ICAR ATRP of Acrylonitrile under Ambient and High Pressure
Abstract
:1. Introduction
2. Experimental Section
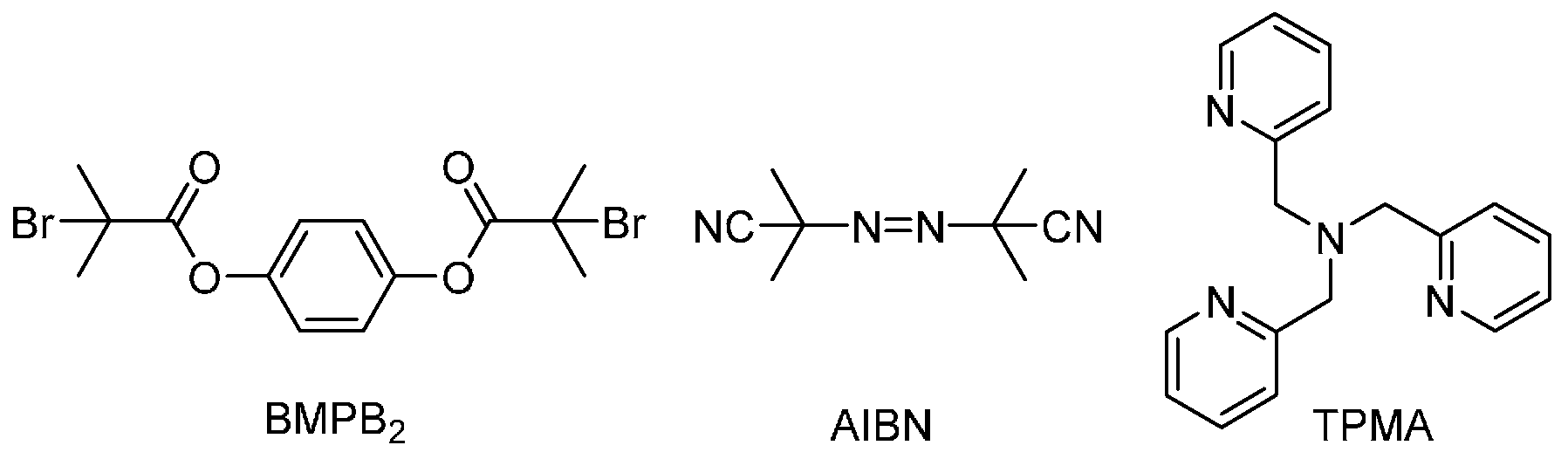
2.1. Materials
2.2. Typical Procedure for Initiators for a Continuous Activator Regeneration Atom Transfer Radical Polymerization (ICAR ATRP) of Acrylonitrile (AN) under Ambient or High Pressure
2.3. Characterizations
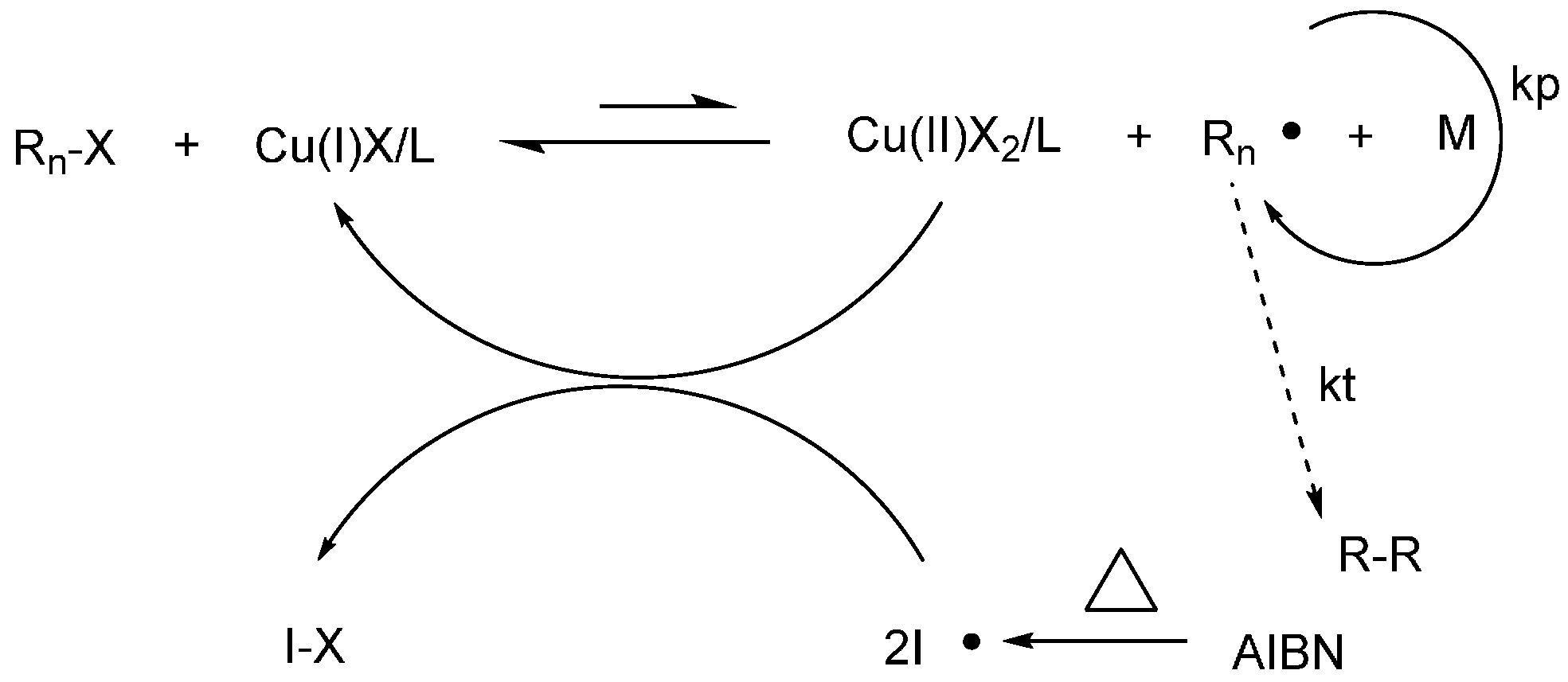
3. Results and Discussion
3.1. Effect of Catalyst Concentration on ICAR ATRP of AN under Ambient Pressure
3.2. Effect of Temperature on ICAR ATRP of AN under Ambient and High Pressure
3.3. Polymerization Kinetics and Chain-End Analysis
3.4. Synthesis of High Molecular Weight Polyacrylonitrile (PAN)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, J.S.; Matyjaszewski, K. Controlled/”living” radical polymerization. Atom-transfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc. 1995, 117, 5614–5615. [Google Scholar] [CrossRef]
- Kato, M.; Kamigaito, M.; Sawamoto, M.; Higashimura, T. Polymerization of methyl methacrylate with the carbon-tetrachloride dichlorotris(triphenylphosphine)ruthenium(II) methylaluminum bis(2,6-di-tert-butylphenoxide) initiating system: Possibility of living radical polymerization. Macromolecules 1995, 28, 1721–1723. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Xia, J.H. Atom transfer radical polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, M.; Terashima, T.; Sawamoto, M. Transition metal-catalyzed living radical polymerization: Toward perfection in catalysis and precision polymer synthesis. Chem. Rev. 2009, 109, 4963–5050. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.H.; Matyjaszewski, K. Homogeneous reverse atom transfer radical polymerization of styrene initiated by peroxides. Macromolecules 1999, 32, 5199–5202. [Google Scholar] [CrossRef]
- Zhu, S.M.; Yan, D.Y.; Zhang, G.S. Reverse atom transfer radical polymerization of methyl methacrylate with a new catalytic system, FeCl3/isophthalic acid. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 765–774. [Google Scholar] [CrossRef]
- Cheng, Z.P.; Zhu, X.L.; Chen, G.J.; Xu, W.J.; Lu, J.M. Reverse atom transfer radical solution polymerization of methyl methacrylate under pulsed microwave irradiation. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 3823–3834. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, L.F.; Jiang, X.W.; Tian, C.; Zhao, X.N.; Ke, Q.; Pan, X.Q.; Cheng, Z.P.; Zhu, X.L. Facile iron-mediated dispersant-free suspension polymerization of methyl methacrylate via reverse ATRP in water. Macromol. Rapid Commun. 2013, 34, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shentu, B.Q.; Weng, Z.X. One-pot synthesis of poly(2,6-dimethyl-1,4-phenylene oxide)/polystyrene alloy with CuCl2/4-dimethylaminopyridine as a versatile catalyst in water. RSC Adv. 2014, 4, 510–515. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Jakubowski, W.; Min, K.; Tang, W.; Huang, J.Y.; Braunecker, W.A.; Tsarevsky, N.V. Diminishing catalyst concentration in atom transfer radical polymerization with reducing agents. Proc. Natl. Acad. Sci. USA 2006, 103, 15309–15314. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.H.; Zhang, L.F.; Zhang, Z.B.; Zhu, J.; Tu, Y.F.; Cheng, Z.P.; Zhu, X.L. Iron-mediated ICAR ATRP of methyl methacrylate. Macromolecules 2011, 44, 3233–3239. [Google Scholar] [CrossRef]
- Ding, M.Q.; Jiang, X.W.; Peng, J.Y.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Diffusion-regulated phase-transfer catalysis for atom transfer radical polymerization of methyl methacrylate in an aqueous/organic biphasic system. Macromol. Rapid Commun. 2015, 36, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.L.; Zhang, B.J.; Jiang, X.W.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Cu(II)-mediated atom transfer radical polymerization of methyl methacrylate via a strategy of thermo-regulated phase-separable catalysis in a liquid/liquid biphasic system: homogeneous catalysis, facile heterogeneous separation, and recycling. Macromol. Rapid Commun. 2014, 35, 1615–1621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.J.; Liu, X.H. ICAR ATRP of acrylonitrile utilizing a moderate temperature radical initiator. Chin. J. Polym. Sci. 2013, 31, 1613–1622. [Google Scholar] [CrossRef]
- Wang, G.X.; Lu, M.; Hou, Z.H.; Gao, Y.; Liu, L.C.; Wu, H. Fe-mediated ICAR ATRP of styrene and acrylonitrile in polyethylene glycol. J. Appl. Polym. Sci. 2014, 131, 40135. [Google Scholar] [CrossRef]
- D’hooge, D.; Konkolewicz, D.; Reyniers, M.; Marin, G.B.; Matyjaszewski, K. Kinetic modeling of ICAR ATRP. Macromol. Theory Simul. 2012, 21, 52–69. [Google Scholar] [CrossRef]
- Souza, F.L.; Sáez, C.; Caňizares, P.; Motheo, A.J.; Rodrigo, M.A. Sonoelectrolysis of wastewaters polluted with dimethyl phthalate. Ind. Eng. Chem. Res. 2013, 52, 9674–9682. [Google Scholar] [CrossRef]
- D’hooge, D.; Steenberge, P.H.M.; Reyniers, M.F.; Marin, G.B. Fed-batch control and visualization of monomer sequences of individual ICAR ATRP gradient copolymer chains. Polymers 2014, 6, 1074–1095. [Google Scholar] [CrossRef]
- Jakubowski, W.; Matyjaszewski, K. Activator generated by electron transfer for atom transfer radical polymerization. Macromolecules 2005, 38, 4139–4146. [Google Scholar] [CrossRef]
- Bai, L.J.; Zhang, L.F.; Zhang, Z.B.; Tu, Y.F.; Zhou, N.C.; Cheng, Z.P.; Zhu, X.L. Iron-mediated AGET ATRP of styrene in the presence of catalytic amounts of base. Macromolecules 2010, 43, 9283–9290. [Google Scholar] [CrossRef]
- Ding, M.Q.; Jiang, X.W.; Peng, J.Y.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. An atom transfer radical polymerization system: catalyzed by an iron catalyst in PEG-400. Green Chem. 2015, 17, 271–278. [Google Scholar] [CrossRef]
- Magenau, A.J.D.; Strandwitz, N.C.; Gennaro, A.; Matyjaszewski, K. Electrochemically mediated atom transfer radical polymerization. Science 2011, 332, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Bortolamei, N.; Isse, A.A.; Magenau, A.J.D.; Gennaro, A.; Matyjaszewski, K. Controlled aqueous atom transfer radical polymerization with electrochemical generation of the active catalyst. Angew. Chem. Int. Ed. 2011, 50, 11391–11394. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.P.; Fu, Y.; Bao, X.C.; Feng, X.S.; Wang, Y.; Liu, W.H. Electrochemically mediated atom transfer radical polymerization of iminodiacetic acid-functionalized poly(glycidyl methacrylate)grafted at carbon fibers for nano-nickel recovery from spent electroless nickel plating baths. J. Appl. Electrochem. 2014, 44, 621–629. [Google Scholar] [CrossRef]
- Shida, N.; Koizumi, Y.; Nishiyama, H.; Tomita, I.; Inagi, S. Electrochemically mediated atom transfer radical polymerization from a substrate surface manipulated by bipolar electrolysis: Fabrication of gradient and patterned polymer brushes. Angew. Chem. Int. Ed. 2015, 54, 3922–3926. [Google Scholar] [CrossRef] [PubMed]
- Treat, N.J.; Sprafke, H.; Kramer, J.W.; Clark, P.G.; Barton, B.E.; de Alaniz, J.R.; Fors, B.P.; Hawker, C.J. Metal-free atom transfer radical polymerization. J. Am. Chem. Soc. 2014, 136, 16096–16101. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.C.; Lamson, M.; Yan, J.J.; Matyjaszewski, K. Photoinduced metal-free atom transfer radical polymerization of acrylonitrile. ACS Macro Lett. 2015, 4, 192–196. [Google Scholar] [CrossRef]
- Miyake, G.M.; Theriot, J.C. Perylene as an organic photocatalyst for the radical polymerization of functionalized vinyl monomers through oxidative quenching with alkyl bromides and visible light. Macromolecules 2014, 47, 8255–8261. [Google Scholar] [CrossRef]
- Ding, M.Q.; Jiang, X.W.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Recent progress on transition metal catalyst separation and recycling in ATRP. Macromol. Rapid Commun. 2015, 36, 1702–1721. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, L.; Cheng, Z.; Zhu, X. Metal-free photoinduced electron transfer–atom transfer radical polymerization (PET–ATRP) via a visible light organic photocatalyst. Polym. Chem. 2016, 7, 689–700. [Google Scholar] [CrossRef]
- Arita, T.; Buback, M.; Janssen, O.; Vana, P. RAFT-polymerization of styrene up to high pressure: Rate enhancement and improved control. Macromol. Rapid Commun. 2004, 25, 1376–1381. [Google Scholar] [CrossRef]
- Wang, R.; Luo, Y.W.; Sun, X.Y.; Zhu, S.P. Design and control of copolymer composition distribution in living radical polymerization using semi-batch feeding policies: A model simulation. Macromol. Theory Simul. 2006, 15, 356–368. [Google Scholar] [CrossRef]
- Barner-Kowollik, C.; Russell, G.T. Chain-length-dependent termination in radical polymerization: Subtle revolution in tackling a long-standing challenge. Prog. Polym. Sci. 2009, 34, 1211–1259. [Google Scholar] [CrossRef]
- D’hooge, D.; Reyniers, M.F.; Marin, G.B. The crucial role of diffusional limitations in controlled radical polymerization. Macromol. React. Eng. 2013, 7, 362–379. [Google Scholar] [CrossRef]
- Monteiro, M.J.; Bussels, R.; Beuermann, S.; Buback, M. High-pressure “living” free-radical polymerization of styrene in the presence of RAFT. Aust. J. Chem. 2002, 55, 433–437. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, X.N.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Reversible addition-fragmentation chain transfer polymerization of vinyl acetate under high pressure. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1430–1436. [Google Scholar] [CrossRef]
- Rzayev, J.; Penelle, J. Controlled/living free-radical polymerization under very high pressure. Macromolecules 2002, 35, 1489–1490. [Google Scholar] [CrossRef]
- Rzayev, J.; Penelle, J. HP-RAFT: A free-radical polymerization technique for obtaining living polymers of ultrahigh molecular weights. Angew. Chem. Int. Ed. 2004, 43, 1691–1694. [Google Scholar] [CrossRef] [PubMed]
- Arita, T.; Kayama, Y.; Ohno, K.; Tsujii, Y.; Fukuda, T. High-pressure atom transfer radical polymerization of methyl methacrylate for well-defined ultrahigh molecular-weight polymers. Polymer 2008, 49, 2426–2429. [Google Scholar] [CrossRef]
- Mueller, L.; Jakubowski, W.; Matyjaszewski, K.; Pietrasik, J.; Kwiatkowski, P.; Chaladaj, W.; Jurczak, J. Synthesis of high molecular weight polystyrene using AGET ATRP under high pressure. Eur. Polym. J. 2011, 47, 730–734. [Google Scholar] [CrossRef]
- Pitto, V.; Voit, B.I.; Loontjens, T.J.A.; van Benthem, R.A.T.M. New star-branched poly(acrylonitrile) architectures: ATRP synthesis and solution properties. Macromol. Chem. Phys. 2004, 205, 2346–2355. [Google Scholar] [CrossRef]
- Liu, X.H.; Wang, J.; Yang, J.S.; An, S.L.; Ren, Y.L.; Yu, Y.H.; Chen, P. Fast copper catalyzed living radical polymerization of acrylonitrile utilizing a high concentration of radical initiator. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 1933–1940. [Google Scholar] [CrossRef]
- Barboiu, B.; Percec, V. Metal catalyzed living radical polymerization of acrylonitrile initiated with sulfonyl chlorides. Macromolecules 2001, 34, 8626–8636. [Google Scholar] [CrossRef]
- Chen, H.; Wang, C.H.; Liu, D.L.; Song, Y.T.; Qu, R.J.; Sun, C.M.; Ji, C.N. AGET ATRP of acrylonitrile using 1,1,4,7,10,10-hexamethyltriethylenetetramine as both ligand and reducing agent. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 128–133. [Google Scholar] [CrossRef]
- Tsai, J.S.; Lin, C.H. The Effect of the side-chain of acrylate comonomers on the orientation, pore-size distribution, and properties of polyacrylonitrile precursor and resulting carbon-fiber. J. Appl. Polym. Sci. 1991, 42, 3039–3044. [Google Scholar] [CrossRef]
- Chae, H.G.; Newcomb, B.A.; Gulgunje, P.V.; Liu, Y.D.; Gupta, K.K.; Kamath, M.G.; Lyons, K.M.; Ghoshal, S.; Pramanik, C.; Giannuzzi, L.; et al. High strength and high modulus carbon fibers. Carbon 2015, 93, 81–87. [Google Scholar] [CrossRef]
- Gulgunje, P.V.; Newcomb, B.A.; Gupta, K.; Chae, H.G.; Tsotsis, T.K.; Kumar, S. Low-density and high-modulus carbon fibers from polyacrylonitrile with honeycomb structure. Carbon 2015, 95, 710–714. [Google Scholar] [CrossRef]
- Ho, R.M.; Wang, T.C.; Lin, C.C.; Yu, T.L. Mesoporous carbons from poly(acrylonitrile)-b-poly(ε-caprolactone) block copolymers. Macromolecules 2007, 40, 2814–2821. [Google Scholar] [CrossRef]
- Tsarevsky, N.V.; Matyjaszewski, K. “Green” atom transfer radical polymerization: From process design to preparation of well-defined environmentally friendly polymeric materials. Chem. Rev. 2007, 107, 2270–2290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.F.; Cheng, Z.P.; Lü, Y.T.; Zhu, X.L. A highly active iron-based catalyst system for the AGET ATRP of styrene. Macromol. Rapid Commun. 2009, 30, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.P.; Huang, J.; Chen, L.D.; Liu, Y.J.; Wang, G.W. Synthesis of thermal degradable poly(alkoxyamine) through a novel nitroxide radical coupling step growth polymerization mechanism. Macromolecules 2014, 47, 7812–7822. [Google Scholar] [CrossRef]
- Dong, H.C.; Tang, W.; Matyjaszewski, K. Well-defined high-molecular-weight polyacrylonitrile via activators regenerated by electron transfer ATRP. Macromolecules 2007, 40, 2974–2977. [Google Scholar] [CrossRef]
- Yuan, Y.X.; Johnson, F.; Cabasso, I. Polybenzimidazole (PBI) molecular weight and mark-houwink equation. J. Appl. Polym. Sci. 2009, 112, 3436–3441. [Google Scholar] [CrossRef]
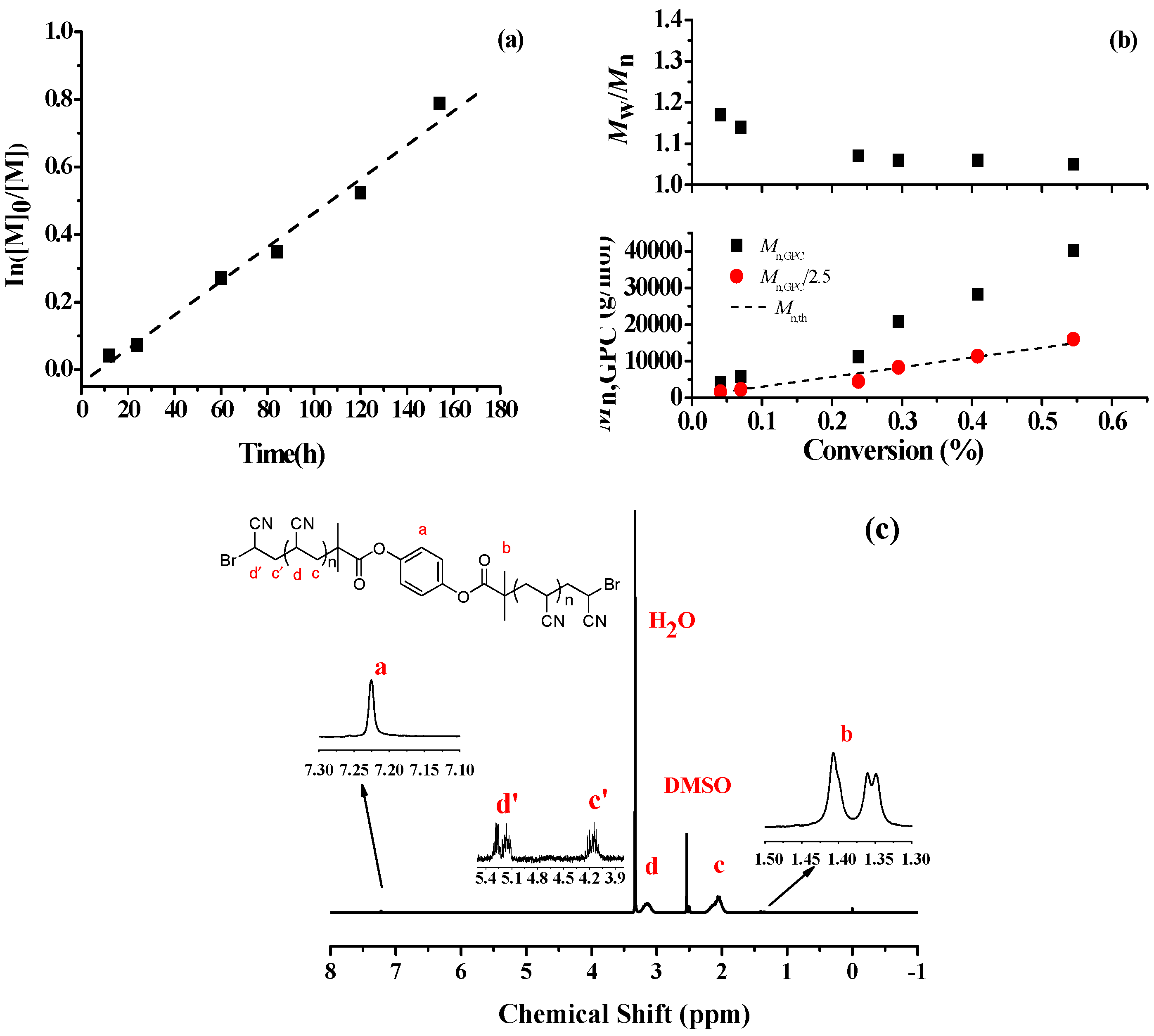
| Entry | x | Cu (ppm) | Time (h) | Conv. (%) | Mn,th b (g/mol) | Mn,GPC/2.5 (g/mol) | Mw/Mn |
|---|---|---|---|---|---|---|---|
| 1 | 0.5 | 250 | 23 | 44.9 | 47,600 | 47,600 | 1.20 |
| 2 | 0.1 | 50 | 9 | 36.5 | 38,600 | 50,400 | 1.28 |
| 3 | 0.02 | 10 | 9 | 74.9 | 79,400 | 69,640 | 1.33 |
| 4 | 0.005 | 2.5 | 9 | 37.7 | 40,000 | 85,560 | 1.80 |
| Entry | Time (h) | T (°C) | Conv. (%) | Mn,th b (g/mol) | Mn,GPC/2.5 (g/mol) | Mw/Mn |
|---|---|---|---|---|---|---|
| 1 | 21 | 60 | 61.9 | 16,400 | 18,200 | 1.06 |
| 2 | 22 | 50 | 54.7 | 14,500 | 16,880 | 1.10 |
| 3 | 45 | 40 | 43.5 | 11,500 | 14,040 | 1.06 |
| 4 | 120 | 30 | 40.8 | 10,800 | 11,300 | 1.06 |
| Entry | Time (h) | T (°C) | Conv. (%) | Mn,th b (g/mol) | Mn,GPC/2.5 (g/mol) | Mw/Mn |
|---|---|---|---|---|---|---|
| 1 | 1 | 60 | 45.1 | 11,900 | 13,880 | 1.14 |
| 2 | 4 | 50 | 41.7 | 11,000 | 13,120 | 1.13 |
| 3 | 7 | 40 | 51.3 | 13,600 | 16,640 | 1.11 |
| 4 | 8 | 30 | 21.3 | 5,600 | 7,520 | 1.12 |
| Entry | x | Conv. (%) | Mn,th d (g/mol) | Mη e (g/mol) | Mn,GPC/2.5 (g/mol) | Mw/Mn |
|---|---|---|---|---|---|---|
| 1 b | 10,000 | 32.1 | 169,900 | – | 190,200 | 1.18 |
| 2 b | 20,000 | 25.3 | 267,800 | – | 260,700 | 1.20 |
| 3 c | 10,000 | 81.4 | 431,600 | 541,200 | – | – |
| 4 c | 20,000 | 72.3 | 766,400 | 1,034,500 | – | – |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Chen, J.; Zhang, L.; Cheng, Z.; Zhu, X. ICAR ATRP of Acrylonitrile under Ambient and High Pressure. Polymers 2016, 8, 59. https://doi.org/10.3390/polym8030059
Huang Z, Chen J, Zhang L, Cheng Z, Zhu X. ICAR ATRP of Acrylonitrile under Ambient and High Pressure. Polymers. 2016; 8(3):59. https://doi.org/10.3390/polym8030059
Chicago/Turabian StyleHuang, Zhicheng, Jing Chen, Lifen Zhang, Zhenping Cheng, and Xiulin Zhu. 2016. "ICAR ATRP of Acrylonitrile under Ambient and High Pressure" Polymers 8, no. 3: 59. https://doi.org/10.3390/polym8030059