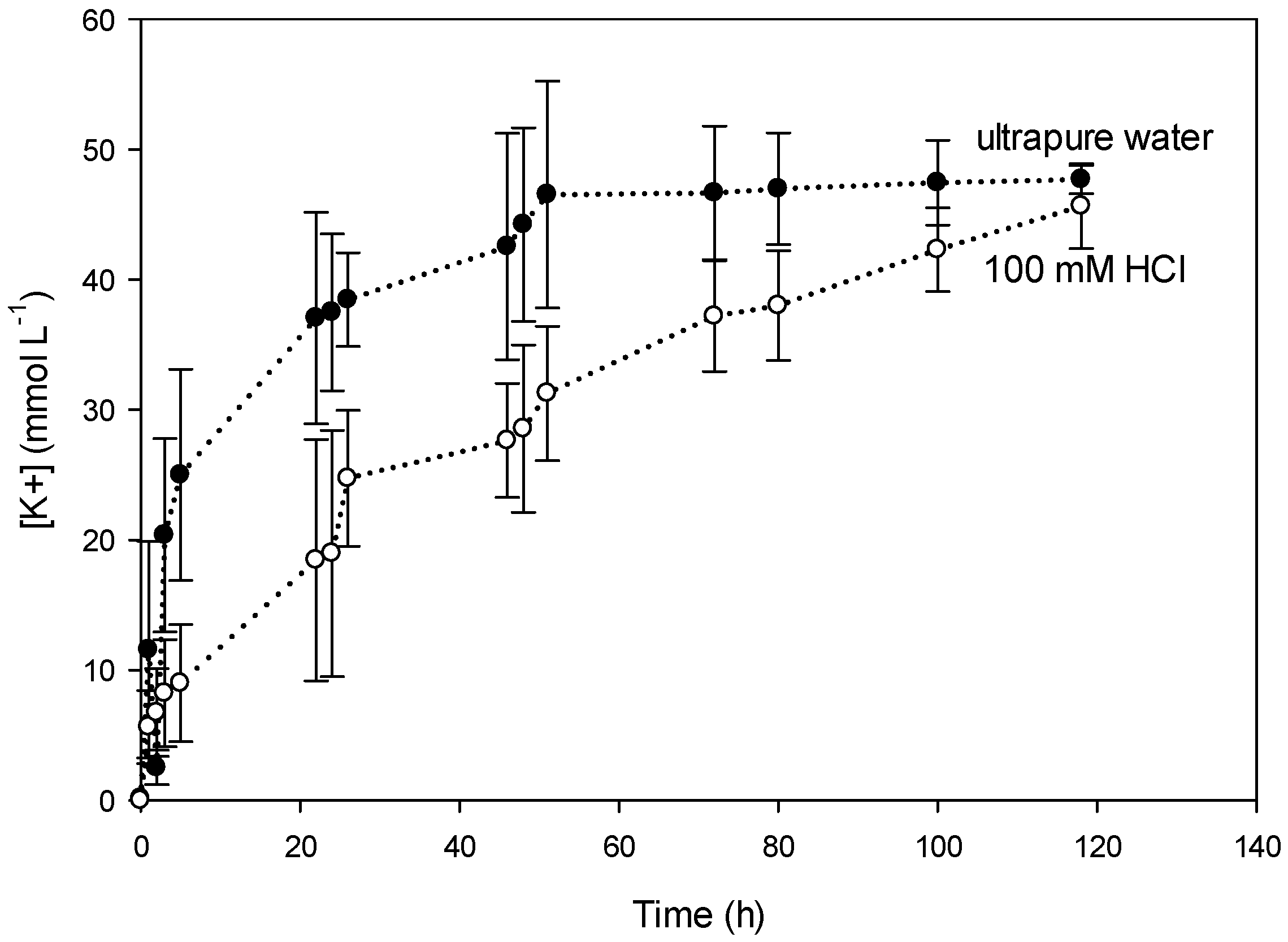
3.3.1. Effect of the Receiving Phase on the Transport Mechanism
3.3.2. Effect of the Receiving Phase on Selectivity
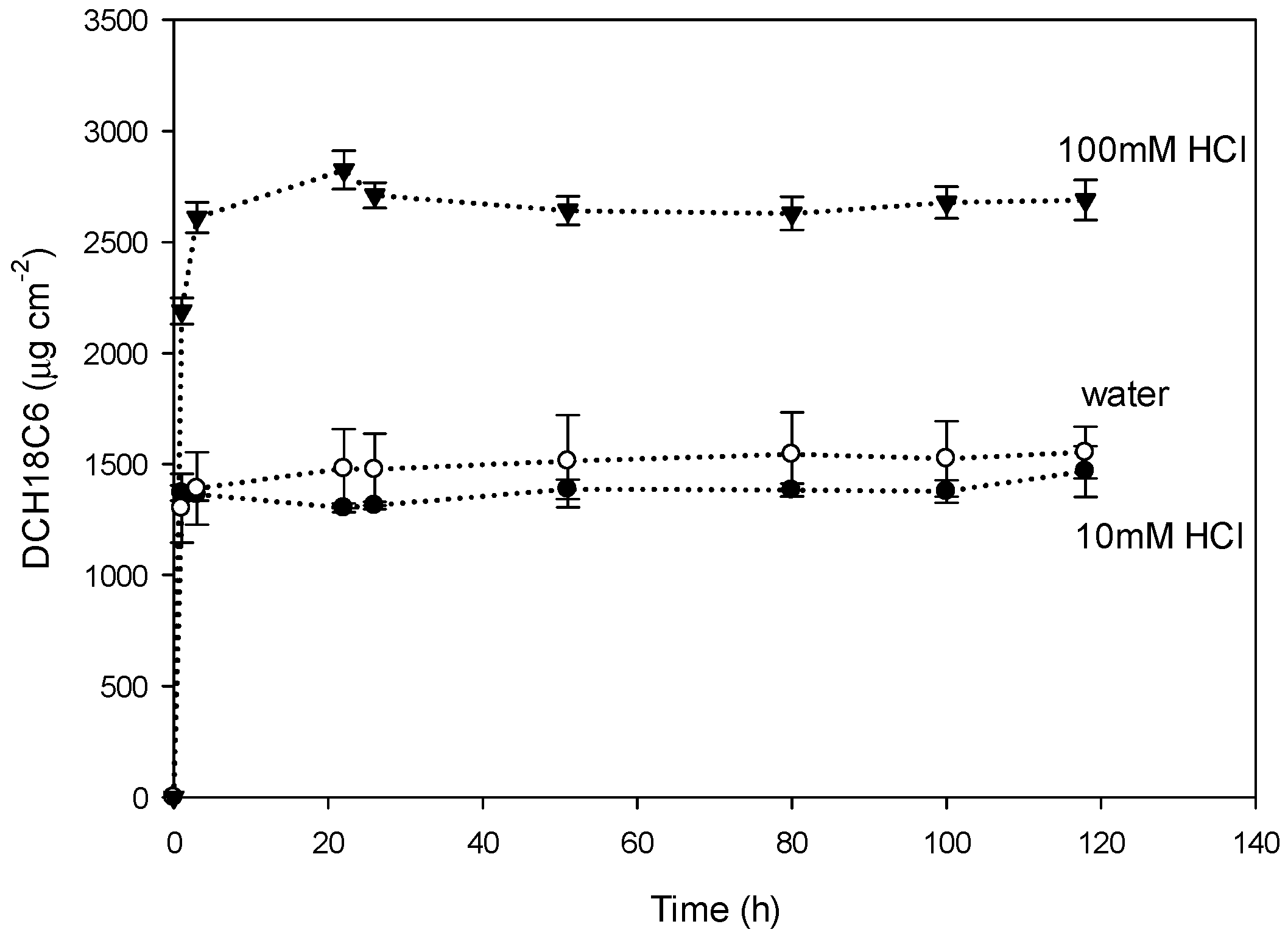
3.3.3. Effect of the Receiving Phase on Membrane Stability
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| A | area |
| BLM | bulk liquid membrane |
| CTA | cellulose triacetate |
| DCM | dichloromethane |
| DCH18C6 | dicyclohexan-18-crown-6 |
| ELM | emulsion liquid membrane |
| ISE | ion-selective electrode |
| Ji | flux ion i |
| LC-MS | liquid chromatography—mass spectrometry |
| Mn+ | cation |
| PIM | polymer inclusion membrane |
| PMMA | poly (methyl) acrylate |
| PVC | polyvinyl chloride |
| SEM | scanning electron microscope |
| SLM | supported liquid membrane |
| V | volume |
| Xn- | anion |
| 2-NPOE | 2-nitrophenyl octyl ether |
| αi,j | relative selectivity |
References
- Korstick, D. Minerals handbook; United States Geological Survey: Moab, UT, USA, 2005.
- Zhang, S.; Lim, C.Y.; Chen, C.-L.; Liu, H.; Wang, J.-Y. Urban nutrient recovery from fresh human urine through cultivation of chlorella sorokiniana. J. Environ. Manag. 2014, 145, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Udert, K.M.; Wächter, M. Complete nutrient recovery from source-separated urine by nitrification and distillation. Water Res. 2012, 46, 453–464. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, J.A.; Boyer, T.H. Phosphate recovery using hybrid anion exchange: Applications to source-separated urine and combined wastewater streams. Water Res. 2013, 47, 5003–5017. [Google Scholar] [CrossRef] [PubMed]
- Tice, R.C.; Kim, Y. Energy efficient reconcentration of diluted human urine using ion exchange membranes in bioelectrochemical systems. Water Res. 2014, 64, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Diem, K.; Lentner, C. Documenta geigy: Scientific Tables, 7st ed.; Georg Thieme Verlag Stuttgart: New York, NY, USA, 1975. [Google Scholar]
- Rockstroem, J.; Steffen, W.; Noone, K.; Persson, A.; Chapin, F.S.; Lambin, E.F.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. A safe operating space for humanity. Nature 2009, 461, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Inoue, Y.; Yamazaki, Y.; Hayashi, F.; Kanbara, S.; Matsuishi, S.; Yokoyama, T.; Kim, S.W.; Hara, M.; Hosono, H. Ammonia synthesis using a stable electride as an electron donor and reversible hydrogen store. Nat. Chem. 2012, 4, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Kuntke, P.; Sleutels, T.H.J.A.; Saakes, M.; Buisman, C.J.N. Hydrogen production and ammonium recovery from urine by a microbial electrolysis cell. Int. J. Hydrog. Energy 2014, 39, 4771–4778. [Google Scholar] [CrossRef]
- Kuntke, P.; Śmiech, K.; Bruning, H.; Zeeman, G.; Saakes, M.; Sleutels, T.; Hamelers, H.; Buisman, C. Ammonium recovery and energy production from urine by a microbial fuel cell. Water Res. 2012, 46, 2627–2636. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Schwegler, P.; Larsen, T.A. Nutrients in urine: Energetic aspects of removal and recovery. Water Sci. Technol. 2003, 48, 37–46. [Google Scholar] [PubMed]
- Nightingale, E.R. Phenomenological theory of ion solvation. Effective radii of hydrated ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Lide, D.R. Handbook of Chemistry and Physics, 85st ed.; CRC Press: London, UK, 2004. [Google Scholar]
- Shukla, J.P.; Kumar, A.; Singh, R.K. Macrocycle-mediated selective transport of plutonium(iv) nitrate through bulk liquid and supported liquid membranes using dicyclohexano-18-crown-6 as mobile carrier. Sep. Sci. Technol. 1992, 27, 447–465. [Google Scholar] [CrossRef]
- Tomar, J.; Awasthy, A.; Sharma, U. Synthetic ionophores for the separation of Li+, Na+, K+, Ca2+, Mg2+ metal ions using liquid membrane technology. Desalination 2008, 232, 102–109. [Google Scholar] [CrossRef]
- Belkhouche, N.E.; Didi, M.A.; Romero, R.; Jönsson, J.A.; Villemin, D. Study of new organophosphorus derivates carriers on the selective recovery of M (II) and M (III) metals, using supported liquid membrane extraction. J. Membr. Sci. 2006, 284, 398–405. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Zand, H.R.K. Selective transport of bismuth ions through supported liquid membrane. Chem. Eng. Technol. 2005, 28, 892–898. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Mornane, P.; Potter, I.D.; Perera, J.M.; Cattrall, R.W.; Kolev, S.D. Extraction and transport of metal ions and small organic compounds using polymer inclusion membranes (PIMs). J. Membr. Sci. 2006, 281, 7–41. [Google Scholar] [CrossRef]
- Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Recent trends in extraction and transport of metal ions using polymer inclusion membranes (PIMs). J. Membr. Sci. 2012, 415–416, 9–23. [Google Scholar] [CrossRef]
- Schow, A.J.; Peterson, R.T.; Lamb, J.D. Polymer inclusion membranes containing macrocyclic carriers for use in cation separations. J. Membr. Sci. 1996, 111, 291–295. [Google Scholar] [CrossRef]
- Vázquez, M.I.; Romero, V.; Fontàs, C.; Anticó, E.; Benavente, J. Polymer inclusion membranes (PIMs) with the ionic liquid (IL) aliquat 336 as extractant: Effect of base polymer and IL concentration on their physical-chemical and elastic characteristics. J. Membr. Sci. 2014, 455, 312–319. [Google Scholar] [CrossRef]
- Almeida, M.I.G.S.; Silva, A.M.L.; Cattrall, R.W.; Kolev, S.D. A study of the ammonium ion extraction properties of polymer inclusion membranes containing commercial dinonylnaphthalene sulfonic acid. J. Membr. Sci. 2015, 478, 155–162. [Google Scholar] [CrossRef]
- Arous, O.; Amara, M.; Trari, M.; Bouguelia, A.; Kerdjoudj, H. Cadmium (II) and lead (II) transport in a polymer inclusion membrane using tributyl phosphate as mobile carrier and CuFeO2 as a polarized photo electrode. J. Hazard. Mater. 2010, 180, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Ulewicz, M.; Szczygelska-Tao, J.; Biernat, J.F. Selectivity of Pb(II) transport across polymer inclusion membranes doped with imidazole azothiacrown ethers. J. Membr. Sci. 2009, 344, 32–38. [Google Scholar] [CrossRef]
- Thunhorst, K.L.; Noble, R.D.; Bowman, C.N. Properties of the transport of alkali metal salts through polymeric membranes containing benzo-18-crown-6 crown ether functional groups. J. Membr. Sci. 1999, 156, 293–302. [Google Scholar] [CrossRef]
- Heng, L.Y.; Hall, E.A.H. Methacrylate-acrylate based polymers of low plasticiser content for potassium ion-selective membranes. Anal. Chim. Acta 1996, 324, 47–56. [Google Scholar] [CrossRef]
- Heng, L.Y.; Toth, K.; Hall, E.A.H. Ion-transport and diffusion coefficients of non-plasticised methacrylic-acrylic ion-selective membranes. Talanta 2004, 63, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Alvarez, G.; Bautista-Flores, A.N.; de San Miguel, E.R.; Muhammed, M.; de Gyves, J. Transport characterisation of a PIM system used for the extraction of Pb(II) using D2EHPA as carrier. J. Membr. Sci. 2005, 250, 247–257. [Google Scholar] [CrossRef]
- Fontàs, C.; Tayeb, R.; Dhahbi, M.; Gaudichet, E.; Thominette, F.; Roy, P.; Steenkeste, K.; Fontaine-Aupart, M.-P.; Tingry, S.; Tronel-Peyroz, E.; et al. Polymer inclusion membranes: The concept of fixed sites membrane revised. J. Membr. Sci. 2007, 290, 62–72. [Google Scholar] [CrossRef]
- Ulewicz, M.; Lesinska, U.; Bochenska, M.; Walkowiak, W. Facilitated transport of Zn(II), Cd(II) and Pb(II) ions through polymer inclusion membranes with calix[4]-crown-6 derivatives. Sep. Purif. Technol. 2007, 54, 299–305. [Google Scholar] [CrossRef]
- Yilmaz, A.; Arslan, G.; Tor, A.; Akin, I. Selectively facilitated transport of Zn(II) through a novel polymer inclusion membrane containing cyanex 272 as a carrier reagent. Desalination 2011, 277, 301–307. [Google Scholar] [CrossRef]
- Zawierucha, I.; Kozlowski, C.; Malina, G. Removal of toxic metal ions from landfill leachate by complementary sorption and transport across polymer inclusion membranes. Waste Manag. 2013, 33, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- Benosmane, N.; Hamdi, S.M.; Hamdi, M.; Boutemeur, B. Selective transport of metal ions across polymer inclusion membranes (PIMs) containing calix[4]resorcinarenes. Sep. Purif. Technol. 2009, 65, 211–219. [Google Scholar] [CrossRef]
- Sugiura, M.; Kikkawa, M.; Urita, S. Carrier-mediated transport of rare earth ions through cellulose triacetate membranes. J. Membr. Sci. 1989, 42, 47–55. [Google Scholar] [CrossRef]
- Reid, C.E.; Breton, E.J. Water and ion flow across cellulosic membranes. J. Appl. Polym. Sci. 1959, 1, 133–143. [Google Scholar] [CrossRef]
- Geise, G.M.; Paul, D.R.; Freeman, B.D. Fundamental water and salt transport properties of polymeric materials. Prog. Polym. Sci. 2014, 39, 1–42. [Google Scholar] [CrossRef]
- Sears, J.K.; Darby, J.R. Technology of Plasticizers; John Wiley & Sons: New York, NY, USA, 1982. [Google Scholar]
- Gherrou, A.; Kerdjoudj, H.; Molinari, R.; Seta, P. Preparation and characterization of polymeric plasticized membranes (PPM) embedding a crown ether carrier application to copper ions transport. Mater. Sci. Eng. C 2005, 25, 436–443. [Google Scholar] [CrossRef]
- Gardner, J.S.; Walker, J.O.; Lamb, J.D. Permeability and durability effects of cellulose polymer variation in polymer inclusion membranes. J. Membr. Sci. 2004, 229, 87–93. [Google Scholar] [CrossRef]
- Gherrou, A.; Kerdjoudj, H.; Molinari, R.; Seta, P.; Drioli, E. Fixed sites plasticized cellulose triacetate membranes containing crown ethers for silver(I), copper(II) and gold(III) ions transport. J. Membr. Sci. 2004, 228, 149–157. [Google Scholar] [CrossRef]





| Cation | Hydrated radii (Å) | Diffusion coefficient (10−5·cm2·s−1) |
|---|---|---|
| K+ | 3.31 | 1.96 |
| Na+ | 3.58 | 1.33 |
| NH4+ | 3.31 | 1.96 |
| Assigned name | DCH18C6 (wt %) | CTA (wt %) | 2-NPOE (wt %) |
|---|---|---|---|
| PIM-1 | 0.0 | 100 | 0.0 |
| PIM-2 | 0.0 | 80.0 | 20.0 |
| PIM-3 | 0.0 | 60.0 | 40.0 |
| PIM-4 | 0.0 | 40.0 | 60.0 |
| PIM-5 | 0.0 | 20.0 | 80.0 |
| PIM-6 | 1.56 | 78.7 | 19.7 |
| PIM-7 | 14.0 | 68.8 | 17.2 |
| PIM-8 | 24.8 | 60.2 | 15.0 |
| PIM-9 | 33.2 | 53.4 | 13.4 |
| Assigned name | Ratio 2-NPOE vs. CTA | Content CTA (wt %) | Content 2-NPOE (wt %) | Content CTA (10−2·g·cm−2) | Content 2-NPOE (10−2·g·cm−2) | JK (10−3 mmol·cm−2·h−1) |
|---|---|---|---|---|---|---|
| PIM-1 | 0.0 | 100 | 0.0 | 4.70 | 0.0 | 0.57 ± 0.04 |
| PIM-2 | 0.25 | 80.0 | 20.0 | 3.76 | 0.94 | 7.02 ± 0.32 |
| PIM-3 | 0.67 | 60.0 | 40.0 | 2.82 | 1.88 | 4.38 ± 0.24 |
| PIM-4 | 1.50 | 40.0 | 60.0 | 1.88 | 2.88 | 1.90 ± 0.08 |
| Assigned name | DCH18C6 (wt %) | Content of DCH181C6 (10−3g cm−2) | αNa,K (-) | αNH4,K (-) |
|---|---|---|---|---|
| PIM-6 | 1.56 | 0.07 | 5.35 | 50.1 |
| PIM-7 | 14.0 | 0.66 | 27.9 | 295 |
| PIM-8 | 24.8 | 1.18 | 21.4 | 86.0 |
| PIM-9 | 33.2 | 1.55 | 7.02 | 133 |
| Feed phase | PIM bulk | Receiving phase |
|---|---|---|
| K+ NO3− → | K+[DCH18C6] ... NO3− → | K+ NO3− |
| H+ Cl− | ← H+ Cl− | ← H+ Cl− |
| Time (h) | Receiving phase | J (10−3 mmol·cm−2·h−1) | JNa (10−3 mmol·cm−2·h−1) | JNH4 (10−3 mmol·cm−2·h−1) | αNa,K (−) | αNH4,K (−) |
|---|---|---|---|---|---|---|
| 50 | water | 6.8 ± 0.4 | 2.4 ± 1.4 | 0.8 ± 0.1 | 21.4 | 86.0 |
| HCl | 3.8 ± 0.6 | 2.0 ± 0.3 | 0.7 ± 0.1 | 11.6 | 38.3 | |
| 120 | water | N/A | N/A | N/A | N/A | N/A |
| HCl | 6.3 ± 0.9 | 5.8 ± 0.7 | 3.8 ± 0.7 | 5.30 | 14.8 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casadellà, A.; Schaetzle, O.; Nijmeijer, K.; Loos, K. Polymer Inclusion Membranes (PIM) for the Recovery of Potassium in the Presence of Competitive Cations. Polymers 2016, 8, 76. https://doi.org/10.3390/polym8030076
Casadellà A, Schaetzle O, Nijmeijer K, Loos K. Polymer Inclusion Membranes (PIM) for the Recovery of Potassium in the Presence of Competitive Cations. Polymers. 2016; 8(3):76. https://doi.org/10.3390/polym8030076
Chicago/Turabian StyleCasadellà, Anna, Olivier Schaetzle, Kitty Nijmeijer, and Katja Loos. 2016. "Polymer Inclusion Membranes (PIM) for the Recovery of Potassium in the Presence of Competitive Cations" Polymers 8, no. 3: 76. https://doi.org/10.3390/polym8030076